间歇性缺氧对大鼠氧化应激及肾缺氧诱导因子-1α mRNA表达的影响
2020-12-23杜艺梁顺柯剑婷魏玉婷裴雪峰
杜艺 梁顺 柯剑婷 魏玉婷 裴雪峰
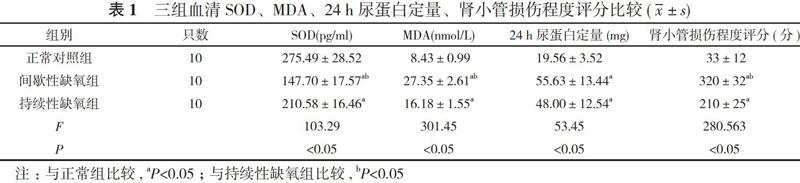
【摘要】 目的 分析在不同缺氧状态下大鼠肾组织中缺氧诱导因子-1αmRNA(HIF-1αmRNA)水平表达和血清超氧化物歧化酶(SOD)及丙二醛(MDA)含量变化, 探讨阻塞性睡眠呼吸暂停低通气综合征(OSAHS)肾损伤的特点。方法 30只大鼠, 采用随机数字表法分為正常对照组、间歇性缺氧组和持续性缺氧组, 每组10只。正常对照组大鼠不予任何处理, 剩余两组分别放入间歇性缺氧/再氧合动物舱建立慢性间歇性缺氧(CIH)和持续性缺氧大鼠模型。造模15 d后, 收集三组大鼠24 h尿液, 检测尿蛋白定量, 酶联免疫吸附试验(ELISA)方法检测各组血清SOD及MDA水平, 苏木精—伊红染色法(HE)染色观察肾组织形态学改变做肾小管损伤评分, 实时荧光定量聚合酶链反应(PCR)检测肾组织HIF- lα mRNA的表达量。比较三组一般情况、血清SOD、MDA、24 h尿蛋白定量、肾小管损伤程度评分、HIF-1αmRNA水平, 分析HIF-1α mRNA和血清MDA与24 h尿蛋白定量、肾小管损伤程度的相关性。结果 正常对照组大鼠反应敏捷, 毛发光亮, 精神状况良好。持续性缺氧组和间歇性缺氧组大鼠行动迟缓, 毛发稀疏, 消瘦。正常对照组体重(210.03±15.71)g明显高于间歇性缺氧组的(185.94±6.79)g和持续性缺氧组的(169.18±10.23)g, 间歇性缺氧组体重高于持续性缺氧组, 差异均有统计学意义(P<0.05)。正常对照组血清SOD水平明显高于间歇性缺氧组和持续性缺氧组, 24 h尿蛋白定量、血清MDA、肾小管损伤程度评分均明显低于间歇性缺氧组和持续性缺氧组, 差异均有统计学意义(P<0.05)。间歇性缺氧组血清MDA、肾小管损伤程度评分均明显高于持续性缺氧组, 血清SOD水平明显低于持续性缺氧组, 差异均有统计学意义(P<0.05)。间歇性缺氧组和持续性缺氧组24 h尿蛋白定量比较差异无统计学意义(P>0.05)。正常对照组HIF-1αmRNA水平(0.23±0.18)明显低于间歇性缺氧组的(0.78±0.33)、持续性缺氧组的(0.52±0.13), 而间歇性缺氧组HIF-1αmRNA水平高于持续性缺氧组, 差异均有统计学意义(P<0.05)。HIF-1α mRNA与24 h尿蛋白定量、肾小管损伤程度呈正相关(r=0.65、0.78, P<0.05);血清MDA与24 h尿蛋白定量、肾小管损伤程度呈正相关(r=0.68、0.72, P<0.05)。结论 慢性缺氧抑制大鼠生长发育, 出现肾功能损伤, CIH以肾小管的损伤更加明显, CIH与持续性缺氧相比较高水平的 HIF-1αmRNA表达提示机体处于缺氧应激状态, CIH肾损害可能与氧化应激状态及高水平的HIF-1αmRNA表达有关。
【关键词】 间歇性缺氧;氧化应激;缺氧诱导因子
DOI:10.14163/j.cnki.11-5547/r.2020.32.084
【Abstract】 Objective To analyze the expression of hypoxia-inducible factor-1 α mRNA (HIF-1 α mRNA) and the changes of superoxide dismutase (SOD) and malondialdehyde (MDA) in rats under different hypoxia conditions, and to discuss the characteristics of renal injury in obstructive sleep apnea hypopnea syndrome (OSAHS). Methods A total of 30 rats were randomly divided into normal control group, intermittent hypoxia group and persistent hypoxia group according to random numerical table, with 10 rats in each group. The rats in the normal control group were not treated, and the remaining two groups were put into intermittent hypoxia/reoxygenation animal cabins to establish chronic intermittent hypoxia (CIH) and persistent hypoxia rat models. After 15 d of modeling, the 24 h urine of the three groups of rats was collected, and the urine protein was measured. The enzyme-linked immunosorbent assay (ELISA) method was used to detect the serum SOD and MDA levels in each group. Hematoxylin-Eosin (HE) staining was used to observe the morphological changes of renal tissues to score renal tubular damage, and real-time fluorescent quantitative polymerase chain reaction (PCR) to detect the expression of HIF-1α mRNA in renal tissues. The general condition, serum SOD, MDA, 24-h urinary protein quantification, renal tubular injury score, HIF-1 α mRNA level were compared among the three groups, and the correlation between HIF-1 α mRNA, serum MDA and 24-h urinary protein quantification, renal tubular injury degree was analyzed. Results The rats in the normal control group had quick response, bright hair and good mental condition. The rats in the persistent hypoxia group and the intermittent hypoxia group were slow, with sparse hair and weight loss. The weight (210.03±15.71) g of normal control group was obviously higher than (185.94±6.79) g of persistent hypoxia group and (169.18±10.23) g of intermittent hypoxia group, and intermittent hypoxia group was higher than persistent hypoxia group, and the difference was statistically significant (P<0.05). The serum SOD of normal control group was obviously higher than intermittent hypoxia group and persistent hypoxia group, and 24-h urinary protein quantification, renal tubular injury score were obviously lower than intermittent hypoxia group and persistent hypoxia group, and the difference was statistically significant (P<0.05). The serum MDA and renal tubular injury score of intermittent hypoxia group were obviously higher than persistent hypoxia group, and serum SOD was obviously lower than persistent hypoxia group, and the difference was statistically significant (P<0.05). There was no statistically significant difference in 24-h urinary protein quantification between intermittent hypoxia group and persistent hypoxia group (P>0.05). HIF-1αmRNA (0.23±0.18) of normal control group was obviously lower than (0.78±0.33) of intermittent hypoxia group and (0.52±0.13) of persistent hypoxia group, and HIF-1αmRNA of intermittent hypoxia group was higher than persistent hypoxia group, and the difference was statistically significant (P<0.05). HIF-1α mRNA was positively correlated with 24-h urinary protein quantification and renal tubular injury (r=0.65, 0.78, P<0.05). Serum MDA was positively correlated with 24-h urinary protein quantification and renal tubular injury (r=0.68, 0.72, P<0.05). Conclusion Chronic hypoxia inhibits the growth and development of rats, resulting in renal function injury. The injury of renal tubules in CIH was more obvious. Compared with persistent hypoxia, the higher level of HIF-1αmRNA expression in CIH suggests that the body is in a state of hypoxic stress. CIH renal damage may be related to oxidative stress and high levels of HIF-1αmRNA expression.
【Key words】 Intermittent hypoxia; Oxidative stress; Hypoxia-inducible factor
阻塞性睡眠呼吸暂停低通气综合征(obstructive sleep apnea hypopnea syndrome, OSAHS)是临床常见的睡眠呼吸疾病, 可导致全身多系统功能损害[1]。与持续性缺氧不同, 睡眠呼吸暂停慢性间歇低氧是OSAHS 特有的特殊低氧模式。反复的低氧-复氧, 类似缺血再灌注, 细胞线粒体产生较多的活性氧簇(reactive oxygen species, ROS), 引起机体氧化应激, 是OSAHS导致机体损害的重要病理生理机制[2]。肾脏因其为高血流、高灌注脏器, 对氧供给与张力的变化更为敏感, 更易受到缺氧的损伤[3]。近年来对OSAHS肾损伤的研究逐渐受到重视。OSAHS肾损伤主要表现为夜间多尿、蛋白尿、肾小管功能受损等, 后期可发展为慢性肾衰竭, 但相关机制尚不清楚[4]。本实验根据OSAHS的典型病理生理特征, 建立慢性间歇性缺氧(chronic intermittent hypoxia, CIH)大鼠模型, 观察与持续缺氧方式对 Wistar大鼠肾功能、缺氧诱导因子-1αmRNA(hypoxia-inducible factor-lamRNA, HIF-1αmRNA)的表达、氧化应激影响的异同, 以期对OSAHS的临床治疗提供实验依据。
1 材料与方法
1. 1 材料来源 健康雄性Wistar大鼠30只, 4~6周龄, 体重18~200 g, 由中山大学实验动物中心提供, 饲养于本院实验动物中心。所有大鼠适应性饲养3 d, 自由饮水饮食, 饲养环境温度19~23℃, 空气湿度为 50%~60%。
1. 2 试剂 ELISA试剂盒购于上海铭睿生物科技有限公司, CIH/再氧合动物舱(广州市华粤仪器有限公司);ABI Prism 7300 SDS PCR仪为美国ABI公司产品;兔抗HIF-1多克隆抗体购自北京博奥森生物有限公司;Trizol购自ABI基因公司;引物序列由上海生物工程技术服务公司合成;绿色荧光核酸染料荧光定量PCR试剂盒购自广州达晖生物公司。
1. 3 分组及模型制备 将30只大鼠采用随机数字表法分为正常对照组、间歇性缺氧组和持续性缺氧组, 每组10只。每天上午8:00至下午4:00将间歇性缺氧组和持续性缺氧组大鼠分别放置于间歇性缺氧组舱和持续性缺氧舱内。间歇性缺氧组模型的建模方法:根据N2稀释原理, 循环充入N2和O2, 每一循环为9 min, 4 min充入N2, 5 min充入O2, 使箱内氧浓度在6%和21%切换;由测氧仪监测间歇性低氧舱中的氧浓度, 调节气体流量, 使每一循环间歇性低氧舱内的最低氧浓度达6%左右, 持续45 s左右, 然后再恢复至21%。该循环每天重复8 h, 共15 d, 建立间歇性低氧大鼠模型[5, 6]。持续性缺氧组放入氧浓度为(7.0±0.5)%的缺氧舱内。在实验过程中将整个实验舱罩上黑布, 模拟夜晚大鼠睡眠环境, 实验舱内及室内温度20~25℃, 湿度45%~55%。实验结束后, 所有大鼠放置普通饲养笼中饲养且给予人工日光照射, 全天饮食及活动自由, 模拟白天状态。正常对照组放置无盖箱内常规饲养, 不予任何处理。每天除实验时间外, 所有大鼠放置相同自然环境下生活和饲养。
1. 4 标本获取及检测 造模15 d后, 所有大鼠称重、代谢笼24 h尿量收集, 磺基水杨酸比浊法测定尿蛋白含量。后麻醉大鼠, 尾静脉收集血清, ELISA方法检测血清超氧化物歧化酶(superoxide dismutase, SOD)、丙二醛( malondialdehyde, MDA), 脊椎脱臼法处死大鼠。行肾静脉注射磷酸缓冲盐溶液(PBS), 肾洗至苍白再将肾取出, 部分肾组织放置于-70℃液氮保存, 用于荧光定量 PCR 检测;剩余组織放置于10%甲醛中性溶液中固定, 用于病理切片观察。
1. 5 肾脏小管损伤指数计算 肾组织常规固定, 脱水, 石蜡包埋, 切成4 μm 厚切片, 行HE染色。光镜下观察形态, 参考Paller等[7]的方法进行评分, 随机选择10个无重叠视野(×200), 每个视野下随机选择10处肾小管, 共100处肾小管计分, 依计分高低判定肾小管损伤程度。
1. 6 实时荧光定量PCR测定HIF-1 mRNA 引物设计使用 Primers 软件, 上游:5'-AGCCCTAGATGGCTTTGTGA-3', 下游:5'-TATCGAGGCTGTGTCGACTG-3'; β-actin:上游引物:5'-GACGGTCAGGTCATCACTATCG-3', 下游引物:5'-ACGGATGTCAACGTCACACTTC-3'。实时定量PCR反应参数:预变性94℃ 15 min, 然后94℃变性20 s, 60℃退火40 s, 72℃延伸30 s, 共35个循环。每个循环后采集荧光生成扩增曲线, 并在反应后生成熔点曲线。计算方法:待测样品mRNA相对表达量=2-ΔCT×100%;ΔCT=目标基因CT值-内参(以管家基因β-actin为内参) CT值, 采用仪器自带软件分析各组HIF-1 mRNA的相对表达量[8]。
1. 7 观察指标 比较三组一般情况、血清SOD、MDA、24 h尿蛋白定量、肾小管损伤程度评分、HIF-1αmRNA水平, 分析HIF-1α mRNA和血清MDA与24 h尿蛋白定量、肾小管损伤程度的相关性。
1. 8 统计学方法 采用SPSS21.0 软件进行统计分析, 定量资料以均数±标准差( x-±s)表示, 各组间均数比较采用单因素方差分析, 以 P<0.05 为差异有统计学意义。利用Pearson检测进行肾组织HIF-1αmRNA、血清MDA水平与尿蛋白定量及肾小管损伤指数评分的相关性分析。
综上所述, 慢性缺氧抑制大鼠生长发育, 出现肾功能损伤, CIH以肾小管的损伤更加明显, CIH与持续性缺氧相比较高水平的 HIF-1αmRNA表达提示机体处于缺氧应激状态, CIH肾损害可能与氧化应激状态及高水平的HIF-1αmRNA表达有关。
参考文献
[1] Carlos Z, Vanesa GP, Alberto R. Obstructive sleep apnea syndrome is a systemic disease. Eur J Intern Med, 2008(19):390-398.
[2] Forman HJ, Torres M, Fukuto J. Redox signaling. Mol Cell Bi, 2002, 23(4):49-62.
[3] 窦占军, 王蓓, 郭婧, 等. 氧化应激对阻塞性睡眠呼吸暂停低通气综合征肾损害的作用与抗氧化治疗现状. 中国药物与临床, 2017, 17(8):1160-1162.
[4] Mavanur M, Sanders M, Unruh M. Sleep disordered breathing in patients with chronic kidney disease. Indian Med Res, 2010(131):277-284.
[5] 马雪, 史清海, 伏建峰. 缺氧动物及细胞实验模型的研究进展. 西北国防医学杂志, 2016, 37(8):535-538.
[6] Costa-Silva JH, Zoccal DB, Machado BH. Chronic intermittent hypoxia alters gluta-matergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol, 2012, 302(6):785-793.
[7] Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest, 1984, 74(4):1156-1164.
[8] 陈凤花, 王琳, 胡丽华. 实时荧光定量RT-PCR内参基因的选择. 临床检验杂志, 2005, 23(5):393-395.
[9] Xie J, Wei YX, Liu S, et al. Obstructive sleep apnea hypopnea syndrome as a reason for active management of pulmonary embolism. Chinese Medical Journal, 2015, 128(16):2147-2153.
[10] Chou YT, Lee PH, Yang CT. Obstructive sleep apnea:a stand alone risk factor for chronic kidney disease. Nephr Di Tr, 2011, 26(7):2244-2250.
[11] 李婷, 李秀翠, 梁冬施, 等. 慢性間歇性低氧对大鼠肾脏氧化应激损伤及HIF-1α表达的影响. 中国病理生理杂志, 2015, 31(2):348-353.
[12] 曾勇, 王跃建, 陈伟雄, 等. 阻塞性睡眠呼吸暂停低通气综合征对儿童身高体重的影响. 临床耳鼻咽喉头颈外科杂志, 2013, 27(4):204-211.
[13] 许晓丽, 何志婷, 刘琼, 等. 肾康丸对慢性间歇性缺氧致肾损伤大鼠西部医学2019, 31(8):1185-1189.
[14] Wang X, Wang Y, Cai Z, et al. Alterations of IGF-1, complement C3 and superoxide dismutase in patients with moderate-to-severe obstructive sleep apnea hypopnea syndrome. Biomarkers in Medicine, 2018, 12(9):217-228.
[15] Zhou YH, Yu JP, Liu YF, et al. Effects of Ginkgo biloba Extract on Inflammatory Mediators(SOD, MDA, TNF-α, NF-κBp65, IL-6)in TNBS-Induced Colitis in Rats. Mediators of Inflammation, 2017, 2006(5):l-9.
[16] Punjabi NM, Soeken JD, Katzel LI. Sleep disordered preq thing and overweight men. Am J Respir Crit Care Med, 2000(165):677-683.
[17] 杨伟, 胡相卡, 陈香, 等. EGCG通过HIF-1α信号通路对顺铂诱导大鼠肾损伤的保护作用. 中药药理与临床, 2016, 32(1):40-43.
[18] Nicholl DD, Ahmed SB, Loewen AH. Declining kidney function increases the prevalence of sleep. Apnea Nocturnal Hypoxia Chest, 2012, 141(6):1422-1430.
[19] 姚军萍, 汪兴洪, 苏贵平. 多发性骨髓瘤中缺氧诱导因子-1α和VEGF的表达及相关性研究. 皖南医学院学报, 2012, 31(1):21-22.
[20] Zhong R, Xu H, Chen G, et al. The role of hypoxia-inducible factor lain radiation-induced autophagic cell death in breast cancer cells. Tumor Biology, 2015, 36(9):7077-7083.
[收稿日期:2020-04-30]
