pEGFP-Egr-1表达质粒构建及其表达抑制HepG2细胞增殖
2016-08-10黄心怡金韵特陈建明
黄心怡,虞 游,金韵特,陈建明
(杭州师范大学生命与环境科学学院,浙江 杭州 310036)
pEGFP-Egr-1表达质粒构建及其表达抑制HepG2细胞增殖
黄心怡,虞游,金韵特,陈建明
(杭州师范大学生命与环境科学学院,浙江 杭州 310036)
摘要:将RT-PCR扩增的Egr-1基因连接到pMD-18载体上并测序鉴定,经测序正确的Egr-1基因亚克隆到绿色荧光蛋白真核表达载体pEGFP-C1上构建成重组质粒pEGFP-Egr-1.重组质粒pEGFP-Egr-1转染HepG2细胞后,运用荧光显微镜及荧光定量PCR技术分析其在HepG2细胞中的表达情况,并进一步运用WST-1法分析其表达对HepG2细胞增殖的影响.结果显示,重组质粒pEGFP-Egr-1构建正确,转染后在荧光显微镜下观察可见HepG2细胞发出绿色荧光信号,荧光定量PCR分析发现转染后HepG2细胞Egr-1基因mRNA水平明显升高.WST-1结果表明,与对照组相比,转染pEGFP-Egr-1质粒的HepG2细胞,其增殖速率明显下降.上述结果表明,pEGFP-Egr-1重组质粒构建成功,外来过表达Egr-1基因能够抑制HepG2细胞增殖.
关键词:Egr-1基因;pEGFP-Egr-1表达质粒;HepG2细胞;细胞增殖
肝癌是一种常见的恶性肿瘤,其发病率及死亡率呈逐年上升趋势,且其发病机制复杂病程较长,肝癌患者目前的治愈率较低[1].姜黄是一种中药,其有效成分是姜黄素,越来越多的研究结果揭示姜黄素对人多种肿瘤细胞的增殖有抑制作用[2-6].本课题组利用姜黄素处理培养的人肝癌细胞系HepG2细胞,结果发现姜黄素能抑制HepG2细胞增殖并能诱导HepG2细胞中早期生长反应因子1(early growth response 1,Egr-1)的表达,这一研究结果提示Egr-1基因可能参与姜黄素对HepG2细胞增殖的抑制作用[7].已知Egr-1基因编码产物是一种转录因子,它能够被生长因子、细胞因子及抗癌药物等众多刺激源所激活,激活后的Egr-1基因编码产物作为转录因子,参与细胞增殖、细胞分化及细胞凋亡等生命活动过程[8-9].有研究报道在一些肿瘤细胞及肿瘤组织中Egr-1基因呈现低表达,并且还报道了Egr-1基因外来过表达对肿瘤细胞增殖有显著抑制作用[10].由此,推测Egr-1基因可能是一种抑癌基因,它在HepG2细胞增殖中起抑制作用,姜黄素抑制HepG2细胞增殖可能是通过诱导Egr-1基因表达而介导的.本文通过构建Egr-1基因的增强绿色荧光蛋白融合表达质粒,并使其在培养的HepG2细胞中外来过表达,利用基因外来过表达探讨Egr-1基因在HepG2细胞增殖中所起的作用.
1材料和方法
1.1主要仪器
Incustar 210型CO2细胞培养箱(BioSource),CKX41倒置相差显微镜(Olympus),BX-51荧光显微镜(Omachi),Tanon Gis System凝胶成像系统(上海天能),MLS-3750 自动高压蒸汽灭菌锅(Sanyo),常规PCR仪(杭州朗基)及液氮生物储存罐等.
1.2主要试剂
DMEM高糖培养基购自武汉博士德,新生牛血清购自杭州四季青,姜黄素购自Bio Basic,细胞消化用胰蛋白酶、青链霉素、Trizol试剂及WST-1细胞增殖检测试剂盒购自碧云天、cDNA第一链合成试剂盒及PCR扩增试剂盒购自上海生工,GeneTran脂质体转染试剂购自BIOMIGA,SYBR®qPCR Mix购自THUNDERBIRD,其余生化试剂均为国产分析纯.
1.3质粒、菌种和细胞系
人肝癌细胞系HepG2细胞及大肠杆菌DH5α为本室保存,T-载体pMD-18购自大连宝生物公司,增强绿色荧光蛋白表达载体pEGFP-C1由浙江大学医学院提供.HepG2细胞贴壁培养于含10%小牛血清及1%青链霉素的DMEM培养基中,在37 ℃及含5%CO2的细胞培养箱中进行培养,每隔2~3 d换一次培养基.
1.4Egr-1基因编码区的克隆
培养的HepG2细胞用20 μmol/L姜黄素处理3 h后,收集细胞用Trizol试剂提取细胞总RNA,取2 μg总RNA逆转录合成cDNA第一链,再用PCR技术扩增Egr-1基因其全长编码区cDNA片段.PCR扩增引物序列为,上游引物:5-CTCGAGGGATGGCCGCGGCCAA-3,下游引物:5- AAGCTTTTAGCAAATTTCAATTGTCC-3;PCR扩增条件为:94 ℃预变性5min后94 ℃变性35s,58 ℃退火35s,72 ℃延伸2min35s,35个循环后72 ℃延伸10min.扩增得到的PCR产物经琼脂糖凝胶电泳后,割胶纯化回收Egr-1目的DNA片段,将回收的目的DNA片段克隆到pMD-18T-载体并进行测序,经测序正确后进行下一步实验.
1.5pEGFP-Egr-1真核表达质粒的构建
经测序正确的pMD18-Egr-1重组质粒用XhoI和HindIII双酶切,电泳后割胶回收Egr-1目的DNA片段,再与经XhoI和HindIII双酶切的带有增强绿色荧光蛋白基因的真核表达载体pEGFP-C1连接,构建得到pEGFP-Egr-1重组表达质粒,然后转化大肠杆菌大量提取制备pEGFP-Egr-1重组表达质粒,经酶切鉴定及测序鉴定正确的质粒用于后续实验.
1.6pEGFP-Egr-1表达质粒转染HepG2细胞
转染前一天,取对数生长期的HepG2细胞接种于6孔细胞培养板中,每孔接种2×105个细胞,第二天当细胞生长汇合达到80%时即可用于转染实验.转染时,首先在超净工作台内取2μg重组表达质粒DNA,将其稀释到50μL不含血清的DMEM培养基中,随后取5μL转染试剂(GeneTran)稀释到50μL不含血清的DMEM中,室温静置5min后,将二者混合并轻轻混匀,配置成转染复合物,再室温静置20min,最后将100μL转染复合物轻轻滴加到6孔板每孔的细胞培养基中,轻轻混匀后将6孔板再放回到培养箱中继续培养24h.
1.7pEGFP-Egr-1质粒在细胞中表达检测
pEGFP-Egr-1表达质粒或pEGFP-C1载体分别转染Hep2细胞24h后,消化收集细胞,分别用荧光显微镜及半定量RT-PCR技术检测pEGFP-Egr-1质粒在Hep2细胞中的表达情况.荧光显微镜检测时,先用PBS漂洗细胞2次,再将细胞放在荧光显微镜下用488nm波长激发光激发后观察细胞发出的绿色荧光信号,并用ProGRes数码系统拍摄细胞荧光照片.半定量RT-PCR检测时,先用Trizol试剂分别提取细胞总RNA,各取2μg细胞总RNA用cDNA合成试剂盒逆转录合成cDNA第一链,再各取2μLcDNA作为模板DNA用PCR技术分析Egr-1基因其mRNA转录情况,以β-actin基因mRNA转录水平作为内对照.PCR扩增Egr-1基因时,其引物序列为上游引物5’-CCAAGACTGAAGACTGAA-3’,下游引物5’-TAGAAGAAAAAGTCTGGTGAAGTTTCCATACTTG-3’,扩增条件为94 ℃预变性4min后94 ℃变性30s,50 ℃退火45s,72 ℃延伸45s,32个循环后72 ℃延伸10min;PCR扩增β-actin基因时,其引物序列为上游引物5’-GCGGGAAATCGTGCGTGACATT-3’,下游引物5’-GATGGAGTTGAAGGTAGTTTCGTG-3’,扩增条件为94 ℃预变性4min后94 ℃变性30s,55 ℃退火30s,72 ℃延伸30s,22个循环后72 ℃延伸10min.
1.8pEGFP-Egr-1重组质粒表达对HepG2细胞增殖影响分析
将对数生长的HepG2细胞按每孔5 000个细胞接种于96孔培养板中培养过夜.第二天进行细胞转染,分别用pEGFP-C1、1×pEGFP-Egr-1、2×pEGFP-Egr-1以及4×pEGFP-Egr-1转染HepG2细胞,转染方法同上.转染培养24h后,收获细胞并用WST-1分析细胞增殖情况.独立实验重复3次,每次实验时每种转染重复3个复孔细胞.
1.9统计方法

2结果
2.1pEGFP-Egr-1真核表达质粒的构建
RT-PCR扩增得到的产物(图1A)经琼脂糖凝胶电泳检测后切胶回收目的片段后TA克隆构建pMD18-Egr-1重组质粒,将重组质粒转化大肠杆菌小提制备pMD18-Egr-1质粒.随后,用XhoI和HindIII双酶切pMD18-Egr-1质粒,电泳后回收小片段,再与经XhoI和HindIII双酶切的pEGFP-C1连接构建pEGFP-Egr-1质粒,然后转化大肠杆菌大提制备pEGFP-Egr-1质粒,经酶切鉴定后的质粒用于测序鉴定(图1B),测序结果正确的质粒用于后续实验.
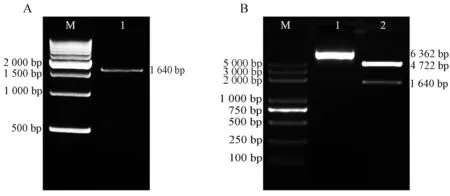
A:M,DNA分子量标记;泳道1,RT-PCR产物.B:M,DNA分子量标记;泳道1,pEGFP-Egr-1;泳道2,pEGFP-Egr-1用Xho I和Hind III双酶切.图1 pEGFP-Egr-1真核表达质粒的构建Fig. 1 Construction of pEGFP-Egr-1 eukaryotic expression plasmid

图2 重组质粒pEGFP-Egr-1在HepG2细胞中表达EGFP-Egr-1融合蛋白Fig. 2 Recombinant plasmid pEGFP-Egr-1 expressed EGFP-EGR-1 fusion protein in HepG2 cells
2.2pEGFP-Egr-1质粒在HepG2细胞中的表达
pEGFP-Egr-1转染HepG2细胞24 h后,在荧光显微镜下观察到HepG2发出清晰的绿色荧光信号(图2),空载体pEGFP-C1转染的HepG2细胞也观察到了清晰的绿色荧光信号,且空载体转染细胞的荧光信号比pEGFP-Egr-1质粒转染细胞的荧光信号要多得多,符合笔者的预期.这是由于空载体比pEGFP-Egr-1质粒小因而其转染效率高之故.荧光显微镜分析结果说明,pEGFP-Egr-1质粒在HepG2细胞中表达了EGFP-Egr-1融合蛋白.pEGFP-Egr-1质粒转染HepG2细胞24 h后,荧光定量PCR分析发现,HepG2细胞Egr-1基因mRNA水平要明显高于空载体转染细胞中Egr-1基因的mRNA 水平(图3),进一步说明,pEGFP-Egr-1质粒在HepG2细胞中表达了EGFP-Egr-1融合蛋白.
2.3pEGFP-Egr-1质粒表达抑制HepG2细胞的增殖
WST-1结果显示,pEGFP-Egr-1转染HepG2细胞后,与空载体pEGFP-C1相比,随着转染质粒浓度的升高,HepG2细胞增殖速率越慢,统计发现差异显著(P<0.05),由此说明,pEGFP-Egr-1质粒表达能抑制HepG2细胞的增殖.上述结果表明,Egr-1基因能够抑制肝癌HepG2细胞的增殖(图4).

GAPDH作为内对照,**表示与pEGFP-C1组相比较P<0.01.图3 荧光定量分析重组质粒pEGFP-Egr-1转染的HepG2细胞Egr-1基因mRNA水平Fig. 3 mRNA level of Egr-1 Gene analyzed by qPCR in recombinant plasmid pEGFP- Egr-1-transfected HepG2 cells

符号(*)表示与pEGFP-C1转染细胞相比有显著差异.;***,P<0.001.图4 重组质粒pEGFP-Egr-1表达抑制HepG2细胞的增殖Fig. 4 Expression of recombinant plasmid pEGFP-Egr-1 inhibited the proliferation of HepG2 cells
3讨论
Egr-1作为一种含有锌指结构的转录因子,定位于细胞核内,主要参与细胞的增殖及分化过程[11-12].在肿瘤细胞及肿瘤组织中,Egr-1基因呈现低表达;而外来过表达Egr-1基因能够抑制肿瘤细胞的增殖,这些研究结果表明Egr-1基因可能是一个潜在的抑癌基因[13-15].姜黄素是中药姜黄的有效成分,越来越多的研究结果揭示姜黄素能够抑制多种肿瘤细胞的增殖并诱导其凋亡[16-18].本课题组的研究也发现姜黄素能够有效抑制HepG2细胞增殖,并且还发现姜黄素能够诱导HepG2细胞中Egr-1基因表达,我们推测姜黄素抑制HepG2细胞增殖是通过诱导Egr-1基因表达而介导的[7].
本研究通过构建Egr-1基因的增强绿色荧光蛋白融合表达质粒,通过转染使其在培养的HepG2细胞中外来过表达Egr-1基因的融合蛋白,研究探讨Egr-1基因在HepG2细胞增殖中所起的作用.首先,利用RT-PCR及基因克隆等技术,成功将Egr-1基因编码区cDNA克隆到增强绿色荧光蛋白表达载体pEGFP-C1中,并利用荧光显微镜及荧光定量PCR技术分析构建质粒在HepG2细胞中的表达情况,最后成功构建了能在HepG2细胞中表达Egr-1基因的绿色荧光融合蛋白的pEGFP-Egr-1重组质粒;随后,将pEGFP-Egr-1重组质粒转染培养的HepG2细胞,利用WST-1法分析pEGFP-Egr-1转染后Egr-1基因外来过表达对HepG2细胞增殖的影响.结果发现,HepG2细胞转染了重组表达质粒pEGFP-Egr-1后与转染空载体pEGFP-C1相比,其增殖速率明显下降,说明Egr-1基因外来过表达能抑制HepG2细胞的增殖,证明Egr-1基因在HepG2细胞增殖中起抑制作用.本研究结果为我们最终揭示Egr-1基因在姜黄素抑制HepG2细胞增殖中所起的作用奠定了研究基础.
参考文献:
[1] SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2015[J]. CA Cancer J Clin,2015,65(1):5-29.
[2] SHISHODIA S. Molecular mechanisms of curcumin action: gene expression[J]. Biofactors,2013,39(1):37-55.
[3] SHEHZAD A, LEE J, LEE Y S. Curcumin in various cancers[J]. Biofactors,2013,39(1):56-68.
[4] GAO W, CHAN J Y, WEI W I, et al. Anti-cancer effects of curcumin on head and neck cancers[J]. Anticancer Agents Med Chem,2012,12(9):1110-1116.
[5] CRETU E, TRIFAN A, VASINCU A, et al. Plant-derived anticancer agents-curcumin in cancer prevention and treatment[J]. Rev Med Chir Soc Med Nat Iasi,2012,116(4):1223-1229.
[6] ZHOU H, BEEVERS C S, HUANG S. The targets of curcumin[J]. Curr Drug Targets,2011,12(3):332-347.
[7] 虞游,蒋汉伟,卢佳伟,等.姜黄素通过诱导Egr-1基因表达抑制HepG2细胞的增殖[J].中国细胞生物学学报,2015,37(6):840-845.
[8] THIEL G, CIBELLI G. Regulation of life and death by the zinc finger transcription factor Egr-1[J]. J Cell Physiol,2002,193(3):287-292.
[9] KRONES-HERZIG A, MITTAL S, YULE K, et al. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53[J]. Cancer Res,2005,65(12):5133-5143.
[10] BARON V, ADAMSON ED, CALOGERO A, et al. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbetal, PTEN, p53, and fibronectin[J]. Cancer Gene Ther,2006,13(2):115-124.
[11] WANG X, MEI Y, JI Q, et al. Early growth response gene-1 decoy oligonucleotides inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia of autogenous vein graft in rabbits[J]. Interact Cardiovasc Thorac Surg,2015,21(1):50-54.
[12] GUO B, TIAN X C, LI D D, et al. Expression, regulation and function of Egr1 during implantation and decidualization in mice[J].Cell Cycle,2014,13(16):2626-2640.
[13] WEI J, OUYANG Y, LI X, et al. Early growth response gene 1, a TRBP binding protein, is involved in miRNA activity of miR-125a-3p in human cells[J]. Cell Signal,2015,27(6):1120-1128.
[14] ZHANG H, CHEN X, WANG J, et al. EGR1 decreases the malignancy of human non-small cell lung carcinoma by regulating KRT18 expression[J]. Sci Rep,2014,4:5416.
[15] MYUNG D S, PARK Y L, KIM N, et al. Expression of early growth response-1 in colorectal cancer and its relation to tumor cell proliferation and apoptosis[J]. Oncol Rep,2014,31(2):788-794.
[16] KUMAR A, SASMAL D, JADAV S S, et al. Mechanism of immunoprotective effects of curcumin in DLM-induced thymic apoptosis and altered immune function: an in silico and in vitro study[J]. Immunopharmacol Immunotoxicol,2015,37(6):488-498.
[17] JIN H, QIAO F, WANG Y, et al. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of P13K/Akt signaling pathway[J]. Oncol Rep,2015,34(5):2782-2789.
[18] PATEL P B, THAKKAR V R, PATEL J S. Cellular effect of curcumin and citral combination on breast cancer cells: induction of apoptosis and cell cycle arrest[J]. J Breast Cancer,2015,18(3):225-234.
收稿日期:2015-10-30
基金项目:杭州市社会发展自主申报项目(20160533B01);国家级大学生创新创业训练计划项目(201510346019).
通信作者:陈建明(1964—),男,副教授,博士,主要从事肿瘤细胞分子生物学研究.E-mail:jianmingchen@hznu.edu.cn
doi:10.3969/j.issn.1674-232X.2016.04.009
中图分类号:Q253;R363.1
文献标志码:A
文章编号:1674-232X(2016)04-0382-05
Construction of pEGFP-Egr-1 Eukaryotic Expression Plasmid and Its Expression Inhibiting HepG2 Cell Proliferation
HUANG Xinyi, YU You, JIN Yunte, CHEN Jianming
(College of Life and Environment Sciences, Hangzhou Normal University, Hangzhou 310036, China)
Abstract:Egr-1 gene amplified by RT-PCR was ligated into the pMD-18 T-vector and confirmed by sequencing. Then the pMD-Egr-1 plasmid was digested and the cloned into the pEGFP-C1 to construct the pEGFP-Egr-1. The pEGFP-Egr-1 plasmid was transfected into HepG2 cells and its expression was analyzed by fluorescent microscope and qPCR. Meanwhile, the effect of pEGFP-Egr-1 expression on HepG2 cells proliferation was measured by WST-1 assay. It was shown that the recombinant plasmid pEGFP-Egr-1 was successfully constructed and the GFP signal was also observed; the result of qPCR showed the mRNA expression of Egr-1 gene was elevated after transfection of pEGFP-Egr-1 and the WST-1 result indicated the proliferation rate of HepG2 cells transfected with pEGFP-Egr-1 was decreased compared with the control cells transfected with pEGFP-C1. Thus, the collected data suggested pEGFP-Egr-1 plasmid was successfully constructed and over-expression of Egr-1 gene could inhibit HepG2 cell proliferation.
Key words:Egr-1 gene; pEGFP-Egr-1 expression plasmid; HepG2 cells; cell proliferation
