小果南烛三萜类化学成分研究
2021-09-12张萌李齐激王雪杨娟杨小生
张萌 李齐激 王雪 杨娟 杨小生
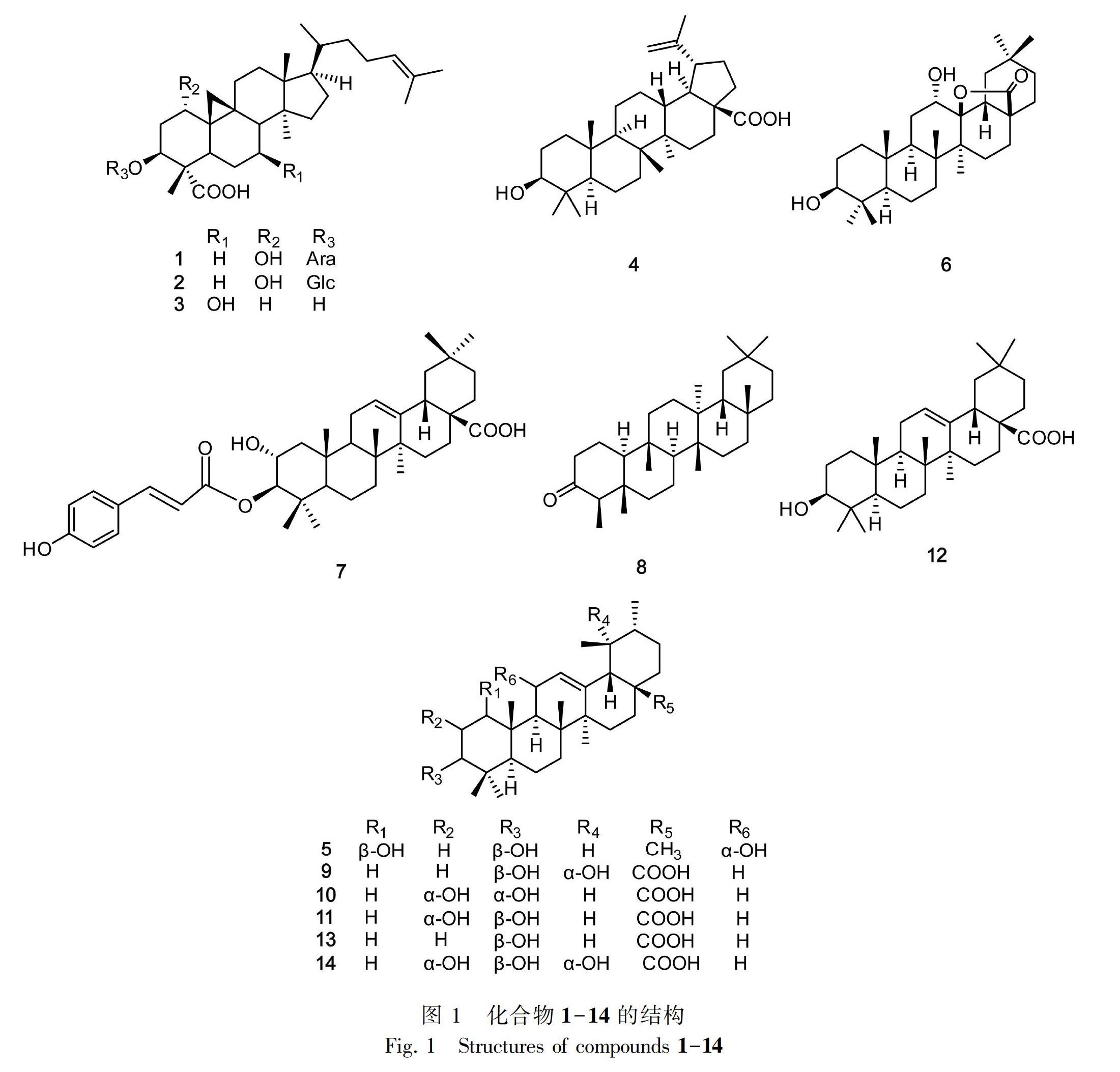
摘 要: 羊毛甾烷三萜是南烛属植物主要活性成分之一。为进一步研究南烛属植物功效物质基础,结合民族民间对小果南烛的应用实际,该文利用硅胶、MCI、Sephadex LH-20、半制备高效液相色谱等植物化学技术手段对该植物95%乙醇提取物进行分离、纯化,综合理化性质和波谱数据对其化合物进行鉴定。结果表明:从小果南烛的茎、叶提取物中分离得到14个三萜及其皂苷,分别鉴定为mollic acid 3-O-α-L-arabinopyranoside (1)、mollic acid 3-O-β-D-glucopyranoside (2)、cycloart-3,7-dihydroxy-24-en-28-oic acid (3)、白桦脂酸 (4)、1β, 3α, 11α-trihydroxy-urs-12-ene (5)、 oleanderolide (6)、(Z)-马斯里酸-3-O-对香豆酸酯 (7)、木栓酮 (8)、坡模酸 (9)、2α, 3α-二羟基乌苏-12-烯-28-酸 (10)、科罗索酸 (11)、齐墩果酸 (12)、熊果酸 (13)、委陵菜酸 (14)。其中,化合物1-7为首次从南烛属植物分离得到,化合物8-11,14为首次从小果南烛植物中分离得到。
关键词: 小果南烛, 化学成分, 三萜, 提取分离, 结构鉴定
中图分类号: Q946; R914.4 文献标识码: A 文章编号: 1000-3142(2021)07-1082-08
Abstract: Lanostane triterpene is one of the main active components of Lyonia Nutt. In order to further study the functional material basis of Lyonia, and in combination with the application of ethnic and folk practices to Lyonia ovalifolia var. elliptica, 95% ethanol extract of the plant was treated with phytochemical techniques such as silica gel, MCI, Sephadex LH-20, HPLC, fourteen lanostane triterpenes and their glycosides were identified by the physical and chemical properties. These fourteen compounds were identified as mollic acid 3-O-α-L-arabinopyranoside (1), mollic acid 3-O-β-D-glucopyranoside (2), cycloart-3,7-dihydroxy-24-en-28-oic acid (3), betulinicacid (4), 1β, 3α, 11α-trihydroxy-urs-12-ene (5), oleanderolide (6), 3-O-cis-p-coumaroyl maslinic acid (7), friedelin (8), pomolic acid (9), 2α, 3α-dihydroxy-urs-12-en-28-oic acid (10), corosolic acid (11), oleanolic acid (12), ursolic acid (13), tormentic acid (14). Compounds 1-7 were isolated from this genus for the first time, and compounds 8-11, 14 are isolated from this plant for the first time.
Key words: Lyonia ovalifolia var. elliptica, chemical constituents, triterpenes, extraction and isolation, structure identification
小果南燭(Lyonia ovalifolia var. elliptica)为杜娟花科(Ericaceae)南烛属(Lyonia Nutt)植物,又名小果珍珠花,生于阳坡灌木丛,主要分布于长江以南的湖南、广西、四川、贵州、云南等地。该植物具有祛风解毒、活血强筋等功效,民间用其治疗闭合性骨折(贵州植物志编辑委员会,1990)。现有的南烛属植物化学成分主要有木藜芦烷、异海松烷二萜、羊毛甾烷三萜及其他类三萜皂苷,此外还含有环烯醚萜、木脂素类、黄酮等成分(Sakakibara et al.,1974;Kashima et al.,2010;Zhao et al.,2018);药理作用主要表现为抗菌、抗病毒、调节钠离子通道、昆虫拒食、镇痛、抗氧化(Lv et al.,2016,2017;Wu et al.,2011;Li et al.,2013a,b)等方面,其活性物质主要为木藜芦烷与异海松烷二萜,羊毛甾烷三萜及其皂苷。
小果南烛是南烛属植物南烛(Lyonia ovalifolia)的变种,与其原植物种主要区别在于叶下有毛,叶较薄,纸质,萼裂片三角状卵形,果实较小。之前该植物的化学研究以分析毒性成分木藜芦烷二萜(Yasue et al.,1970)为主,但其三萜类活性成分报道甚少。作为贵州苗族地区同胞偶有应用的、具有小毒的植物药,明确其物质基础是其用药安全和研究开发的前提。为丰富小果南烛中三萜类成分,加深对其功效物质的认识和为进一步研究开发提供参考,本文利用多种柱层析、半制备高效液相色谱等技术从小果南烛茎、叶的95%乙醇提取物中分离得到14个三萜及其皂苷(图1),其中,化合物1-7为首次从南烛属植物分离得到,化合物8-11,14为首次从小果南烛中分离得到。
1 材料与仪器
藥材于2017年9月采自贵州省贵阳市花溪区,经贵州中医药大学孙庆文教授鉴定为杜鹃花科南烛属植物小果南烛(Lyonia ovalifolia var. elliptica)。
Bruker 600 MHz核磁共振测试仪(TMS内标)(美国Bruker公司);500 MHz 液体核磁共振谱仪(中国科学院武汉物理数学研究所);INOVA 400 MHz核磁共振测试仪(TMS内标)(美国Varian公司);Hewlett Pakard 110质谱仪(美国惠普公司);汉邦NS4101型高效液相色谱仪(江苏汉邦科技有限公司);旋转蒸发仪N-1100型(日本Eyela公司);Sephadex LH-20(瑞士安发玛生物技术公司);硅胶(青岛海洋化工有限公司);高效液相色谱仪所用溶剂为色谱纯,其他溶剂经工业纯重蒸处理后使用。
2 提取与分离
小果南烛茎、叶共14 kg,阴干粉粹后,用95% 乙醇回流提取3次,每次2 h,减压浓缩回收溶剂后得到浸膏约4.2 kg。将浸膏分散在25 L蒸馏水中,分别用2倍体积的石油醚、乙酸乙酯萃取3次,减压浓缩并回收溶剂,分别得到石油醚层浸膏(210 g)、乙酸乙酯层浸膏(500 g)。
对石油醚层浸膏进行硅胶柱层析,以石油醚∶乙酸乙酯(体积比1∶0 ~ 0∶1)为洗脱体系梯度,洗脱得到Fr.1-Fr.31。小果南烛石油醚层Fr.19、Fr.20合并经柱层析、洗涤、重结晶得到化合物12(200 mg);Fr.16通过MCI柱子以除去其色素并对其甲醇部分进行硅胶柱层析,以石油醚∶乙酸乙酯(体积比1∶0~0∶1) 进行梯度洗脱,得到Fr.16.1-Fr.16.10。Fr.16.1生成结晶,对结晶部分进行柱层析纯化得到化合物8(40 mg);对Fr.16.8进行硅胶柱层析,以氯仿为洗脱剂得到4个流分Fr.16.8.1-Fr.16.8.4,Fr.16.8.3以V(石油醚)∶V(丙酮)=10∶1进行硅胶柱层析得到化合物4(30 mg);Fr.23、Fr.24合并经过硅胶柱层析得到Fr.23.1-Fr.23.15,Fr.23.14经Sephadex LH-20柱色谱[V(三氯甲烷)∶V(甲醇)=1∶1]、制备型HPLC[V(乙腈)∶V(水)=85∶15]分离纯化后得到化合物5(180 mg)、6(8 mg)、9(25 mg)。
乙酸乙酯层浸膏用硅胶柱层析进行分离,以乙酸乙酯-甲醇(体积比1∶0~0∶1)梯度洗脱得到4个流分Fr.1-Fr.4。 Fr.3以二氯甲烷-甲醇(体积比1∶0~0∶1)得到Fr.3.1-Fr.3.18。Fr.3.14、Fr.3.15、 Fr.3.17分别结晶得到化合物1(1.25 g)、2(36 mg)、3(10 mg); Fr.2以二氯甲烷-甲醇 (体积比1∶0~0∶1) 得到Fr.2.1~Fr.2.15, Fr.2.4结晶得到化合物13(90 mg);Fr.2.7经硅胶柱层析二氯甲烷∶甲醇(体积比1∶0~0∶1)分离得到Fr.2.7.1-Fr.2.7.5,其中Fr.2.7.2、Fr.2.7.4分别经制备型高效液相色谱[V(乙腈)∶V(水)=20∶80]纯化得到7(15 mg)、10(8 mg);Fr.2.8经硅胶柱层析[V(石油醚)∶V(丙酮)为1∶0~0∶1]后,Fr.2.8.8经MCI柱色谱[V(甲醇)∶V(水)=3∶7]、Sephadex LH-20柱色谱[V(三氯甲烷)∶V(甲醇)=1∶1]、制备型高效液相色谱[V(乙腈)∶V(水)为70∶30~95∶5]纯化得到化合物11(8 mg)、14(75mg)。
3 结构鉴定
化合物1 白色粉末,ESI-MS m/z: 627 [M + Na]+ 。1H NMR (600 MHz, DMSO-d6) δ: 0.38 (1H, d, J = 4.3 Hz, H-19), 0.56 (1H, d, J = 4.3 Hz, H-19), 0.85 (3H, d, J = 6.2 Hz, H-21), 0.88 (3H, s, H-30), 0.90 (3H, s, H-18), 0.98 (3H, s, H-29), 1.55 (3H, s, H-26), 1.63 (3H, s, H-27), 3.27-3.63 (H-2′, 3′, 4′, 5′), 4.19 (1H, d, J = 5.6 Hz, H-1′), 4.39 (1H, dd, J = 11.2, 3.4 Hz, H-3), 5.06 (1H, t, J = 7.2 Hz, H-24); 13C NMR (150 MHz, DMSO-d6) δ: 70.8 (C-1), 40.1 (C-2), 78.8 (C-3), 51.7 (C-4), 35.3 (C-5), 20.0 (C-6), 27.7 (C-7), 47.4 (C-8), 21.9 (C-9), 32.4 (C-10), 24.4 (C-11), 36.6 (C-12), 44.8 (C-13), 48.5 (C-14), 35.1 (C-15), 28.7 (C-16), 52.9 (C-17), 18.0 (C-18), 28.9 (C-19), 36.1 (C-20), 19.1 (C-21), 36.0 (C-22), 25.0 (C-23), 124.9 (C-24), 130.3 (C-25), 25.5 (C-26), 17.5 (C-27), 177.9 (C-28), 9.3 (C-29), 18.1 (C-30), 103.3 (C-1′), 72.2 (C-2′), 70.6 (C-3′), 66.8 (C-4′), 63.9 (C-5′)。以上数据与文献(Rogers,1989)报道一致,故鉴定化合物1为mollic acid 3-O-α-L-arabinopyranoside 。
化合物2 白色粉末,ESI-MS m/z: 657 [M + Na]+ 。1H NMR (600 MHz, DMSO-d6) δ: 0.38 (1H, d, J = 4.3 Hz, H-19), 0.56 (1H, d, J = 4.3 Hz, H-19), 0.85 (3H, d, J = 6.2 Hz, H-21), 0.89 (3H, s, H-30), 0.90 (3H, s, H-18), 0.98 (3H, s, H-29), 1.55 (3H, s, H-26), 1.63 (3H, s, H-27), 3.19-4.31 (H-2′, 3′, 4′, 5′, 6′), 4.40 (1H, dd, J = 12.1, 4.3 Hz, H-3), 4.59 (1H, d, J = 7.6 Hz, H-1′), 5.06 (1H, t, J = 7.3 Hz, H-24); 13C NMR (150 MHz, DMSO-d6) δ: 73.3 (C-1), 40.1 (C-2), 79.0 (C-3), 51.7 (C-4), 35.3 (C-5), 20.0 (C-6), 27.7 (C-7), 47.4 (C-8), 21.9 (C-9), 32.5 (C-10), 24.5 (C-11), 36.8 (C-12), 44.8 (C-13), 48.5 (C-14), 35.3 (C-15), 28.8 (C-16), 53.0 (C-17), 18.0 (C-18), 28.8 (C-19), 36.1 (C-20), 19.1 (C-21), 36.0 (C-22), 24.8 (C-23), 124.9 (C-24), 130.3 (C-25), 25.5 (C-26), 17.5 (C-27), 178.0 (C-28), 9.3 (C-29), 18.1 (C-30), 104.2 (C-1′), 75.1 (C-2′), 71.1 (C-3′), 70.6 (C-4′), 67.9 (C-5′), 60.2 (C-6′)。以上數据与文献(Rogers & Thevan,1986)报道一致,故鉴定化合物2为mollic acid 3-O-β-D-glucopyranoside。
化合物3 白色粉末,ESI-MS m/z: 495 [M + Na]+。1H NMR (600 MHz, DMSO-d6) δ: 0.29 (1H, d, J = 4.2 Hz, H-19), 0.47 (1H, d, J = 4.2 Hz, H-19), 0.79 (3H, d, J = 6.2 Hz, H-21), 0.82 (3H, s, H-30), 0.83 (3H, s, H-18), 0.84 (3H, s, H-29), 1.49 (3H, s, H-26), 1.56 (3H, s, H-27), 1.93 (1H, d, J = 7.9 Hz, H-8), 2.37 (1H, dd, J = 12.6, 4.5 Hz, H-5), 4.15 (1H, dd, J = 11.3, 3.0 Hz, H-3), 5.00 (1H, t, J = 7.2 Hz, H-24); 13C NMR (150 MHz, DMSO-d6) δ: 32.5 (C-1), 29.0 (C-2), 71.0 (C-3), 51.6 (C-4), 40.4 (C-5), 35.3 (C-6), 69.1 (C-7), 53.8 (C-8), 19.8 (C-9), 24.5 (C-10), 27.8 (C-11), 35.5 (C-12), 44.8 (C-13), 47.6 (C-14), 37.5 (C-15), 25.0 (C-16), 48.5 (C-17), 17.5 (C-18), 25.5 (C-19), 35.9 (C-20), 18.1 (C-21), 36.5 (C-22), 22.3 (C-23), 125.0 (C-24), 130.4 (C-25), 17.9 (C-26), 25.0 (C-27), 178.3 (C-28), 8.7 (C-29), 19.0 (C-30)。以上数据与文献(Milena et al.,2009)报道一致,故鉴定化合物3为cycloart-3, 7-dihydroxy-24-en-28-oic acid。
化合物4 白色粉末,ESI-MS m/z: 479 [M + Na]+。1H NMR (600 MHz, DMSO-d6) δ: 0.64 (3H, s, H-27), 0.75 (3H, s, H-24), 0.86 (3H, s, H-25), 0.86 (3H, s, H-26), 0.92 (3H, s, H-23), 1.64 (3H, s, H-30), 2.96 (1H, dd, J = 10.7, 5.3 Hz, H-3α), 4.55 (1H, brs, H-29b), 4.68 (1H, brs, H-29a); 13C NMR (150 MHz, DMSO-d6) δ: 38.3 (C-1), 27.2 (C-2), 76.9 (C-3), 38.6 (C-4), 55.0 (C-5), 18.0 (C-6), 34.0 (C-7), 40.3 (C-8), 50.0 (C-9), 36.8 (C-10), 20.5 (C-11), 25.2 (C-12), 37.7 (C-13), 42.1 (C-14), 30.2 (C-15), 31.8 (C-16), 55.5 (C-17), 46.7 (C-18), 48.6 (C-19), 150.4 (C-20), 29.3 (C-21), 36.4 (C-22), 28.2 (C-23), 15.8 (C-24), 15.9 (C-25), 16.0 (C-26), 14.4 (C-27), 177.4 (C-28), 109.7 (C-29), 19.0 (C-30)。以上数据与文献(武蕊娟等,2015)报道一致,故鉴定化合物4为白桦脂酸。
化合物5 白色粉末,ESI-MS m/z: 481 [M + Na]+。 1H NMR (500 MHz, CDCl3) δ: 0.81 (3H, s, H-28), 0.82 (3H, s, H-26), 0.89 (3H, d, J = 6.6 Hz, H-29), 0.92 (3H, d, J = 6.5 Hz, H-30), 1.00 (3H, s, H-25), 1.08 (3H, s, H-27), 1.12 (3H, s, H-24), 1.20 (3H, s, H-23), 2.07 (1H, d, J = 11.4 Hz, H-18), 2.20 (1H, dt, J = 13.5, 3.6 Hz, H-1), 4.18 (1H, dd, J = 8.2, 5.5 Hz, H-11), 4.50 (1H, s, OH-11); 13C NMR (125 MHz, CDCl3) δ: 70.9 (C-1), 28.9 (C-2), 78.8 (C-3), 38.3 (C-4), 55.4 (C-5), 18.5 (C-6), 33.4 (C-7), 39.8 (C-8), 54.7 (C-9), 39.3 (C-10), 70.4 (C-11), 116.2 (C-12), 145.2 (C-13), 43.1 (C-14), 27.7 (C-15), 27.3 (C-16), 34.0 (C-17), 51.1 (C-18), 41.2 (C-19), 41.1 (C-20), 31.4 (C-21), 41.8 (C-22), 27.8 (C-23), 16.7 (C-24), 15.8 (C-25), 16.9 (C-26), 24.4 (C-27), 28.5 (C-28), 18.1 (C-29), 21.4 (C-30)。以上數据与文献(Topcu & Nulubele,1999)报道一致,故鉴定化合物5为1β, 3α, 11α-trihydroxy-urs-12-ene。
化合物6 白色粉末,ESI-MS m/z: 495 [M + Na]+。1H NMR (600 MHz, CDCl3) δ: 0.78 (3H, s, H-24), 0.88 (3H, s, H-25), 0.90 (3H, s, H-30), 0.98 (3H, s, H-29), 0.99 (3H, s, H-23), 1.14 (3H, s, H-26), 1.30 (3H, s, H-27), 1.72 (ddd, J = 13.0 , 3.4, 3.4 Hz, H-1), 2.04 ( 1H, m, H-18), 2.13 (ddd, J = 13.4, 13.4, 5.9 Hz, H-16), 3.22 (1H, dd, J = 10.9, 5.1 Hz, H-3), 3.89 (1H, brs, H-12); 13C NMR (150 MHz, CDCl3) δ: 39.0 (C-1), 27.6 (C-2), 78.9 (C-3), 39.0 (C-4), 55.3 (C-5), 17.9 (C-6), 34.1 (C-7), 42.3 (C-8), 44.8 (C-9), 36.7 (C-10), 29.0 (C-11), 76.7 (C-12), 90.8 (C-13), 42.4 (C-14), 28.2 (C-15), 21.3 (C-16), 44.8 (C-17), 51.3 (C-18), 39.7 (C-19), 31.8 (C-20), 34.4 (C-21), 27.3 (C-22), 28.2 (C-23), 15.5 (C-24), 16.5 (C-25), 18.8 (C-26), 18.7 (C-27), 180.0 (C-28), 33.4 (C-29), 24.0 (C-30)。以上数据与文献(Fu et al.,2005)报道一致,故鉴定化合物6为oleanderolide。
化合物7 白色粉末,ESI-MS m/z: 641 [M + Na]+ 。1H NMR (600 MHz, CD3OD) δ: 0.78 (3H, s, H-26), 0.85 (3H, s, H-23), 0.88 (3H, s, H-29), 0.95 (6H, s, H-24, 30), 0.99 (3H, s, H-25), 1.16 (3H, s, H-27), 2.82 (1H, m, H-18), 3.80 (1H, m, H-2), 4.54 (1H, d, J = 3.5 Hz, H-3), 5.22 (1H, t, J = 4.6 Hz, H-12), 5.81 (1H, d, J = 12.7 Hz, H-2′), 6.71 (2H, d, J = 8.6 Hz, H-3′′, 5′′), 6.84 (1H, d, J = 12.8 Hz, H-3′), 7.60 (2H, d, J = 8.6 Hz, H-2′′, 6′′); 13C NMR (150 MHz, CD3OD) δ: 47.7 (C-1), 67.5 (C-2), 85.2 (C-3), 40.6 (C-4), 56.4 (C-5), 19.4 (C-6), 33.6 (C-7), 40.4 (C-8), 48.5 (C-9), 39.5 (C-10), 24.0 (C-11), 124.3 (C-12), 144.8 (C-13), 43.1 (C-14), 28.8 (C-15), 24.6 (C-16), 48.2 (C-17), 42.8 (C-18), 47.3 (C-19), 31.5 (C-20), 35.0 (C-21), 33.9 (C-22), 29.6 (C-23), 17.7 (C-24), 17.0 (C-25), 18.2 (C-26), 26.4 (C-27), 181.0 (C-28), 33.8 (C-29), 24.0 (C-30), 168.6 (C-1′), 117.4 (C-2′), 140.1 (C-3′), 129.4 (C-1′′), 133.6 (C-2′′), 115.8 (C-3′′), 159.8 (C-4′′), 115.8 (C-5′′), 133.6 (C-6′′)。以上数据与文献(许琼明等,2010)报道一致,故鉴定化合物7为(Z)-马斯里酸-3-O-对香豆酸酯。
化合物8 针状结晶(氯仿),ESI-MS m/z: 449 [M + Na]+。1H NMR (400 MHz, CDCl3) δ: 0.72 (3H, s, H-24), 0.87 (3H, d, J = 6.4 Hz, H-25), 0.96 (3H, s, H-29), 1.01 (3H, s, H-30), 1.01 (3H, s, H-26), 1.05 (3H, s, H-27), 1.17 (3H, s, H-28); 13C NMR (100 MHz, CDCl3) δ: 22.0 (C-1), 41.5 (C-2), 213.2 (C-3), 58.3 (C-4), 42.0 (C-5), 41.2 (C-6), 18.2 (C-7), 53.0 (C-8), 37.5 (C-9), 59.5 (C-10), 35.5 (C-11), 30.6 (C-12), 39.7 (C-13), 38.1 (C-14), 32.3 (C-15), 36.0 (C-16), 29.8 (C-17), 42.6 (C-18), 35.2 (C-19), 28.0 (C-20), 32.8 (C-21), 39.1 (C-22), 6.9 (C-23), 14.7 (C-24), 18.0 (C-25), 20.1 (C-26), 18.7 (C-27), 32.0 (C-28), 35.0 (C-29), 37.4 (C-30)。以上数据与文献(徐菁等,2014)报道一致,故鉴定化合物8为木栓酮。
化合物9 白色粉末,ESI-MS m/z: 495 [M + Na]+。1H NMR (600 MHz, DMSO-d6) δ: 0.56 (3H, s, H-25), 0.58 (3H, s, H-23), 0.73 (3H, d, J = 6.0 Hz, H-30), 0.78 (3H, s, H-26), 0.97 (3H, s, H-24), 1.15 (3H, s, H-29), 1.47 (3H, s, H-27), 2.25 (1H, s, H-18), 3.62 (1H, m, H-3α), 5.04 (1H, s, H-12); 13C NMR (150 MHz, DMSO-d6) δ: 38.5 (C-1), 27.0 (C-2), 77.1 (C-3), 38.3 (C-4), 55.0 (C-5), 18.2 (C-6), 32.8 (C-7), 41.2 (C-8), 47.0 (C-9), 36.7 (C-10), 23.2 (C-11), 126.9 (C-12), 138.7 (C-13), 41.5 (C-14), 28.3 (C-15), 25.3 (C-16), 46.8 (C-17), 53.4 (C-18), 71.8 (C-19), 37.5 (C-20), 25.9 (C-21), 38.2 (C-22), 28.2 (C-23), 15.2 (C-24), 16.1 (C-25), 16.8 (C-26), 24.1 (C-27), 179.0 (C-28), 26.6 (C-29), 16.5 (C-30)。以上数据与文献(An et al.,2005)报道一致,故鉴定化合物9为pomolic acid。
化合物10 白色粉末,ESI-MS m/z: 495 [M + Na]+。1H NMR (600 MHz, DMSO-d6) δ: 0.71 (3H, s, H-25), 0.75 (3H, s, H-24), 0.79 (3H, d, J = 6.5 Hz, H-30), 0.86 (3H, d, J = 6.5 Hz, H-29), 0.88 (3H, s, H-26), 0.89 (3H, s, H-27), 1.02 (3H, s, H-23), 2.09 (1H, d, J = 11.3 Hz, H-18), 3.74 (1H, d, J = 8.7 Hz, H-3β), 3.99 (1H, m, H-2β), 5.11 (1H, s, H-12); 13C NMR (150 MHz, DMSO-d6) δ: 38.0 (C-1), 64.7 (C-2), 77.9 (C-3), 38.5 (C-4), 47.6 (C-5), 17.6 (C-6), 32.7 (C-7), 38.5 (C-8), 46.9 (C-9), 37.8 (C-10), 23.4 (C-11), 123.5 (C-12), 139.6 (C-13), 41.8 (C-14), 28.9 (C-15), 21.8 (C-16), 46.7 (C-17), 52.3 (C-18), 40.2 (C-19), 37.4 (C-20), 29.1 (C-21), 35.3 (C-22), 28.9 (C-23), 23.4 (C-24), 16.3 (C-25), 17.6 (C-26), 22.0 (C-27), 179.2 (C-28), 17.0 (C-29), 21.2 (C-30)。以上数据与文献(王福东等,2005)报道一致,故鉴定化合物10为2α, 3α-二羟基乌苏-12-烯-28-酸。
化合物11 白色粉末,ESI-MS m/z: 495 [M + Na] +。1H NMR (600 MHz, DMSO-d6) δ: 0.39 (3H, s, H-24), 0.45 (3H, s, H-25), 0.50 (3H, d, J = 6.5 Hz, H-30), 0.56 (3H, d, J = 6.5 Hz, H-29), 0.60 (3H, s, H-26), 0.61 (3H, s, H-27), 0.72 (3H, s, H-23), 4.12 (1H, d, J = 8.9 Hz, H-3α), 4.81 (1H, m, H-2α), 5.38 (1H, s, H-12); 13C NMR (150 MHz, DMSO-d6) δ: 47.2 (C-1), 67.5 (C-2), 82.6 (C-3), 38.8 (C-4), 55.0 (C-5), 18.3 (C-6), 32.9 (C-7), 40.0 (C-8), 47.3 (C-9), 37.8 (C-10), 23.2 (C-11), 124.6 (C-12), 138.7 (C-13), 42.0 (C-14), 27.7 (C-15), 23.5 (C-16), 48.8 (C-17), 52.7 (C-18), 38.7 (C-19), 38.5 (C-20), 30.6 (C-21), 36.6 (C-22), 29.0 (C-23), 17.4 (C-24), 17.4 (C-25), 17.5 (C-26), 24.3 (C-27), 178.6 (C-28), 21.3 (C-29), 16.8 (C-30)。以上數据与文献(陈龙胜等,2008)报道一致,故鉴定化合物11为科罗索酸。
化合物12 白色粉末,ESI-MS m/z: 479 [M + Na]+。1H NMR (600 MHz, CDCl3) δ: 0.74 (3H, s, H-24), 0.77 (3H, s, H-25), 0.90 (3H, s, H-29), 0.91 (3H, s, H-30), 0.92 (3H, s, H-23), 0.98 (3H, s, H-23), 1.13 (3H, s, H-26), 2.82 (1H, m, H-18), 3.22 (1H, m, H-3), 5.27 (1H, brs, H-12); 13C NMR (150 MHz, CDCl3) δ: 38.7 (C-1), 27.5 (C-2), 79.4 (C-3), 39.1 (C-4), 55.6 (C-5), 18.6 (C-6), 32.9 (C-7), 39.6 (C-8), 48.0 (C-9), 37.4 (C-10), 23.2 (C-11), 123.0 (C-12), 144.0 (C-13), 41.2 (C-14), 27.9 (C-15), 23.6 (C-16), 47.0 (C-17), 42.0 (C-18), 46.2 (C-19), 31.0 (C-20), 34.1 (C-21), 32.8 (C-22), 28.4 (C-23), 15.7 (C-24), 15.9 (C-25), 17.5 (C-26), 26.3 (C-27), 184.0 (C-28), 33.4 (C-29), 23.9 (C-30)。以上数据与文献(刘普等,2006)报道一致,故鉴定化合物12为齐墩果酸。
化合物13 白色粉末,ESI-MS m/z: 479 [M + Na]+。 1H NMR (600 MHz, DMSO-d6) δ: 0.66 (3H, s, H-24), 0.74 (3H, s, H-25), 0.80 (3H, d, J = 6.4 Hz, H-29), 0.86 (3H, d, J = 4.0 Hz, H-30), 0.88 (3H, s, H-23), 0.90 (3H, s, H-26), 1.03 (3H, s, H-27), 2.09 (1H, d, J = 11.3 Hz, H-18), 3.02 (1H, dd, J = 11.1, 5.0 Hz, H-3), 5.11 (1H, t, J = 3.7 Hz, H-12); 13C NMR (150 MHz, DMSO-d6) δ: 36.5 (C-1), 27.0 (C-2), 79.2 (C-3), 38.2 (C-4), 54.8 (C-5), 18.0 (C-6), 32.7 (C-7), 40.1 (C-8), 47.0 (C-9), 38.5 (C-10), 23.3 (C-11), 124.6 (C-12), 138.2 (C-13), 41.7 (C-14), 28.3 (C-15), 23.8 (C-16) , 46.8 (C-17) , 52.4 (C-18), 38.4 (C-19), 38.4 (C-20), 30.2 (C-21), 36.3 (C-22), 27.6 (C-23), 15.2 (C-24), 16.1 (C-25), 17.0 (C-26), 22.9 (C-27), 178.3 (C-28), 16.9 (C-29), 21.1 (C-30)。以上数据与文献(李火云等,2014)报道一致,故鉴定化合物13为熊果酸。
化合物14 白色粉末,ESI-MS m/z: 511 [M + Na]+ 。1H NMR (600 MHz, DMSO-d6) δ: 0.62 (3H, s, H-24), 0.64 (3H, s, H-25), 0.77 (3H, d, J = 6.4 Hz, H-29), 0.83 (3H, d, J = 4.0 Hz, H-30), 0.85 (3H, s, H-23), 1.01 (3H, s, H-26), 1.21 (3H, s, H-27), 2.30 (1H, s, H-18), 2.67 (1H, dd, J = 9.4, 3.8 Hz, H-3), 3.70 (1H, m, H-2), 5.10 (1H, s, H-12); 13C NMR (150 MHz, DMSO-d6) δ: 46.8 (C-1), 67.2 (C-2), 82.4 (C-3), 39.0 (C-4), 54.9 (C-5), 18.2 (C-6), 32.7 (C-7), 40.4 (C-8), 47.0 (C-9), 37.7 (C-10), 23.3 (C-11), 126.8 (C-12), 138.7 (C-13), 41.5 (C-14), 28.1 (C-15), 25.2 (C-16), 47.0 (C-17), 53.2 (C-18), 71.7 (C-19), 41.2 (C-20), 26.0 (C-21), 37.3 (C-22), 28.9 (C-23), 16.4 (C-24), 16.4 (C-25), 17.2 (C-26), 24.0 (C-27), 177.7 (C-28), 26.4 (C-29), 16.7 (C-30)。以上数据与文献(郑光海和朴惠顺,2012)报道一致,故鉴定化合物14为委陵菜酸。
4 讨论与结论
本研究从小果南烛茎、叶乙醇提取物中获得14个三萜及其皂苷类物質,其中,化合物1-7为首次从南烛属植物分离得到,化合物8-11,14首次从小果南烛分离得到。
从该属植物化学研究报道来看,羊毛甾烷三萜是该属植物中主要的三萜类型,且具有较好的抗恶性细胞增殖活性(Teng et al.,2018),但该类成分未从小果南烛中分离得到。本研究发现的环阿屯烷三萜(1-3)为首次从南烛属植物分离得到,该类成分对人乳腺癌、肝癌、前列腺癌细胞系具有较好的抑制作用及抗骨质疏松和抗补体活性(Yang et al.,2016;李延勋等, 2017)。化合物1在小果南烛中含量较大(克级),且能有效抑制Ca-Ski宫颈癌细胞的生长(Wong et al.,2012)。这不仅可作为该药材质量标志物候选物质用于质量控制,还可作为该植物化学分类的重要参考。此外,化合物2对经热诱导和化学诱导的小鼠伤害性疼痛以及大鼠足跖肿胀具有抑制作用(Ojewole,2008);化合物3对革兰氏阳性菌、革兰氏阴性菌及病原真菌具有抑制作用(Milena et al.,2009);化合物11、12、13具有抗炎(鞠建华等,2003)、抗肿瘤(Chiang et al.,2005)等活性。本研究结果丰富了南烛属植物三萜成分类型,为小果南烛的后续研究与应用提供了物质基础。
参考文献:
AN RB, KIM HC, JEONG GS, et al., 2005. Constituents of the aerial parts of Agrimonia pilosa [J]. Nat Prod Sci, 11 (4): 196-198.
CHEN LS, L Y, XU SW, et al., 2008. Study on the triterpene acids in fruit of Crataegus pinnatifida [J]. Lishizhen Med Mat Med Res, 19(12): 2909-2910. [陈龙胜, 吕杨, 许舒雯, 等, 2008. 山楂中三萜酸成分的研究 [J]. 时珍国医国药, 19(12): 2909-2910.]
Editorial Committee of Flora of Guizhou, 1990. Flora of Guizhou [M]. Guiyang: Guizhou Peoples Publishing House, 3: 260-262. [贵州植物志编辑委员会, 1990. 贵州植物志 [M]. 贵阳: 贵州人民出版社, 3: 260-262.]
CHIANG YM, CHANG JY, KUO CC, et al., 2005. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa [J]. Phytochemistry, 66: 495-501.
FU LW, ZHANG SJ, LI N, et al., 2005. Three new triterpenes from Nerium oleander and biological activity of the isolated compounds [J]. J Nat Prod, 68(2): 198-206.
JU JH, ZHOU L, LIN G, et al., 2003. Studies on constituents of triterpene acids from Eriobotrya japonica and their anti-inflammatory and antitussive effects [J]. Chin Pharm J, 38(10): 752-757. [鞠建华, 周亮, 林耕, 等, 2003. 枇杷叶中三萜酸类成分及其抗炎、镇咳活性研究 [J]. 中国药学杂志, 38(10): 752-757.]
KASHIMA K, SANO K, YUN YS, et al., 2010. Ovafolinins A-E, five new lignans from Lyonia ovalifolia [J]. Chem Pharm Bull, 58(2): 191-194.
LI HY, JIAO K, ZHANG P, et al., 2014. Chemical constituents from Isodon excisoides [J]. Chin Trad Herb Drug, 45(2): 154-160. [李火云, 焦珂, 張鹏, 等, 2014. 拟缺香茶菜化学成分研究 [J]. 中草药, 45(2): 154-160.]
LI Y, LIU YB, ZHANG JJ, et al., 2013a. Grayanoids from the Ericaceae family: structures, biological activities and mechanism of action [J]. Phytochem Rev, 12(2): 305-325.
LI Y, LIU YB, ZHANG JJ, et al., 2013b. Mollolide A, a diterpenoid with a new 1, 10: 2, 3-disecograyanane skeleton from the roots of Rhododendron molle [J]. Org Lett, 15(12): 3074-3077.
LI YX, LI ZP, SU YF, 2017. Research progress on secocycloartane triterpenoids [J]. Chin Trad Herb Drug, 48(15): 3198-3209. [李延勋, 栗章彭, 苏艳芳, 2017. 裂环环阿屯烷型三萜的研究进展 [J]. 中草药, 48(15): 3198-3209.]
LIU P, DUAN HQ, PAN Q, et al., 2006. Triterpenes from herb of Potentilla chinesis [J]. Chin J Chin Mat Med, 31(22): 187. [刘普, 段宏泉, 潘勤, 等, 2006. 委陵菜三萜成分研究 [J]. 中国中药杂志, 31(22): 187.]
LV XJ, LI Y, MA SG, et al., 2016. Antiviral triterpenes from the twigs and leaves of Lyonia ovalifolia [J]. J Nat Prod, 79(11): 2824-2837.
LV XJ, LI Y, MA SG, et al., 2017. Isopimarane and nor-diterpene glucosides from the twigs and leaves of Lyonia ovalifolia [J]. Tetrahedron, 73(6): 776-784.
MILENA P, POPOVA A, IOANNA B, et al., 2009. Terpenes with antimicrobial activity from Cretan propolis [J]. Phytochemistry, 70(10): 1262-1271.
OJEWOLE JAO, 2008. Analgesic and antiinflammatory effects of mollic acid glucoside, a 1α-hydroxycycloartenoid saponin extractive from Combretum molle R. Br. ex G. Don (Combretaceae) Leaf [J]. Phytother Res, 22: 30-35.
ROGERS CB, THEVAN I, 1986. Identification of the mollic acid α-L-arabinoside, a 1α-hydroxycycloartenoid from Combretum molle leaves [J]. Phytochemistry, 25(7): 1759-1761.
ROGERS CB, 1989. Isolation of the 1α-hydroxycycloartenoid mollic acid α-L-arabinoside from Combretum edwardsii leaves [J]. Phytochemistry, 28(1): 279-281.
SAKAKIBARA J, HOTTA Y, YASUE M, 1974. Studies on the constituents of Lyonia ovalifolia Drude var. elliptica Hand.-Mazz. XVII. structure of a triterpene arabinoside, ovalifolioside. 2 [J]. PSJ, 94(2): 170-175.
SU Y, CHI WC, WU L, et al., 2016. Photochemistry and pharmacology of 9, 19-cyclolanostane glycosides isolated from genus Cimicifuga [J]. Chin J Nat Med, 14(10): 721-731.
TENG Y, ZHANG HQ, ZHOU JF, et al., 2017. Triterpenoid glycosides from the leaves of Lyonia ovalifolia var. hebecarpa and their antitumor activities [J]. Chin J Org Chem, 37(9): 2416-2422. [滕楊, 张涵琪, 周俊飞, 等, 2017. 毛果南烛中三萜皂苷化合物及其抗肿瘤活性 [J]. 有机化学, 37(9): 2416-2422.]
TENG Y, ZHANG HQ, ZHOU JF, et al., 2018. Hebecarposides A-K, antiproliferative lanostane-type triterpene glycosides from the leaves of Lyonia ovalifolia var. hebecarpa [J]. Phytochemistry, 151: 32-41.
TOPCU G, NULUBELE A, 1999. Terpenoids from Salvia kronenburgii [J]. J Nat Prod, 62(12): 1605-1608.
WANG FD, DING L, WANG HQ, 2005. Studies on triterpenoid constituents from Rabdosia japonica var. galaucocalyx [J]. Chin J Chin Mat Med, 30(24): 1929-1932. [王福东, 丁兰, 汪汉卿, 2005. 兰萼香茶菜三萜成分研究 [J] . 中国中药杂志, 30(24): 1929-1932.]
WONG YH, ABDUL KADIR H, LING SK, 2012. Induction of mitochondria-mediated apoptosis in ca-ski human cervical cancer cells triggered by mollic acid arabinoside isolated from Leea indica [J]. Evid-Based Compl Alt, (5): 11.
WU RJ, SONG LQ, TAN L, et al., 2015. Chemical constituents from Paliurus ramosissimus [J]. Chin Trad Herb Drug, 46(19): 2834-2838. [武蕊娟, 宋联强, 谭镭, 等, 2015. 马甲子的化学成分研究 [J]. 中草药, 46(19): 2834-2838.]
WU ZY, LI HZ, WANG WG, et al., 2011. Lyonin A, a new 9, 10-Secograyanotoxin from Lyonia ovalifolia [J]. Chem Biodivers, 8(6): 1182-1187.
XU J, GAO HY, MA SL, et al., 2014. Chemical constituents and bioactivity of Kalimeris indica [J]. Chin Trad Herb Drugs, 45(22): 3246-3250. [徐菁, 高鸿悦, 马淑丽, 等, 2014. 马兰化学成分及生物活性研究 [J]. 中草药, 45(22): 3246-3250.]
XU QM, TANG LH, LI X, et al., 2010. Isolation and identification of 3-O-p-coumaroyloxyl pentacyclic triterpenoids from Lysimachia clethroides Duby [J]. Chin Pharm J, 45(11): 825-828. [许琼明, 唐丽华, 李夏, 等, 2010. 珍珠菜中五环三萜-3-O-对香豆酸酯类化学成分的分离鉴定 [J]. 中国药学杂志, 45(11): 825-828.]
YASUE M, SAKAKIBARA J, KAIYA T, 1970. Studies on the consituents of Lyonia ovalifolia Drude varelliptica Hand.-Mazz. X. on the consitutents of the flowers] [J]. PSJ, 90(9): 1174-1177.
ZHAO DR, SU LH, LI RT, et al., 2018. Chemical constituents from the twigs and leaves of Lyonia ovalifolia [J]. Biochem System Ecol, 78: 1-4.
ZHENG GH, PIAO HS, 2012. Study on chemical constituents from Potentilla supina [J]. Chin Trad Herb Drug, 43(7): 1285-1288. [鄭光海, 朴惠顺, 2012. 朝天委陵菜化学成分研究 [J]. 中草药, 43(7): 1285-1288.]
(责任编辑 何永艳)
