2例妊娠相关乳腺癌患者的病例报道及相关文献复习
2020-05-06金司爻赵志刚霍记平
金司爻 赵志刚 霍记平
摘 要 目的:為妊娠相关乳腺癌(PABC)的早期诊断和治疗方案的选择提供参考。方法:对我院2例PABC患者的疾病特征、治疗过程和预后情况等进行分析;检索PubMed数据库中1986年1月-2019年4月发表的相关文献,纳入标题、关键词或摘要中包括“Breast cancer and pregnancy”“Pregnancy-associated breast cancer”“Breast cancer during pregnancy”“Breast carcinoma during pregnancy”“Case reports”等的病例报告,排除不符合PABC定义的病例报告,对其中患者的基本资料、肿瘤临床特征、药物治疗方案、母胎/婴预后等信息进行汇总及描述性统计分析。结果与结论:我院2例患者均于哺乳期确诊,经新辅助化疗和手术切除后,预后良好。通过文献检索与筛选获得共36篇病例报告,共45例患者(39例患者在妊娠期间确诊,6例患者在哺乳期间确诊)的临床资料。排除未报道相关信息的病例后,35.0%(14/40)的病例应用了新辅助化疗AC方案(多柔比星+环磷酰胺);59.5%(22/37)的病例进行了择期剖腹产手术,37.8%(14/37)的病例经阴道分娩,1例终止妊娠;患者存活率为80.8%(21/26),婴儿平均出生体质量为2 407 g(1 015~3 830 g)。分别有6例和9例患者在妊娠期和产后使用了紫杉烷类药物。PABC化疗方案的确定应综合考虑多方因素,需要全面权衡母亲及婴儿的受益风险,尽量避免在妊娠早期进行化疗,尤其要兼顾化疗对胎儿的影响。化疗方案仍以蒽环类药物为主导,可在此基础上制订个体化方案,且使用紫杉醇类药物时应充分权衡利弊并进行严密监测。
关键词 妊娠相关乳腺癌;病例报道;文献综述;新辅助化疗;化疗相关不良反应;预后
ABSTRACT OBJECTIVE: To provide reference for the early diagnosis and selection of treatment regimens of pregnancy- associated breast cancer (PABC). METHODS: The disease characteristics, treatment process and prognosis of 2 cases of PABC were analyzed in our hospital. The relevant literature published from Jan. 1986 to Apr. 2019 in PubMed database was retrieved. The case reports that the title, keywords or abstracts involved “Breast cancer and pregnancy”“Pregnancy-associated breast cancer”“Breast cancer during pregnancy”“Breast carcinoma during pregnancy”“Case reports” were included. Cases which didnt meet the definition of PABC were excluded. The general information, tumor clinical characteristics, drug treatment plan, maternal/fetal prognosis and other information of patients were extracted for summary and descriptive statistical analysis. RESULTS & CONCLUSIONS: Two patients were both diagnosed during lactation. The prognosis was good after neoadjuvant chemotherapy and surgical resection. A total of 36 case reports were obtained through literature search and screening, as well asclinical data of 45 patients (39 diagnosed during pregnancy and 6 diagnosed during lactation).Neoadjuvant chemotherapy AC regimen (doxorubicin+cyclophosphamide) was used in 35.0% (14/40) of cases after excluding the cases without relevant information;elective caesarean section was performed in 59.5% (22/37) of cases, 37.8% (14/37) of cases were delivered, and 1 case chose to terminate pregnancy;survival rate of patients was 80.8% (21/26), and the average weight of newborns was 2 407 g (1 015-3 830 g). Six patients each received taxanes during pregnancy and 9 patients during postpartum. The determination of chemotherapy for PABC should comprehensively consider a variety of factors. It is necessary to comprehensively weigh the benefit risks of the mother and child, try to avoid chemotherapy in early pregnancy, and especially consider the impact of chemotherapy on the fetus. The chemotherapy regimen is still dominated by anthracyclines. Based on this, an individualized regimen is formulated and close monitoring should be performed when using paclitaxel.
KEYWORDS Pregnancy-associated breast cancer; Case report; Literature review; Neoadjuvant chemotherapy; Chemotherapy- related adverse reactions; Prognosis
妊娠期乳腺癌或妊娠相关乳腺癌(Pregnancy-associated breast cancer,PABC)是指妊娠期间、产后第1年或哺乳期内任何时间确诊的乳腺癌。据报道,肿瘤占到孕期死亡的0.02%~0.1%,PABC是妊娠期肿瘤中较为常见的一种,发病率约0.4%~1%,并且近年来发病率在逐步升高[1]。由于妊娠及哺乳期患者生理的特殊性以及PABC发病及预后的复杂性,在临床治疗时还需兼顾胎儿或新生儿的安全,故该病的治疗到目前为止仍是临床一大挑战。本文报道了2例在首都医科大学附属北京天坛医院(以下简称“我院”)就诊的PABC患者的药物治疗过程,并对以往的病例报道和相关文献进行了分析与综述,旨在为PABC的早期诊断和化疗方案的选择提供参考。
1 病例报道
1.1 临床资料
病例1:28岁女性,妊娠32周时发现左乳肿物,约鸡蛋大小,随后2个月肿物逐渐增大,未作处理,产一子,体健。产后来我院就诊,查体示左乳外上象限2点钟方向、距乳头4 cm处可触及一大小约6.5 cm×7.0 cm肿物,左侧腋窝触及肿大淋巴结;乳腺B超、胸部CT显示左侧乳腺实性占位,双侧腋窝淋巴结增大;肿物穿刺活检,结果为左乳浸润性导管癌,雌激素受体(ER)(-),孕激素受体(PR)(-),HER-2基因表达(+),增殖细胞核抗原(Ki-67)阳性率80%。术前,给予患者新辅助化疗表柔比星60 mg/m2+多西他赛75 mg/m2静脉滴注6周期(21 d为1个周期),期间患者停止哺乳。出现恶心、呕吐时,给予盐酸托烷司琼注射液5 mg,qd;出现药物性粒细胞减少时,常规给予重组人粒细胞刺激因子2 μg/kg。6周期后患者完成化疗,肿物缩小;全麻下行左乳改良根治术及左侧腋窝淋巴结清扫,术后pTNM分期为T3N1M0Ⅲa期。患者术后恢复良好,于术后2周行紫杉醇175 mg/m2+卡铂400 mg/m2静脉滴注4周期(21 d为1周期)辅助化疗,用药后出现胸闷、憋气、关节疼痛、皮疹、腹泻,考虑为药物不良反应,给予对症治疗后好转。截至本文投稿时(已随访6个月),该患者术后辅助化疗4周期结束,恢复良好,无复发。
病例2:31岁女性,产后3个月哺乳时发现右乳肿物,约蚕豆大小,近2个月以来自觉肿物逐渐增大,入院采集病史显示婴儿情况良好,查体右乳下象限,6点钟方向、距乳头4 cm处可触及一大小约3.5 cm×3.0 cm肿物;乳腺超声、胸部CT检查显示右乳腺实性肿块,右腋窝多发肿大淋巴结;病理结果显示为(右乳)浸润性癌伴坏死,非特殊类型,乳腺癌组织学分型Ⅱ级[2],局灶呈导管原位癌改变。免疫组化:ER(-),PR(-),HER-2基因表达(+++),Ki-67阳性率70%。初步诊断为右乳浸润性癌T2N1M0Ⅱb期。术前给予新辅助化疗多西他赛75 mg/m2+多柔比星50 mg/m2+环磷酰胺500 mg/m2,静脉滴注3周期(21 d为1个周期),因肿瘤缩小未达满意療效,在新辅助化疗第4周期时将方案调整为多西他赛75 mg/m2+环磷酰胺600 mg/m2静脉滴注(21 d为1个周期)联合曲妥珠单抗2 mg/kg静脉滴注,每周1次。患者在化疗过程中出现恶心呕吐时给予静脉滴注昂丹司琼8 mg,bid,后因疗效不佳换为托烷司琼5 mg,qd对症治疗。在完成6周期化疗后,肿物缩小,遂在全麻下行右乳癌保乳术,完整切除乳腺,并行腋窝淋巴结清扫术。患者术后恢复良好。出院后未执行医嘱(曲妥珠单抗联合放疗),仅继续口服利可君、乌苯美司、甲钴胺、昂丹司琼等药物1周,以预防在院化疗期间可能引起的不良反应。截至本文投稿时(已随访3个月),患者恢复良好。
两例患者基本信息情况见表1(表中,G1P1指怀孕、分娩各1次;E:表柔比星;T:多西他赛;P:紫杉醇;CBP:卡铂;A:多柔比星;C:环磷酰胺;TCH:曲妥珠单抗,表2、表3同表1)。
1.2 两例患者治疗方案分析
两例患者均为年轻女性,首发症状分别为妊娠期/哺乳期自觉胸部肿物,且肿物不断增大,经影像学检查、病理学检查和免疫标志物检验确诊。两例患者均为哺乳期确诊,肿瘤病理分级均较高,分别为Ⅲa和Ⅱb期。两例患者在确诊乳腺癌后立即开始术前新辅助化疗,分别为ET方案和TAC方案,均为指南推荐的常用辅助化疗方案[2]。其中,TAC为最常用的治疗方案,但环磷酰胺可经乳汁排出,对婴儿有一定损伤,其说明书明确要求用药时应停止哺乳[3],但考虑病例1中的患者仍在哺乳期,故给予该患者ET方案。在化疗期间,针对患者出现的化疗药物相关不良反应(如粒细胞减少、心悸、恶心呕吐等)均进行了对症治疗。新辅助化疗结束后,根据肿瘤改善情况以及患者需求分别制定了改良根治术和保乳术的手术方案。病例1中的患者术后继续进行紫杉醇+卡铂的联合化疗方案;病例2中的患者术后,医师推荐继续进行曲妥珠单抗治疗并联合放疗,但患者由于经济原因并未采纳。两例患者分别随访6个月和3个月,复查结果均良好,婴儿均体健。
2 文献综述
2.1 文献资料检索
计算机检索PubMed数据库中1986年1月-2019年4月发表的相关文献,纳入标题、关键词或摘要中包括“Breast cancer and pregnancy”“Pregnancy-associated breast cancer”“Breast cancer during pregnancy”“Breast carcinoma during pregnancy”“Case reports”等的病例报告,排除不符合PABC定义的病例报告。提取患者的基本资料、肿瘤临床特征、药物治疗方案、母胎/婴预后等信息,并进行汇总及描述性统计分析。
2.2 文献资料分析
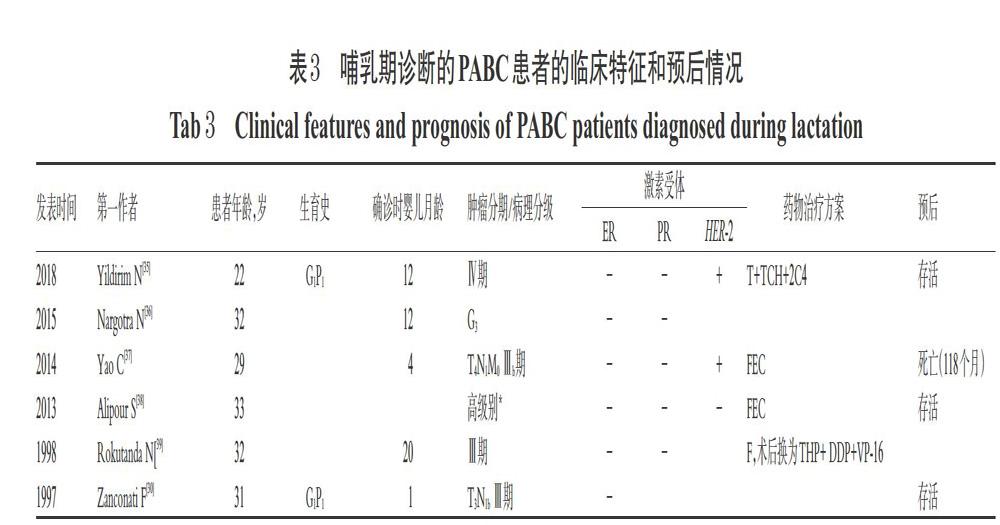
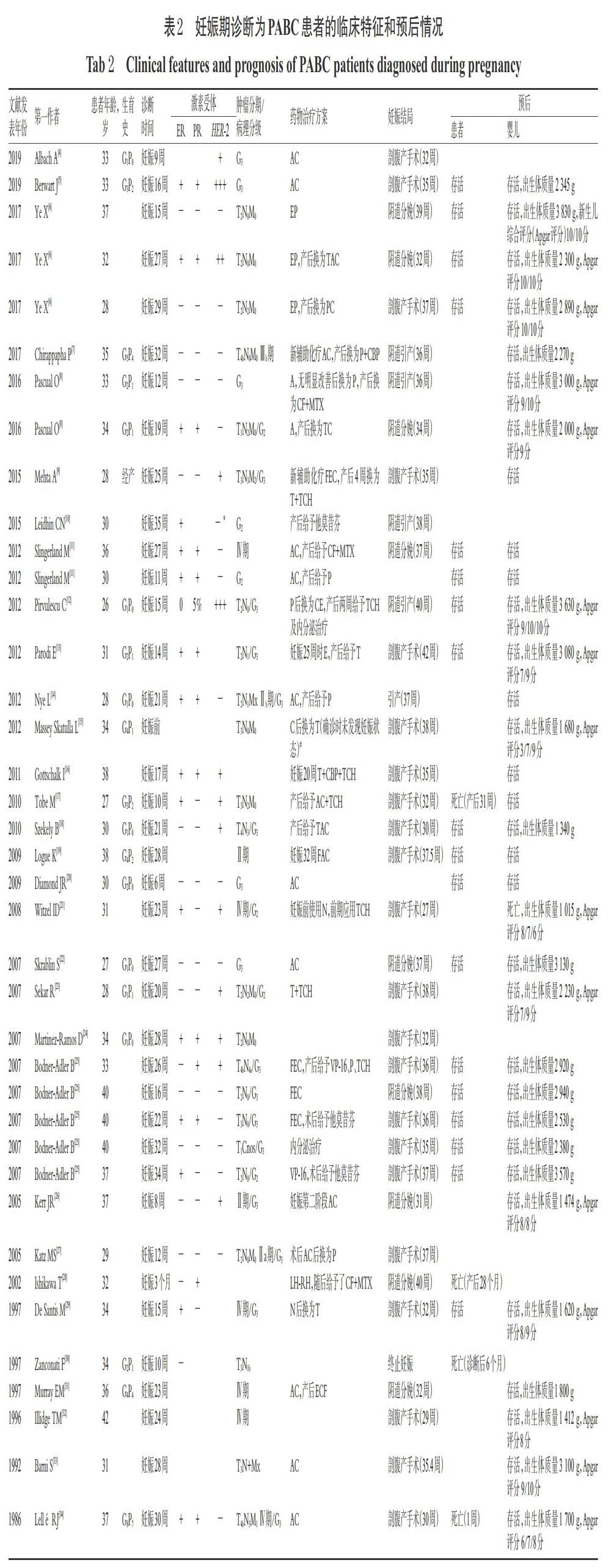
本研究共纳入36篇病例报告[4-39],合计45例患者,其中39例患者在妊娠期即诊断为乳腺癌[4-34],6例患者在哺乳期诊断为乳腺癌[30,35-39]。有研究显示,与妊娠期诊断的乳腺癌相比,产后诊断的乳腺癌预后更差,主要是由于发现较晚,肿瘤恶性程度相对较高[3]。妊娠期和哺乳期诊断的乳腺癌患者的治疗和预后情况分别见表2和表3(表中G为肿瘤组织异型性分级,3级为最高级)。45例患者确诊时的平均年龄为33岁(22~42岁);在妊娠期确诊的乳腺癌患者平均妊娠时间为20周。某些病例报告因未提供病理检查结果、治疗方案、患者及婴儿预后结局的信息而未计入统计数据中,排除这类病例后,ER、PR均为阴性/HER-2为阳性的和ER、PR、HER-2均为阴性(以下简称为“三阴性”)肿瘤的患者共占42.5%(17/40),G3级别肿瘤占76.0%(19/25)。35.0%(14/40)的病例中应用了新辅助化疗AC方案;有6例患者在妊娠期间使用了紫杉烷类药物(胎儿结局良好),有9例患者在产后应用了紫杉烷类药物。59.5%(22/37)的病例进行了择期的剖腹产手术,37.8%(14/37)的病例经阴道分娩或引产,平均生产时间为妊娠35.1周(27~42周);另有1例终止妊娠。患者预后良好,存活率为80.8%(21/26);婴儿总体良好,平均出生体质量为2 407 g(1 015~3 830 g)。
文献报道的化疗方案基本是在遵照指南[2]推荐方案的基础上综合考虑患者身体情况、肿瘤恶性程度、胎儿发育情况及家属意愿来制订的,有关药物对胎儿安全性情况的描述较少,分析原因可能为检测手段有限,只能根据既往案例及实际经验选择药物;如遇病情危重的情况,也会优先考虑使用紫杉醇类药物治疗,必要时终止妊娠。由此可见,PABC应以母婴受益最大化为主要目标,严密监测母婴状况,且需给予个体化治疗。
3 分析与讨论
3.1 PABC的流行病学
乳腺癌是我国最常见的肿瘤,据2016年公共卫生机构对过去5年的统计,我国每10万人中即有29例乳腺癌患者(年龄标准化后),占报告的女性癌症患者总数的17%[40]。乳腺癌的发病率随着年龄的增长而升高,而PABC的患病年龄显著低于一般乳腺癌,相关回顾性研究表明,PABC诊断时的患者平均年龄为33.7岁[41]。有文献报道,未经产妇女患乳腺癌的风险高于多产妇女;同样,未生育女性患乳腺癌的风险也略高于长期暴露于较高雌激素水平的女性[42]。此外,月经初潮早于12岁和绝经时间晚于55岁也是重要的危险因素[43]。随着时间的推移,周期性激素水平也会使女性患上乳腺癌,这可能是生殖细胞因为乳腺细胞响应激素(如雌激素)而生长分裂以及怀孕会导致正常的周期性激素水平中断而引起周期性高激素水平[44]。乳腺癌家族史也是PABC发病的重要危险因素,患有PABC的女性,肿瘤具有更深的组织浸润,更高程度的淋巴结受累和较大的组织异型性,以及更高的HER-2阳性率和三阴性率[45-46]。本文除5例未报道病理检查结果,其余纳入的40名病例中,ER和PR均为阴性/HER-2阳性的肿瘤和三阴性肿瘤的比例共为42.5%;组织异型性G3级别肿瘤比例为76.0%,与文献报道一致。
3.2 PABC早期诊断的重要性
病例中患者均为育龄期女性,此年龄段一般乳腺癌发病率较低,所以在疾病的预防方面非常容易被忽视,并且患者均是在妊娠及哺乳期自觉乳房肿物后就诊,而此时发现的乳腺癌多为晚期,因此建议育龄期女性最好在孕前进行相关筛查,以尽早发现、确诊乳腺癌。
3.3 PABC的化療方案
3.3.1 化疗时机的选择 化疗药物暴露的时机至关重要。妊娠早期阶段是胎儿器官形成的时期,在此阶段如果暴露于化疗药物下,胎儿发生畸形、死亡和自然流产的风险最高。据报道,在孕早期使用环磷酰胺可导致胎儿畸形,包括脚趾缺失、眼睛异常、耳垂低、腭裂[47-48]等。妊娠中晚期胎儿器官已经大致成形,在此阶段进行化疗,先天性畸形的发生率较低[49],因此化疗多在此阶段进行,但可能会增加胎儿子宫内生长受限、早产以及低出生体质量发生的风险[50]。对50例PABC患者的回顾性分析显示,妊娠前3个月接受化疗的患者,胎儿发生严重先天性异常和自然流产的比例高,而在妊娠中期或晚期接受化疗的患者中未发现胎儿畸形[51]。
3.3.2 化疗用药的选择及其对母胎的影响 妊娠期的生理变化可能会一定程度地改变化疗药物在母体的药动学和药效学特点,包括肝脏代谢、肾脏血浆流动和血浆蛋白结合率等,从而可能影响药物清除[52]。而羊水可延迟甲氨蝶呤等药物的消除,从而导致毒性增加[53]。此外,所有药物都有可能根据胎盘的物理和化学特性穿过胎盘对胎儿产生影响,因此在选择化疗药物时需要充分考虑上述因素。而分娩时若发生骨髓抑制则可能使母胎面临发生败血症和出血的风险,因此建议在分娩前至少3周避免化疗,以确保母体血细胞计数达到最佳。
在国家卫生健康委员会发布的2018年版《乳腺癌诊疗规范》[2]中,推荐的首选化疗方案为含蒽环类药物联合或序贯化疗方案(ET/TAC),主要药物有环磷酰胺、氟尿嘧啶以及紫杉醇类等药物。本研究报道的2例患者在确诊PABC后立即开始术前新辅助化疗,方案分别为ET和TAC。在笔者收集的文献综述中,35.0%的病例应用了新辅助化疗AC方案,而紫杉烷类药物多在产后的序贯疗法以及肿瘤分级较高的肿瘤中使用。
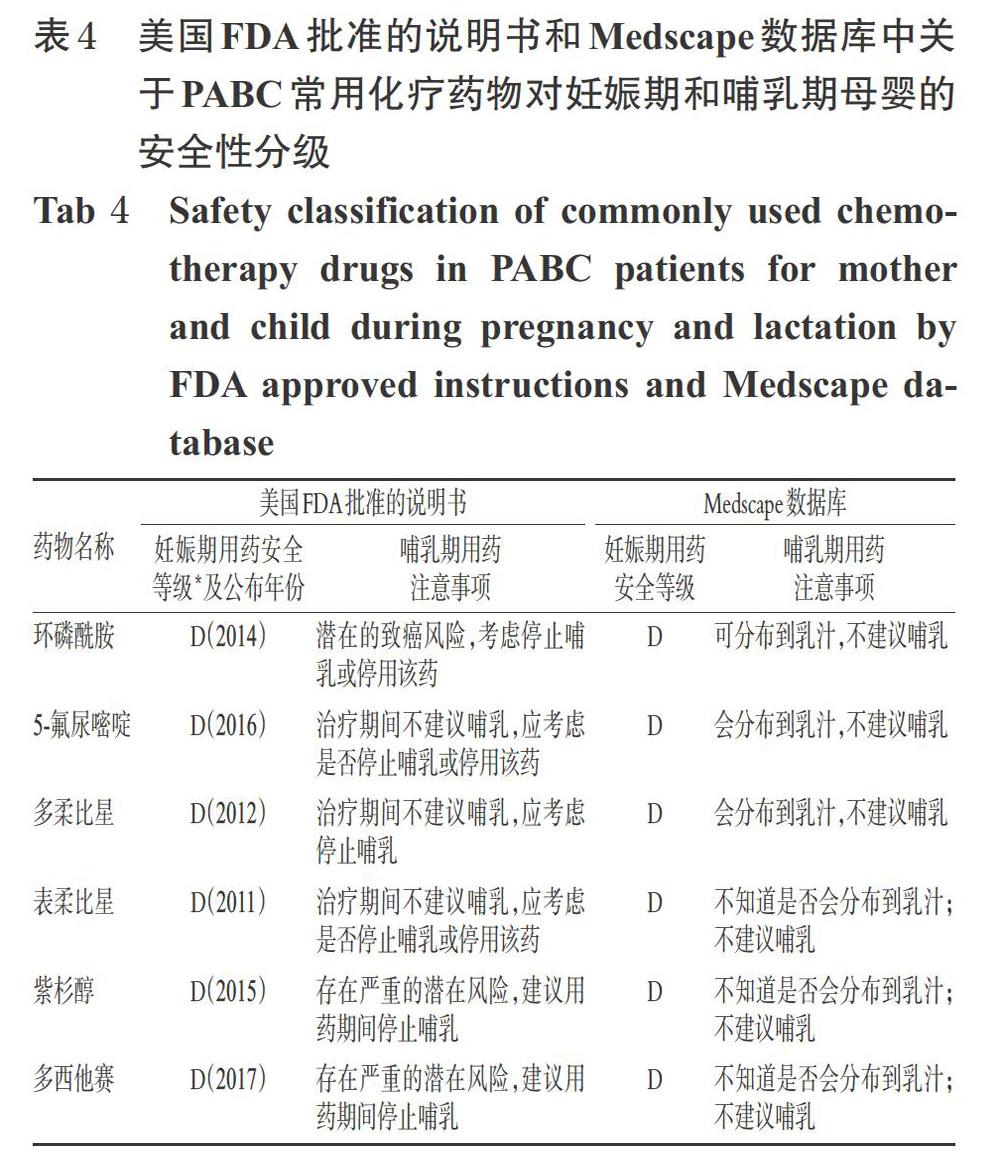
蒽环类是乳腺癌化疗方案中的主要药物,在妊娠中、晚期使用是安全的[54]。其最主要的不良反应是造成母亲以及胎儿的心脏毒性,其严重程度与药物种类及其毒性累积剂量有关。因此,在实施化疗的过程中应根据蒽环类药物使用的累积剂量和患者的耐受程度考虑是否需要换用其他蒽环类药物或者其他种类的化疗药物。紫杉醇在妊娠期的使用目前尚存在争议[55],其最常见的并发症是羊水过多或过少[56]。有病例报告指出,紫杉醇在妊娠28周与顺铂联用、在14周与表柔比星联用时,均未见胎儿或母体出现并发症[57];还有研究指出,在妊娠中晚期使用紫杉烷类药物对母亲、胎儿及新生儿的毒性较小,一般建议在中晚期使用[58]。一项前瞻性研究显示,在进行FAC方案治疗的24例中晚期妊娠患者中没有检测到胎儿先天性异常,并且没有母体或胎儿死亡[59]。曲妥珠单抗因既往出现过导致患儿肺发育不全、骨骼异常、肾功能不全、视力缺失和新生儿死亡的病例而禁用于妊娠期患者[16,60]。美国FDA批准的说明书和Medscape数据库中关于PABC常用化疗药物对妊娠期和哺乳期母婴的安全性分级见表4。
化療可能对患者产生一些长期影响,包括性腺功能障碍、生殖细胞诱变以及后代的致畸性(包括胎儿/婴儿身体和神经发育受损)等[50]。对于处于哺乳期的乳腺癌患者,一方面应建议患者停止哺乳,另一方面需要考虑药物在哺乳期使用的安全性。虽然相关指南不建议母乳喂养,但有研究显示,在保乳术和放射性治疗后,对侧乳房的乳汁产生并不受影响[61-62],因此对于坚持母乳喂养的患者,推荐用对侧乳房进行喂养。
综合上述病例报道、临床资料和相关指南及文献综述,笔者认为无论是治疗妊娠期还是哺乳期确诊的乳腺癌,目前仍将蒽环类药物作为化疗方案中的主要药物,然后根据肿瘤特征、胎儿发育状况、患者耐受情况和药物安全性制订个体化治疗方案。由于需要同时考虑多方因素,并且相关药物试验受医学伦理限制,目前对药物的选择尚无更详细的推荐,仍以严密监控用药后的母婴情况为主。对于紫杉醇的使用,笔者综合各方资料认为应在权衡利弊后使用。
3.4 PABC其他治疗方案的选择
PABC的治疗与一般乳腺癌无差异,治疗手段除化疗外还包括手术、放疗、内分泌治疗等,但均应遵循非妊娠相关乳腺癌患者的治疗指南,如美国国家综合癌症网络(NCCN)乳腺癌临床实践治疗指南[63]以及国家卫生健康委员会发布的乳腺癌诊疗规范[2],且应进行部分调整,以保护胎儿和新生儿。方案制订需综合考虑患者个体情况、耐受性、肿瘤的特点、术后复发风险等,充分权衡治疗的风险-受益后确定。Shachar SS等[64]学者认为,由于PABC的预后与其诊断及治疗时机显著相关,因妊娠而延迟治疗是没有必要的。而对于PABC患者考虑终止妊娠时应由充分知情的患者和医师共同决定。目前有研究显示,早期终止妊娠并不能改变乳腺癌患者的预后,甚至终止妊娠后患者的生存率更低[52,65]。而放射治疗在分娩前是有禁忌的[66],但如果能对腹部做到很好的防护,那么对于存在局部高复发风险的患者则可以考虑放疗。
一般在妊娠早期不建议进行手术治疗[67],最好将手术推迟至妊娠中晚期或胎儿器官发育之后。一般手术至少延迟至妊娠第12周,以降低自然流产的高风险,并根据胎龄密切监测胎儿发育情况。对于妊娠3个月的妊娠中期患者,纳入文献[4,5,34-35]中的大多数患者均接受了腋窝淋巴清扫的乳房切除术。对于在妊娠中期和晚期患者,有研究指出在分娩后进行乳房肿瘤切除术和腋窝淋巴结清扫术联合放疗的效果优于腋窝淋巴结清扫术[68]。
3.5 PABC的预后
到目前为止,PABC患者的生存率与普通乳腺癌相比是否较低仍存在争议,且研究证据不足[69]。生存率较低的原因可能是诊断和治疗较晚,而诊断时的妊娠状态似乎不是一个独立的预后因素[56]。本研究中,我院的2例患者治疗过程均较顺利,术后随访3、6个月其状况均良好,目前以定期复查为主,以监测其肿瘤复发情况。一些多中心研究结果显示,PABC患者接受化疗后其早产率会升高,胎儿出生时体质量较低,且大多数新生儿并发症与早产有关[70-71]。
对于术后有再次生育需求的女性,再次妊娠发病的风险评估以及妊娠的咨询是必不可少的[15]。大部分研究表明,在成功治疗乳腺癌后再次妊娠的女性不会使其乳腺癌的预后变差[72],并且乳腺癌后妊娠可能对这些女性有保护作用[73],但这些数据可能存在一定的选择偏倚,原因是能够怀孕的女性均为肿瘤分级较低且预后良好的女性,即存在“健康妈妈效应”,因此该结论尚需谨慎对待。由于大多数乳腺癌会在初始诊断和治疗后的2年内复发,因此临床通常建议至少治疗后2年再考虑再次妊娠[74]。
4 结语
PABC化疗方案的确定应综合考虑多方因素,尤其是胎儿的发育及药物对胎儿的影响。应尽量避免在妊娠早期进行化疗,慎重选择化疗用药,明确各药物对母婴的影响后再使用;化疗方案仍以蒽环类药物为主导,在此基础上制订个体化方案,且使用紫杉醇类药物时应充分权衡利弊并进行严密监测;胎儿结局主要与患者接受化疗时妊娠的时间、选用的药物等有关。
参考文献
[ 1 ] PEREG D,KOREN G,LISHNER M. Cancer in pregnancy:gaps,challenges and solutions[J]. Cancer Treat Rev,2008,34(4):302-312.
[ 2 ] 国家卫生健康委员会.乳腺癌诊疗规范:2018年版 [J/CD].肿瘤综合治疗电子杂志,2019,5(3):70-99.
[ 3 ] HARTMAN EK,ESLICK GD. The prognosis of women diagnosed with breast cancer before,during and after pregnancy:a meta-analysis[J]. Breast Cancer Res Treat,2016,160(2):347-360.
[ 4 ] ALBACH A,SADRUDDIN S. Diagnosis and management of metastatic breast cancer in a 33-year-old pregnant female:a case report[J]. Cureus,2019. DOI:10.7759/cureus.5240.
[ 5 ] BERWART J,PECCATORI FA. Chemotherapy and anti- HER2 therapy in metastatic breast cancer in pregnancy followed by surgical treatment[J]. Ecancermedicalscience,
2019. DOI:10.3332/ecancer.2019.930.
[ 6 ] YE X,HE Q,ZHOU X. Study on the adverse effects following chemotherapy for breast cancer diagnosis during pregnancy:the first case report in China[J]. Medicine:Baltimore,2017. DOI:10.1097/MD.0000000000008582.
[ 7 ] CHIRAPPAPHA P,THAWEEPWORADEJ P,NGAMPHAIBOON N,et al. Breast reconstruction in pregnancy:a case report of multidisciplinary team approach in immediate autologous flap reconstruction for pregnancy-associated breast cancer[J]. Clin Case Rep,2017,5(9):1450- 1453.
[ 8 ] PASCUAL O,URIARTE M,AGUSTIN MJ,et al. Two cases of breast carcinoma during pregnancy and review of the literature[J]. J Oncol Pharm Pract,2016,22(4):652- 656.
[ 9 ] MEHTA A,STALEY H,SALEEM A,et al. Breast cancer in pregnancy-enough vigilance?[J]. J Obstet Gynaecol,2015. DOI:10.3109/01443615.2014.958447.
[10] LEIDHIN CN,HEENEY A,CONNOLLY C,et al. A rare case of BRCA2-associated breast cancer in pregnancy[J]. Ir Med J,2015,108(7):217-218.
[11] SLINGERLAND M,KROEP J,LIEFERS GJ,et al. Pregnancy-associated breast cancer:current opinions on diagnosis and treatment[J]. Ned Tijdschr Geneeskd,2012,156(40):A5286.
[12] PIRVULESCU C,MAU C,SCHULTZ H,et al. Breast cancer during pregnancy:an interdisciplinary approach in our institution[J]. Breast Care:Basel,2012,7(4):311- 314.
[13] PARODI E,ALLUTO A,MOGGIO G,et al. Transient ventricular hypocinesia after in utero anthracyclines exposure:a case-report and review of the literature[J]. J Matern Fetal Neonatal Med,2012,25(2):189-192.
[14] NYE L,HUYCK TK,GRADISHAR WJ. Diagnostic and treatment considerations when newly diagnosed breast cancer coincides with pregnancy:a case report and review of literature[J]. J Natl Compr Canc Netw,2012,10(2):145-148.
[15] MASSEY SKATULLA L,LOIBL S,SCHAUF B,et al. Pre-eclampsia following chemotherapy for breast cancer during pregnancy:case report and review of the literature[J]. Arch Gynecol Obstet,2012,286(1):89-92.
[16] GOTTSCHALK I,BERG C,HARBECK N,et al. Fetal renal insufficiency following trastuzumab treatment for breast cancer in pregnancy:case report und review of the current literature[J]. Breast Care:Basel,2011,6(6):475-478.
[17] TOBE M,STEPHEN C,VASANTHA K,et al. Breast cancer in pregnancy:case report[J]. Pan Afr Med J,2010. DOI:10.4314/pamj.v521.56195.
[18] SZEKELY B,LANGMAR Z,SOMLAI K,et al. Treatment of pregnancy associated breast cancer[J]. Orv Hetil,2010,151(32):1299-1303.
[19] LOGUE K. Pregnancy-associated breast cancer[J]. Clin J Oncol Nurs,2009,13(1):25-27.
[20] DIAMOND JR,FINLAYSON CA,THIENELT C,et al. Early-stage BRCA2-linked breast cancer diagnosed in the first trimester of pregnancy associated with a hypercoagulable state[J]. Oncology:Williston Park,2009,23(9):784-791.
[21] WITZEL ID,MULLER V,HARPS E,et al. Trastuzumab in pregnancy associated with poor fetal outcome[J]. Ann Oncol,2008,19(1):191-192.
[22] SKRABLIN S,BANOVIC V,MATKOVIC V. Adriamycin and cyclophosphamide chemotherapy in advanced breast cancer in pregnancy[J]. Eur J Obstet Gynecol Reprod Biol,2007,133(2):251-252.
[23] SEKAR R,STONE PR. Trastuzumab use for metastatic breast cancer in pregnancy[J]. Obstet Gynecol,2007,110(2 Pt 2):507-510.
[24] MARTINEZ-RAMOS D,FERRARIS C,GRECO M,et al. Breast carcinoma during pregnancy[J]. Cir Esp,2007,82(5):305-307.
[25] BODNER-ADLER B,BODNER K,ZEISLER H. Breast cancer diagnosed during pregnancy[J]. Anticancer Res,2007,27(3b):1705-1707.
[26] KERR JR. Neonatal effects of breast cancer chemotherapy administered during pregnancy[J]. Pharmacotherapy,2005,25(3):438-441.
[27] KATZ MS,SCHAPIRA L,HARISINGHANI MG,et al. Palpable right breast mass in a pregnant woman[J]. Nat Clin Pract Oncol,2005,2(4):218-221.
[28] ISHIKAWA T,HAMAGUCHI Y,MOMIYAMA N,et al. Pregnancy-associated breast cancer with multiple metastases[J]. Eur J Surg,2002,168(7):428-430.
[29] DE SANTIS M,LUCCHESE A,DE CAROLIS S,et al. Metastatic breast cancer in pregnancy:first case of chemotherapy with docetaxel[J]. Eur J Cancer Care:Engl,2000,9(4):235-237.
[30] ZANCONATI F,ZANELLA M,FALCONIERI G,et al. Gestational squamous cell carcinoma of the breast:an unusual mammary tumor associated with aggressive clinical course[J]. Pathol Res Pract,1997,193(11):783-787.
[31] MURRAY EM,WERNER ID. Pregnancy and abortion in breast cancer patients:two case reports and a literature review[J]. S Afr Med J,1997,87(11):1538-1539.
[32] ILLIDGE TM,HUSSEY M,GODDEN CW. Malignant hypercalcaemia in pregnancy and antenatal administration of intravenous pamidronate[J]. Clin Oncol:R Coll Radiol,1996,8(4):257-258.
[33] BARNI S,ARDIZZOIA A,ZANETTA G,et al. Weekly doxorubicin chemotherapy for breast cancer in pregnancy:a case report[J]. Tumori,1992,78(5):349-350.
[34] LELL? RJ,BEISLER G,UNLU C. Effect of pregnancy on metastasizing breast cancer:a case report[J]. Z Geburtshilfe Perinatol,1986,190(5):229-231.
[35] YILDIRIM N,BAHCECI A. Use of pertuzumab and trastuzumab during pregnancy[J]. Anticancer Drugs,2018,29(8):810-813.
[36] NARGOTRA N,KALITA D. Pregnancy associated breast cancer:awareness is the key to diagnosis:a case report[J]. J Clin Diagn Res,2015,9(11):9-11.
[37] YAO C,XIA H,WANG Y,et al. Long-term treatment after preoperative high-dose chemotherapy in a lactating breast cancer patient[J]. Cell Biochem Biophys,2014,69(1):61-64.
[38] ALIPOUR S,SEIFOLLAHI A,ANBIAEE R. Lactating breast abscess:a rare presentation of adenosquamous breast carcinoma[J]. Singapore Med J,2013,54(12):247-249.
[39] ROKUTANDA N,IINO Y,YOKOE T,et al. Primary squamous cell carcinoma of the breast during lactation:a case report[J]. Jpn J Clin Oncol,2000,30(6):279-282.
[40] LI T,MELLO-THOMS C,BRENNAN PC. Descriptive epidemiology of breast cancer in China:incidence,mortality,survival and prevalence[J]. Breast Cancer Res Treat,2016,159(3):395-406.
[41] LANGER A,MOHALLEM M,STEVENS D,et al. A single-institution study of 117 pregnancy-associated breast cancers(PABC):presentation,imaging,clinicopathological data and outcome[J]. Diagn Interv Imaging,2014,95(4):435-441.
[42] OPDAHL S,ALSAKER MD,JANSZKY I,et al. Joint effects of nulliparity and other breast cancer risk factors[J]. Br J Cancer,2011,105(5):731-736.
[43] DURRANI S,AKBAR S,HEENA H. Breast cancer during pregnancy[J]. Cureus,2018. DOI:10.7759/cureus.2941:e2941.
[44] TRAVIS RC,KEY TJ. Oestrogen exposure and breast cancer risk[J]. Breast Cancer Res,2003. DOI:10.1186/bcr628.
[45] JOHANSSON ALV,ANDERSSON TM,HSIEH CC,et al. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer[J]. Int J Cancer,2018,142(7):1343-1354.
[46] BORGES VF,SCHEDIN PJ. Pregnancy-associated breast cancer:an entity needing refinement of the definition[J]. Durham:Cancer,2012,118(13):3226-3228.
[47] BRIGGS GG,FREEMAN RK,YAFFE SJ. Drugs in pregnancy and lactation:a reference guide to fetal and neonatal risk[M]. Philadelphia:Lippincott Williams & Wilkins,2012:15-16.
[48] TOLEDO T,HARPER R,MOSER R. Fetal effects during cyclophosphamide and irradiation therapy[J]. Ann Intern Med,1971,74(1):87-91.
[49] WOO JC,YU T,HURD TC. Breast cancer in pregnancy:a literature review[J]. Arch Surg,2003,138(1):91-98.
[50] CARDONICK E,IACOBUCCI A. Use of chemotherapy during human pregnancy[J]. Lancet Oncol,2004,5(5):283-291.
[51] SOKAL JE,LESSMANN EM. Effects of cancer chemotherapeutic agents on the human fetus[J]. J Am Med Assoc,1960,172(16):1765-1771.
[52] NUGENT P,OCONNELL TX. Breast cancer and pregnancy[J]. JAMA Surgery,1985,120(11):1221-1224.
[53] SAHAKYAN V,POZZO E,DUELEN R,et al. Methotrexate and valproic acid affect early neurogenesis of human amniotic fluid stem cells from myelomeningocele[J]. Stem Cells International,2017. DOI:10.1155/2017/6101609.
[54] TURCHI JJ,VILLASIS C. Anthracyclines in the treatment of malignancy in pregnancy[J]. Cancer,1988,61(3):435-440.
[55] 吳尚谕.妊娠相关乳腺癌的临床特点及预后分析[D].乌鲁木齐:新疆医科大学,2016.
[56] KNABBEN L,MUELLER MD. Breast cancer and pregnancy[J]. Horm Mol Biol Clin Investig,2017. DOI:10.1515/hmbci-2017-0026.
[57] LOIBL S. New therapeutic options for breast cancer during pregnancy[J]. Breast Care:Basel,2008,3(3):171- 176.
[58] AMANT F,DECKERS S,VAN CALSTEREN K,et al. Breast cancer in pregnancy:recommendations of an international consensus meeting[J]. Eur J Cancer,2010,46(18):3158-3168.
[59] BERRY DL,THERIAULT RL,HOLMES FA,et al. Management of breast cancer during pregnancy using a standardized protocol[J]. J Clin Oncol,1999,17(3):855-855.
[60] ZAGOURI F,SERGENTANIS TN,CHRYSIKOS D,et al. Trastuzumab administration during pregnancy:a systematic review and meta-analysis[J]. Breast Cancer Res Treat,2013,137(2):349-357.
[61] HIGGINS S,HAFFTY BG. Pregnancy and lactation after breast-conserving therapy for early stage breast cancer[J]. Cancer,1994,73(8):2175-2180.
[62] MORAN MS,COLASANTO JM,HAFFTY BG,et al. Effects of breast-conserving therapy on lactation after pregnancy[J]. Cancer J,2005,11(5):399-403.
[63] WILLIAM G,JAME A,HAROLD B,et al. NCCN clinical practice guidelines in oncology:breast cancer[R].New York:NCCN,2019.
[64] SHACHAR SS,GALLAGHER K,MCGUIRE K,et al. Multidisciplinary management of breast cancer during pregnancy[J]. Oncol,2017,22(3):324-334.
[65] LITTON JK,THERIAULT RL. Breast cancer and pregnancy:current concepts in diagnosis and treatment[J]. Oncol,2010,15(12):1238-1247.
[66] DAVIES FA. Pregnancy and breast cancer greentop gui- deline:No. 12[R]. London: Royal College of Obstetricians and Gynaecologists,2011:15.
[67] ROJAS KE,BILBRO N,MANASSEH DM,et al. A review of pregnancy-associated breast cancer:diagnosis,local and systemic treatment,and prognosis[J]. J Womens Health,2018. DOI:10.1089/jwh.2018.7264.
[68] HICKEY M,PEATE M,SAUNDERS CM,et al. Breast cancer in young women and its impact on reproductive function[J]. Hum Reprod,2009,15(3):323-339.
[69] AMANT F,VON MINCKWITZ G,HAN SN,et al. Prognosis of women with primary breast cancer diagnosed during pregnancy:results from an international collaborative study[J]. J Clin Oncol,2013,31(20):2532-2539.
[70] LOIBL S,HAN SN,VON MINCKWITZ G,et al. Treatment of breast cancer during pregnancy:an observational study[J]. Lancet Oncol,2012,13(9):887-896.
[71] VAN CALSTEREN K,HEYNS L,DE SMET F,et al. Cancer during pregnancy:an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes[J]. J Clin Oncol,2010,28(4):683-689.
[72] MUELLER BA,SIMON MS,DEAPEN D,et al. Childbearing and survival after breast carcinoma in young women[J]. Cancer,2003,98(6):1131-1140.
[73] THERIAULT RL, JENNIFER KL. Safety of pregnancy following breast cancer diagnosis:a meta-analysis of 14 studies[J]. Eur J Cancer,2011,47(1):74-83.
[74] HELEWA M,LEVESQUE P,PROVENCHER D,et al. Breast cancer,pregnancy,and breastfeeding[J]. J Obstet Gynaecol Can,2002,24(2):164-180.
(收稿日期:2019-05-28 修回日期:2020-02-06)
(編辑:孙 冰)
