全基因组关联分析筛选鹅蛋品质相关分子标记
2023-10-25高广亮张克山赵献芝许国洋谢友慧周莉张昌莲王启贵
高广亮,张克山,赵献芝,许国洋,谢友慧,周莉,张昌莲,王启贵
全基因组关联分析筛选鹅蛋品质相关分子标记
高广亮1, 2,张克山1, 2,赵献芝1, 2,许国洋1,谢友慧1, 2,周莉3,张昌莲1, 2,王启贵
1重庆市畜牧科学院,重庆 402460;2重庆市肉鹅遗传改良工程技术研究中心,重庆 402460;3重庆綦江区动物疫病预防控制中心,重庆 401420
【目的】通过筛选与鹅蛋品质性状相关的分子标记和候选基因,为解析蛋品质性状的遗传机制及分子标记辅助选择提供理论支撑。【方法】采用同批次健康四川白鹅群体(209只)作为研究对象。收集了每只鹅在产蛋高峰期连续生产的5枚蛋,并测定了蛋重、蛋形指数、蛋壳强度、蛋壳厚度、蛋壳重和蛋黄重量等6个蛋品质性状。基于前期209只四川白鹅(母鹅)2.896 Tb全基因组重测序数据(12.44×/个体),采用全基因组关联分析的方法,筛选与蛋品质性状相关的SNP位点和重要候选基因,并通过核酸飞行时间质谱方法检测了这些SNP位点的基因型频率。 【结果】经过筛选过滤,共有9 279 339个SNPs和209个个体用于后续研究。GWAS研究发现,48个SNP位点与6个鹅蛋品质性状显著或建议性显著相关(阈值分别为5.43×10-9和1.09×10-7),并注释出27个蛋品质性状相关的候选基因,包括妊娠相关血浆蛋白A(pappalysin1, PAPPA)、蛋白丝氨酸/苏氨酸磷酸酶调节亚基2基因(serine/threonine-protein phosphatase 4 regulatory subunit 2,PP4R2)、乙醇胺磷酸转移酶1(ethanolamine phosphotransferase 1, EPT1)和离子型谷氨酸受体K2(glutamate receptor ionotropic, kainate 2,GRIK2)等,其中候选基因参与蛋白质代谢,促进生长因子IGF生成,在的11bp范围内存在5个SNPs与蛋壳厚度显著相关,另外在上有6个SNPs与蛋黄重量显著相关,和分别与机体血钙维持功能以及胆固醇代谢相关。功能富集研究发现,候选基因主要参与了response to growth factor(GO:0070848)、intracellular chemical homeostasis(GO:0055082)、response to hormone(GO:0009725)和regulation of the monoatomic ion transport(GO:43269)等代谢通路。【结论】经GWAS方法筛选出和分别作为蛋重、蛋黄重、蛋壳强度等蛋品质性状潜在功能基因,为鹅蛋品质性状的改良提供了分子遗传标记的理论参考。
全基因组关联分析;分子标记辅助选择;鹅;蛋品质性状
0 引言
【研究意义】我国是世界上养鹅量最大的国家,国家水禽产业体系2022年报告显示我国鹅年出栏量已达4.68亿只,产值526.73亿元,因此针对直接影响鹅蛋经济效益的蛋品质性状研究具有重要的现实意义[1]。【前人研究进展】目前研究表明:品种、年龄、营养、环境、疾病等都可影响蛋品质性状[2-7]。全基因组关联分析(genome-wide association study, GWAS)方法是在全基因组水平上关联定位分析重要经济性状的分子遗传标记,筛选有生物学意义的候选基因。目前GWAS已广泛运用到家禽的分子遗传标记选择中,如:ZHU等[8-9]对北京鸭体尺和屠体性状等进行GWAS分析,发现高皮脂北京鸭抗脂肪生成基因存在特定的突变位点,并发现屠体重、全净膛重等5个体组成性状都有1个相同的全基因组显著位点,候选基因为;Zhang等[10]在两个高低腹脂专门化肉鸡品系中用60K SNP芯片作单倍型GWAS研究,定位了7个可能控制腹脂含量的候选基因,包括、1、和等;LIU等采用600 K高密度SNP阵列,研究了产蛋后期72和80周龄的母鸡蛋品质的全基因关联分析,结果发现上的8.95—9.31 Mb(约0.36 Mb)的基因组区域与白蛋白高度和白蛋白单位密切相关,两个最重要的SNPs占了3.12%—5.75%的表型变异,并筛选了3个与蛋壳颜色的相关的候选基因、和[11];前期本课题组通过GWAS分析筛选了与48周龄鹅蛋数、60周龄鹅蛋数候选基因以及与鹅蛋黄颜色显著相关的、等4个基因[12]。【本研究切入点】由于鹅蛋品质性状是一个复杂的多因素组成数量性状,其蛋重、蛋形指数、蛋比重、蛋壳质量等性状的遗传力都较高,可通过遗传分析来定位主效基因区间。【拟解决的关键问题】在本研究笔者通过GWAS挖掘鹅蛋品质性状的相关分子标记,定位功能基因区间,为解析蛋品质性状的遗传机制及分子标记辅助选择提供理论支撑。
1 材料与方法
1.1 实验动物和表型测定
以重庆市家禽科研基地同批次健康四川白鹅(母鹅)群体(209只)为实验动物。为准确记录鹅蛋品质性状,每只鹅从出雏后进行系统的脚号和翅号标记,鹅群28周龄时转移到个体笼(600 mm×800 mm×900 mm)中饲养至65周(休产期),饲养过程自由饮水和采食全价饲料,收集产蛋高峰期每只鹅连续生产的5个鹅蛋,测定其蛋品质性状(蛋重、蛋形指数、相对密度、蛋壳强度、蛋壳重、蛋壳厚度和蛋黄重)并统计其平均值作为后续GWAS研究的表型性状。利用电子天平称量蛋壳重、蛋黄重;利用游标卡尺测量蛋壳的钝部、尖部和中间部位,并取上述三者的平均值计算蛋壳厚度;利用游标卡尺检测蛋长直径和短径,计算蛋形指数;利用罗氏比色卡进行蛋黄颜色的测定;蛋壳强度利用蛋壳测力计(EFG-0502,Robotmation 公司)进行检测。
1.2 DNA抽提及GWAS测序
抗凝真空管采集上述209只鹅翅静脉血液2 mL,利用血液基因组提取试剂盒(北京天根公司,DP332)提取血液基因组DNA,NanoDrop 2000分光光度计质检通过后,委托天津诺禾生物有限公司利用Illumina HiSeq X Ten平台进行全基因重测序。数据过滤后,BWA软件比对鹅基因组(ASM1303099v1)[13],GATK软件进行SNP 基因型数据检出[14];Plink软件对获得的SNP数据进行质量控制(参数设置:geno 0.1 mind 0.1 MAF 0.05 hwe 0.0000001)和主成分分析方法检测群体结构分层情况[15]。
1.3 表型数据关联与单倍型分析
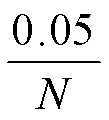

表1 验证筛选的SNPs用引物序列表
1.4 SNPs注释
BEDTools软件[18]在鹅染色体基因组上提取鉴定的显著SNPs位点上下游500 kb区域序列,Annovar软件注释SNP所在基因及邻近基因[19],Metascape(http://metascape.org/)在线对候选基因进行功能分析[20]。
2 结果
2.1 蛋品质表型数据统计分析
209只四川白鹅个体高峰期平均蛋重为132.20 g;蛋形指数在1.24—1.60之间,平均蛋形指数为1.45;蛋壳强度平均值为67.20 kg·cm‑2;蛋壳重平均为22.87 g;蛋壳厚度平均在0.42 cm;蛋黄重量平均值为41.20 g(表2)。

表2 统计分析鹅蛋品质性状
2.2 测序数据分析
209只四川白鹅母鹅全基因组重测序共获得2.896 Tb数据,测序平均覆盖深度为12.44×。经数据过滤,2.891 Tb高质量测序数据比对到鹅参考基因组序列,比对率为96.58%—98.38%,共检出了16 687 310个初始SNP位点;经过质量控制,最终检出9 279 339个SNPs[12]。GWAS结果显示,48个SNPs与上述6个蛋品质性状显著或建议性显著关联,并定位到27个基因(表3,图1)。
以各性状显著关联SNPs上下游500 k范围内注释基因,功能富集分析发现:候选基因主要聚类到response to growth factor(GO:0070848, Log(P)=-8.49)、intracellular chemical homeostasis(GO:0055082, Log(P)= -7.77)、response to hormone(GO:0009725, Log(P)=-7.44)和regulation of the monoatomic ion transport(GO: 43269, Log(P)= -6.08)。富集差异基因P值前20的GO分类条目如图2所示。

表3 筛选与蛋品质性状显著相关的SNPs表

续表3 Continued table 3
: Pregnancy-associated plasma protein A;: Alpha-2C adrenergic receptor;: Epsilon-sarcoglycan;: Thioredoxin-like protein 1;: BCL-6 corepressor;: Mid1-interacting protein 1;: Glutaredoxin-3;: Pre-mRNA-splicing factor ATP-dependent RNA helicase DHX15;: TBC1 domain family member 14;: Dystrobrevin beta;: Ethanolamine phosphotransferase 1;: Nuclear factor of activated T-cells, cytoplasmic 1;: Cytoplasmic dynein 1 intermediate chain 1;: Protein bassoon;: NADPH oxidase 5;: KAT8 regulatory NSL complex subunit 1;: Uncharacterized protein KIAA1257;: Cytochrome P450 4V2;: Kinesin-like protein KIF16B;: Tribbles homolog 2;: Gamma-aminobutyric acid receptor subunit beta-3;: Uncharacterized protein KIAA1522;: Serine/threonine-protein phosphatase 4 regulatory subunit 2;: Activating signal cointegrator 1 complex subunit 3;: Glutamate receptor ionotropic, kainate 2;: Transcription elongation factor B polypeptide 1;:Transcription factor SOX-13
显著关联(阈值5.43×10-9),*潜在显著关联(阈值1.09×10-7)
Significant correlation (p-value 5.43×10-9), *potential significant correlation (p-value 1.09×10-7)

图1 鹅蛋品质性状全基因组关联分析的曼哈顿图和Q-Q plot图
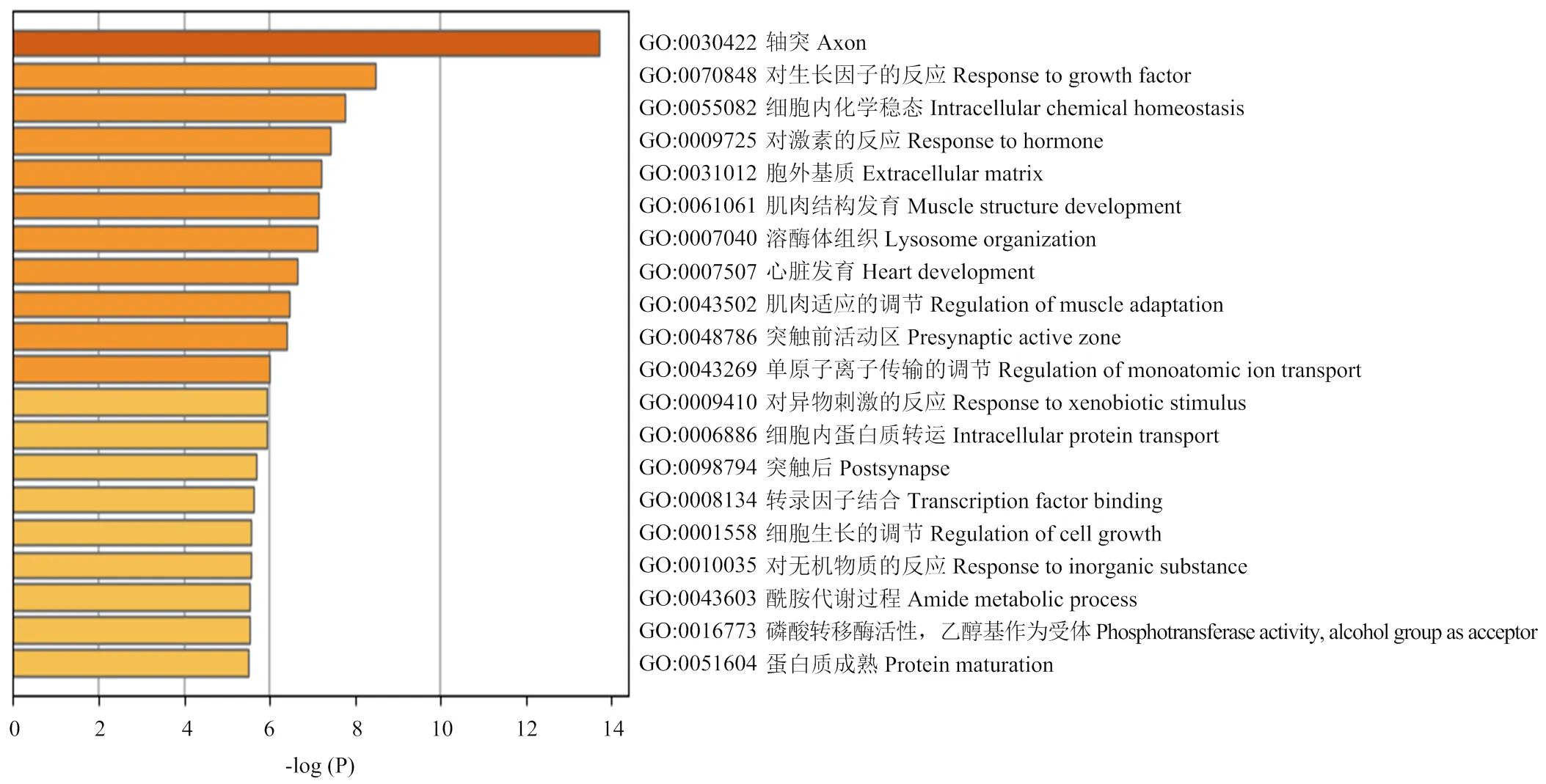
图2 差异显著SNPs上下游500k注释基因聚类分析
2.3 显著相关SNPs的MALDI-TOF MS验证结果
针对重要候选SNPs合成合适的引物作MALDI- TOF MS验证,结果如表4所示:对于蛋重性状,chr29: 3653854 AA基因型个体极显著(<0.01)高于AG和GG基因型个体;对于蛋形指数性状,chr15: 20262147 GG基因型极显著(<0.01)高于GT和TT基因型个体;对于蛋壳厚度性状,chr18:18353514 CC基因型个体极显著(<0.01)高于其他基因型个体;对于蛋黄重量性状,chr34:3206857 GG基因型个体极显著(<0.01)低于其他基因型个体;chr1:36829464 AA基因型个体极显著(<0.01)高于其他基因型个体。
3 讨论
3.1 鹅蛋品质性状表型与其他家禽蛋品质的比较分析
蛋品质性状是鹅蛋经济效益的直接影响因素,蛋重决定了产蛋总重的大小,蛋形指数、蛋黄比例影响消费者对商品蛋的选择,蛋重、蛋形指数、蛋壳质量等均会影响种蛋的合格率及孵化率[21-23]。本研究测定了四川白鹅产蛋高峰期蛋品质性状,发现检测群体的平均蛋重(132.20±18.90)g,显著高于鸡蛋和鸭蛋,但蛋形指数平均值为1.45,与鸡、鸭蛋相当,正常鸡蛋的蛋形指数一般为1.32—1.39,鸭蛋为1.20—1.58[24]。鹅蛋的平均蛋壳强度为(67.20±10.19)kg·cm‑2,平均蛋壳厚度为(0.42±0.06)cm,明显高于鸡蛋,这可能解释了生产中相同数量受精蛋鸡蛋的孵化率可达90%以上,而鹅蛋孵化率一般在80%左右,更厚和强度更高的蛋壳增加了新生雏鹅的破壳难度而导致破壳失败,降低了孵化率。鹅蛋的蛋黄平均重为(41.20±6.73)g,蛋黄比例达到30%以上,最大的蛋黄重量达到62.75 g。早成鸟的蛋黄比例一般比鸽子、企鹅等后代需要照顾的晚成鸟蛋黄比例高,鹅是早成鸟的一种,鹅蛋的高蛋黄比例可为幼雏供应更多的营养物质,使其出壳后能够独立生存[25-26]。
3.2 影响鹅蛋品质性状表型的GWAS分析
蛋品质性状是一个复杂的多因素控制数量性状,遗传因素是主要因素之一,已有的研究显示:鸡蛋的蛋重遗传力约为0.5,壳颜色遗传力为 0.45—0.75,蛋壳强度遗传力约为0.3[21]。全基因组关联技术能对数量性状遗传控制区间作有效定位。本研究通过GWAS在全基因组水平分析了鹅蛋品质遗传控制因素,笔者对209只四川白鹅血样DNA进行全基因组重测序,基于前期本课题组组装了一个染色体水平的鹅基因组[24],有利于将高质量的全基因组重测序数据比对到鹅基因组序列并进行了GWAS分析。最终筛选并使用MALDI-TOF MS验证了48个与蛋重、蛋壳厚度、蛋壳强度等蛋品质性状显著相关的SNPs。

表4 部分SNPs的基因型对鹅6个蛋品质性状的影响
不同字母代表差异显著(<0.05);相同字母代表差异不显著(>0.05)
Different letters in the same row indicate a significant difference between the genotypes (<0.05); the same letter indicates no significant difference between genotypes (>0.05)
通过GWAS分析在12、15以及29号染色体上共鉴定4个SNPs与蛋重显著相关,分别位于、、和。妊娠相关血浆蛋白A(pregnancy-associated plasma protein A,)基因编码一种分泌型金属蛋白酶,可裂解胰岛素样生长因子结合蛋白(),而激活IGF信号通路,参与蛋白质代谢以及对胰岛素样生长因子(IGF)运输和摄取的调节过程[27]。在卵泡发育过程中卵丘颗粒细胞中mRNA表达水平上调是卵母细胞成熟的标志之一[28]。MAROULI等[29]通过全基因组关联研究发现了83个与人身高相关的编码变体,其中增高等位基因促进了的蛋白酶活性,增加了对的裂解,从而导致的生物利用度提高。在家禽中,体型和蛋重一般有着正向相关的关系,能够促进身高,这提示可能作为蛋重正向选择的候选基因。值得注意的是,在筛选的48个候选SNPs中有5个与蛋壳厚度相关的SNPs都定位在chr18的蛋白丝氨酸/苏氨酸磷酸酶调节亚基2基因(serine/ threonine-protein phosphatase 4 regulatory subunit 2,),是PP4复合体5个调节亚基中的一个,参与许多关键的细胞途径,包括DNA损伤反应(DNA修复、细胞周期调节和细胞凋亡)、葡萄糖代谢、细胞迁移、肿瘤发生和免疫反应等[30-31]。家禽无论在排卵还是在蛋壳形成过程中都存在着明确的时序性,这种时序性受周期调节基因的控制,而调控核心节律基因/活性[32],另外,高浓度的葡萄糖影响Ca2+吸收[33],而参与葡萄糖代谢,由此推测可能参与了蛋壳形成。通过GWAS分析发现chr1:1466542和chr1: 1467878与蛋壳强度显著相关的SNPs都定位乙醇胺磷酸转移酶1(ethanolamine phosphotransferase 1, EPT1)基因上,该基因编码蛋白将CDP-乙醇胺催化形成磷脂酰乙醇胺,是磷脂代谢的重要组成部分[34]。如表3所示,6个SNPs定位在chr1上的(glutamate receptor ionotropic, kainate 2)与蛋黄重量显著相关(P=3.46*10-8—2.87* 10-9),ZEMUNIK等[35]通过全基因组关联分析方法在一群孤立的人群中鉴别了与生化性状(总胆固醇、低密度脂蛋白胆固醇、高密度脂蛋白胆固醇和甘油三酯等)相关的遗传变异基础,发现、和等是重要的候选基因。另外,两个定位到(activating signal cointegrator 1 complex subunit 3, ASSC3)上的SNPs chr1 rs36803702C/T和rs36829464 A/G 同样与蛋黄重量显著相关,前期研究发现蛋白酶前体转化酶亚硫酸酯酶/切割酶9型(Proprotein convertase subtilisin/kexin type 9,PCSK9)是最主要的低密度脂蛋白胆固醇调节子,可减少低密度脂蛋白胆固醇的含量,而和能作用核糖体,保护细胞免受因PCSK9抑制剂(PF8503)引起的细胞毒性[36]。蛋黄主要由胆固醇和磷脂组成,本研究发现的候选基因、等均参与胆固醇代谢,由此推测、可作为蛋黄重量相关的候选基因。
3.3 候选基因的功能聚类分析
家禽的产蛋行为受激素调控,FSH促进卵泡生长成熟,LH促进排卵, PRL与抱窝性相关,性激素维持鹅的生殖特征,甲状旁腺激素和降钙素调控蛋壳中钙的沉积[37-39]。在本研究中笔者通过对注释的功能基因进行聚类分析也发现response to hormone(GO: 0009725)是P值最高的GO分类条目之一。每个鸡蛋中的钙量通常占鸡体内钙储存总量的10%左右,身体中绝大多数钙存在于细胞内,仅有不足0.1%位于细胞外,这部分包括电离钙、与蛋白质结合的钙以及与阴离子结合的钙三种形式,其中电离钙是生理活性形式,家禽能在24 h内将身体10%的钙形成蛋壳[40- 41],而不引起机体出现低血钙症,这与家禽独特的血清钙平衡维持密切相关,在进行GWAS分析的时候笔者也发现与蛋品质显著相关的候选基因明显聚到intracellular chemical homeostasis (GO:0055082)、regulation of the monoatomic ion transport(GO:0043269)等通路。
4 结论
本研究通过GWAS方法在全基因组水平上筛选与蛋品质性状显著相关的分子遗传标记,注释筛选了重要候选基因、、和分别作为蛋重、蛋黄重、蛋壳强度等蛋品质性状潜在功能基因。研究结果促进了鹅蛋品质性状分子标记选择研究与运用,也将为四川白鹅蛋品质性能的选育提供理论支撑。
[1] 侯水生, 刘灵芝. 2022年水禽产业现状、未来发展趋势与建议. 中国畜牧杂志, 2023, 59(3): 274-280.
HOU S S, LIU L Z. Present situation, future development trend and suggestions of waterfowl industry in 2022. Chinese Journal of Animal Science, 2023, 59(3): 274-280. (in Chinese)
[2] 王一冰, 陈芳, 苟钟勇, 李龙, 林厦菁, 张盛, 蒋守群. 快大型黄羽肉种鸡VD3需要量研究. 中国农业科学, 2021, 54(16): 3549-3560.
WANG Y B, CHEN F, GOU Z Y, LI L, LIN X J, ZHANG S, JIANG S Q. Requirement of vitamin D3on fast-growing yellow-feathered breeder hens. Scientia Agricultura Sinica, 2021, 54(16): 3549-3560. (in Chinese)
[3] KOWALSKA E, KUCHARSKA-GACA J, KUŹNIACKA J, LEWKO L, GORNOWICZ E, BIESEK J, ADAMSKI M. Egg quality depending on the diet with different sources of protein and age of the hens. Scientific Reports, 2021, 11: 2638.
[4] NASRI H, VAN DEN BRAND H, NAJJAR T, BOUZOUAIA M. Egg storage and breeder age impact on egg quality and embryo development. Journal of Animal Physiology and Animal Nutrition, 2020, 104(1): 257-268.
[5] SUN C J, LIU J N, YANG N, XU G Y. Egg quality and egg albumen property of domestic chicken, duck, goose, Turkey, quail, and pigeon. Poultry Science, 2019, 98(10): 4516-4521.
[6] DA SILVA TEIXEIRA M, TRIGINELLI M V, DE ATAÍDE COSTA T, LARA L J C, SOTO-BLANCO B. Effects of caffeine on egg quality and performance of laying hens. Frontiers in Veterinary Science, 2020, 7: 545359.
[7] WANG J, YUE H Y, WU S G, ZHANG H J, QI G H. Nutritional modulation of health, egg quality and environmental pollution of the layers. Animal Nutrition, 2017, 3(2): 91-96.
[8] ZHU F, CUI Q Q, HOU Z C. SNP discovery and genotyping using Genotyping-by-Sequencing in Pekin ducks. Scientific Reports, 2016, 6: 36223.
[9] ZHU F, YIN Z T, WANG Z, SMITH J, ZHANG F, MARTIN F, OGEH D, HINCKE M, LIN F B, BURT D W, ZHOU Z K, HOU S S, ZHAO Q S, LI X Q, DING S R, LI G S, YANG F X, HAO J P, ZHANG Z D, LU L Z, YANG N, HOU Z C. Three chromosome-level duck genome assemblies provide insights into genomic variation during domestication. Nature Communications, 2021, 12: 5932.
[10] ZHANG H, SHEN L Y, XU Z C, KRAMER L M, YU J Q, ZHANG X Y, NA W, YANG L L, CAO Z P, LUAN P, REECY J M, LI H. Haplotype-based genome-wide association studies for carcass and growth traits in chicken. Poultry Science, 2020, 99(5): 2349-2361.
[11] LIU Z, SUN C J, YAN Y Y, LI G Q, SHI F Y, WU G Q, LIU A Q, YANG N. Genetic variations for egg quality of chickens at late laying period revealed by genome-wide association study. Scientific Reports, 2018, 8: 10832.
[12] GAO G L, GAO D F, ZHAO X Z, XU S S, ZHANG K S, WU R, YIN C H, LI J, XIE Y H, HU S L, WANG Q G. Genome-wide association study-based identification of SNPs and haplotypes associated with goose reproductive performance and egg quality. Frontiers in Genetics, 2021, 12: 602583.
[13] LI H, DURBIN R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics, 2010, 26(5): 589-595.
[14] MCKENNA A, HANNA M, BANKS E, SIVACHENKO A, CIBULSKIS K, KERNYTSKY A, GARIMELLA K, ALTSHULER D, GABRIEL S, DALY M, DEPRISTO M A. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research, 2010, 20(9): 1297-1303.
[15] PURCELL S, NEALE B, TODD-BROWN K, THOMAS L, FERREIRA M A R, BENDER D, MALLER J, SKLAR P, DE BAKKER P I W, DALY M J, SHAM P C. PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics, 2007, 81(3): 559-575.
[16] ZHOU X, STEPHENS M. Genome-wide efficient mixed-model analysis for association studies. Nature Genetics, 2012, 44(7): 821-824.
[17] KARSSEN L C, VAN DUIJN C M, AULCHENKO Y S. The GenABEL project for statistical genomics. F1000Research, 2016, 5: 914.
[18] QUINLAN A R. BEDTools: the swiss-army tool for genome feature analysis. Current Protocols in Bioinformatics, 2014, 47: 11.12.1- 11.1234.
[19] WANG K, LI M Y, HAKONARSON H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Research, 2010, 38(16): e164.
[20] ZHOU Y Y, ZHOU B, PACHE L, CHANG M, KHODABAKHSHI A H, TANASEICHUK O, BENNER C, CHANDA S K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nature Communications, 2019, 10: 1523.
[21] KETTA M, TŮMOVÁ E. Eggshell structure, measurements, and quality-affecting factors in laying hens: a review. Czech Journal of Animal Science, 2016, 61(7): 299-309.
[22] HISASAGA C, GRIFFIN S E, TARRANT K J. Survey of egg quality in commercially available table eggs. Poultry Science, 2020, 99(12): 7202-7206.
[23] PICARD DRUET D, VARENNE A, HERRY F, HÉRAULT F, ALLAIS S, BURLOT T, LE ROY P. Reliability of genomic evaluation for egg quality traits in layers. BMC Genetics, 2020, 21(1): 17.
[24] LI Y, GAO G L, LIN Y, HU S L, LUO Y, WANG G S, JIN L, WANG Q G, WANG J W, TANG Q Z, LI M Z. Pacific Biosciences assembly with Hi-C mapping generates an improved, chromosome- level goose genome. GigaScience, 2020, 9(10): 114.
[25] LIKER A, SZÉKELY T. Mortality costs of sexual selection and parental care in natural populations of birds. International Journal of Organic Evolution, 2005, 59(4): 890-897.
[26] WILLIAMS T D. Physiology, activity and costs of parental care in birds. The Journal of Experimental Biology, 2018, 221(Pt 17): jeb169433.
[27] GYRUP C, OXVIG C. Quantitative analysis of insulin-like growth factor-modulated proteolysis of insulin-like growth factor binding protein-4 and -5 by pregnancy-associated plasma protein-A. Biochemistry, 2007, 46(7): 1972-1980.
[28] KORDUS R J, HOSSAIN A, CORSO M C, CHAKRABORTY H, WHITMAN-ELIA G F, LAVOIE H A. Cumulus cell pappalysin-1, luteinizing hormone/choriogonadotropin receptor, amphiregulin and hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta- isomerase 1 mRNA levels associate with oocyte developmental competence and embryo outcomes. Journal of Assisted Reproduction and Genetics, 2019, 36(7): 1457-1469.
[29] MAROULI E, GRAFF M, MEDINA-GOMEZ C, LO K S, WOOD A R, KJAER T R, FINE R S, LU Y C, SCHURMANN C, HIGHLAND H M, RÜEGER S, et al. Rare and low-frequency coding variants alter human adult height. Nature, 2017, 542(7640): 186-190.
[30] LEE J, ADELMANT G, MARTO J A, LEE D H. Dephosphorylation of DBC1 by protein phosphatase 4 is important for p53-mediated cellular functions. Molecules and Cells, 2015, 38(8): 697-704.
[31] PARK J, LEE D H. Functional roles of protein phosphatase 4 in multiple aspects of cellular physiology: a friend and a foe. BMB Reports, 2020, 53(4): 181-190.
[32] KLEMZ S, WALLACH T, KORGE S, ROSING M, KLEMZ R, MAIER B, FIORENZA N C, KAYMAK I, FRITZSCHE A K, HERZOG E D, STANEWSKY R, KRAMER A. Protein phosphatase 4 controls circadian clock dynamics by modulating CLOCK/BMAL1 activity. Genes & Development, 2021, 35(15/16): 1161-1174.
[33] POHOREC V, KRIŽANČIĆ BOMBEK L, SKELIN KLEMEN M, DOLENŠEK J, STOŽER A. Glucose-stimulated calcium dynamics in beta cells from male C57BL/6J, C57BL/6N, and NMRI mice: a comparison of activation, activity, and deactivation properties in tissue slices. Frontiers in Endocrinology, 2022, 13: 867663.
[34] AHMED M Y, AL-KHAYAT A, AL-MURSHEDI F, AL-FUTAISI A, CHIOZA B A, PEDRO FERNANDEZ-MURRAY J, SELF J E, SALTER C G, HARLALKA G V, RAWLINS L E, et al. A mutation ofunderlies a new disorder of Kennedy pathway phospholipid biosynthesis. Brain, 2017: aww318.
[35] ZEMUNIK T, BOBAN M, LAUC G, JANKOVIĆ S, ROTIM K, VATAVUK Z, BENCIĆ G, DOGAS Z, BORASKA V, TORLAK V, SUSAC J, ZOBIĆ I, RUDAN D A, PULANIĆ D, MODUN D, MUDNIĆ I, GUNJACA G, BUDIMIR D, HAYWARD C, VITART V, WRIGHT A F, CAMPBELL H, RUDAN I. Genome-wide association study of biochemical traits in Korcula Island, Croatia. Croatian Medical Journal, 2009, 50(1): 23-33.
[36] LIAUD N, HORLBECK M A, GILBERT L A, GJONI K, WEISSMAN J S, CATE J H D. Cellular response to small molecules that selectively stall protein synthesis by the ribosome. PLoS Genetics, 2019, 15(3): e1008057.
[37] CHEN Q Y, DUAN J D, WU H Z, LI J B, JIANG Y L, TANG H, LI X Y, KANG L. Expression dynamics of gonadotropin-releasing hormone-I and its mutual regulation with luteinizing hormone in chicken ovary and follicles. General and Comparative Endocrinology, 2019, 270: 96-102.
[38] GHANEM K, JOHNSON A L. Follicle dynamics and granulosa cell differentiation in the Turkey hen ovary. Poultry Science, 2018, 97(10): 3755-3761.
[39] TANIMOTO R, SEKII K, MOROHAKU K, LI J Z, PÉPIN D, OBATA Y. Blocking estrogen-induced AMH expression is crucial for normal follicle formation. Development, 2021, 148(6): dev197459.
[40] DE MATOS R. Calcium metabolism in birds. Veterinary Clinics of North America: Exotic Animal Practice, 2008, 11(1): 59-82.
[41] DESCALZO E, CAMARERO P R, SÁNCHEZ-BARBUDO I S, MARTINEZ-HARO M, ORTIZ-SANTALIESTRA M E, MORENO- OPO R, MATEO R. Integrating active and passive monitoring to assess sublethal effects and mortality from lead poisoning in birds of prey. The Science of the Total Environment, 2021, 750: 142260.
Identification of Molecular Markers Associated with Goose Egg Quality Through Genome-Wide Association Analysis

1Chongqing Academy of Animal Science, Chongqing 402460;2Chongqing Engineering Research Center of Goose Genetic Improvement, Chongqing 402460;3Chongqing Qijiang Animal Disease Control Center, Chongqing 401420
【Objective】 The objective of this study was to screen molecular markers and candidate genes related to goose egg quality traits, to provide a theoretical support for the analysis of the genetic mechanism of egg quality traits and marker-assisted selection. 【Method】 In this study, a batch of healthy Sichuan White Geese (209 individuals) was selected as the research subjects. Five eggs from each goose during the peak egg production period were collected, and then six egg quality traits were measured, including egg weight, egg shape index, eggshell strength, eggshell thickness, eggshell weight, and egg yolk weight. Based on the whole-genome resequencing data (2.896 Tb, 12.44×/individual) of 209 Sichuan White Geese (female geese), a genome-wide association analysis was conducted to identify SNP loci and important candidate genes associated with egg quality traits. The genotype frequencies of the SNP loci were determined using the nucleic acid flight time mass spectrometry method. 【Result】After filtering, a total of 9 279 339 SNPs and 209 individuals were included for further analysis. The GWAS analysis identified 48 SNP loci significantly or suggestively associated with six egg quality traits (thresholds: 5.43×10-9and 1.09×10-7). These loci were annotated to 27 candidate genes related to egg quality traits, including Pregnancy-associated plasma protein A (), Serine/threonine-protein phosphatase 4 regulatory subunit 2 (), Ethanolamine phosphotransferase 1 (), and Glutamate receptor ionotropic, kainate 2 (). Among them, the candidate genewas involved in protein metabolism and promotes the generation of insulin-like growth factor. Five SNPs within the 11 bp range ofwere significantly associated with eggshell thickness. Additionally, six SNPs on thegene were significantly associated with yolk weight.andwere respectively associated with blood calcium homeostasis and cholesterol metabolism in organisms. Functional enrichment analysis revealed that the candidate genes were mainly annotated to “response to growth factor” (GO:0070848), “intracellular chemical homeostasis” (GO:0055082), “response to hormone” (GO:0009725), and “regulation of monoatomic ion transport” (GO:43269). 【Conclusion】 The GWAS analysis showed that theandare potential functional genes associated with various egg quality traits, such as egg weight, egg yolk weight, and shell strength, providing theoretical references for molecular genetic marker-assisted selection of goose egg quality traits.
genome-wide association analysis; molecular marker-assisted selection; goose; egg quality traits
10.3864/j.issn.0578-1752.2023.19.015
2022-05-10;
2023-08-08
重庆市科研机构绩效激励引导专项项目(cstc2022jxjl80007)、重庆市科研院所绩效激励引导专项项目(22527J)、财政部和农业部:国家现代农业产业技术体系(CARS-42-51)、重庆市技术创新与应用发展专项重点项目(cstc2019jscx-gksbX0097)
高广亮,E-mail:guanglianggaocq@hotmail.com。张克山,E-mail:zhangkshLK1988@163.com。高广亮和张克山为同等贡献作者。通信00作者王启贵,E-mail:wangqigui@hotmail.com
(责任编辑 林鉴非)
