无水保活运输温度对史氏鲟氧化应激的影响
2021-09-05周文白婵王炬光柴毅鉏晓艳廖涛熊光权
周文 白婵 王炬光 柴毅 鉏晓艳 廖涛 熊光权

摘 要:為了提高史氏鲟的保活时间和存活率,选取合适质量浓度MS-222麻醉史氏鲟,研究不同无水保活运输温度(4、8、12 ℃)对史氏鲟氧化应激的影响,分别记录模拟保活运输12 h和24 h后各温度组的存活率,分析保活运输前后应激指标和抗氧化指标变化。结果表明:MS-222麻醉史氏鲟,较佳镇静质量浓度为100 mg/L、镇静时间为(2.1±0.1) min,较佳麻醉质量浓度为110 mg/L、麻醉时间为(2.1±0.3) min;保活运输12 h时,12 ℃条件下存活率最高,为85%,保活运输24 h时,4 ℃条件下存活率最高,为62.5%;保活运输12 h后12 ℃条件下血糖浓度和皮质醇含量显著低于其他组,超氧化物歧化酶和谷胱甘肽过氧化物酶(glutathione peroxidase,GPX)活性显著低于其他组,同时丙二醛(malondialdehyde,MDA)含量最低(P<0.05),表明受到的应激最小;保活运输24 h时,各温度组过氧化氢酶活性无明显差异,4 ℃条件下GPX活性较低,同时MDA含量显著低于其他组(P<0.05)。因此在史氏鲟短时间(12 h)保活运输时,较高温度(12 ℃)减少应激效果明显,是较适的保活温度;在较长时间(24 h) 保活运输时,低温能更好保持低代谢和麻醉状态,4 ℃是较适的保活温度。
关键词:MS-222;史氏鲟;温度;无水保活运输;氧化应激
Abstract: In order to improve the survival time and survival rate of Amur sturgeon, the fish were anaesthetized with an appropriate concentration of MS-222 to explore the effects of different temperatures (4, 8 and 12 ℃) during waterless live transportation on oxidative stress in it. The survival rate of the fish was recorded after 12 and 24 h of simulated live transportation, and the changes in oxidative stress and antioxidant indicators were analyzed before and after live transportation. The results showed that the appropriate sedative concentration of MS-222 was 100 mg/L and the sedation time was (2.1 ± 0.1) min; the appropriate anaesthetic concentration was 110 mg/L and the anesthesia time was (2.1 ± 0.3) min. After 12 h live transportation, the highest survival rate of 85% was observed at 12 ℃. After 24 h live transportation, the highest survival rate of 62.5% was observed at 4 ℃. After 12 h live transportation, blood sugar, cortisol and malondialdehyde (MDA) levels as well as the activity of superoxide dismutase (SOD) and glutathione peroxidase (GPX) were significantly lower in the 12 ℃ group than in the other temperature groups (P < 0.05), indicating that the lowest level of oxidative stress occurred in the 12 ℃ group. After 24 h live transportation, there were no significant differences in catalase (CAT) activity among all groups. GPX activity was lower in the 4 ℃ group, and MDA content was significantly lower in this group than in the other groups (P < 0.05). Therefore, when the sturgeon was transported without water for a short time (12 h), a significantly lower level of oxidative stress was observed at higher temperature (12 ℃), suggesting that the appreciate survival temperature was 12 ℃. When the sturgeon was transported for a long time (24 h), low metabolism and anesthesia were maintained better at lower temperature (4 ℃), so that the fish could survive better.
Keywords: tricaine methanesulfonate (MS-222); Acipenser schrenckii; temperature; waterless live transportation; oxidative stress
DOI:10.7506/rlyj1001-8123-20210408-095
中图分类号:TS254.4 文献标志码:A 文章编号:1001-8123(2021)07-0032-06
引文格式:
周文, 白婵, 王炬光, 等. 无水保活运输温度对史氏鲟生化指标的影响[J]. 肉类研究, 2021, 35(7): 32-37. DOI:10.7506/rlyj1001-8123-20210408-095. http://www.rlyj.net.cn
ZHOU Wen, BAI Chan, WANG Juguang, et al. Effects of different temperatures during waterless live transportation on oxidative stress in Amur sturgeon (Acipenser schrencki)[J]. Meat Research, 2021, 35(7): 32-37. DOI:10.7506/rlyj1001-8123-20210408-095. http://www.rlyj.net.cn
史氏鲟(Acipenser schrencki)隶属于鲟形目、鲟亚科,被认为是现存最古老的生物之一,具有很高的经济和营养价值,近几十年来,产量逐渐增加[1]。中国是鲟鱼产量最大的国家,在世界上占有很大的比重,2010—2017年,其产量占世界鲟鱼产量80%左右[1]。活鱼在中国特别受欢迎,因为在中国文化中,新鲜杀死的鱼比死鱼更美味[2-4]。如今,高价值品种的活鱼销量大幅增长,其销售价格比大宗淡水鱼高5~10 倍,带来了可观的经济效益[5]。为了提高利润率和口感质量,鲟鱼通常是鲜活销售[6-7]。然而,活鱼运输是一个相对复杂的过程,需要更好地控制潜在的运输刺激,防止鱼的生理应激反应,否则过度的生理应激会导致鱼的活力下降,甚至死亡[8-9]。
作为一种新颖的鱼类运输策略,鱼类无水运输是实现较高成活率和大容量的绿色经济解决方案[10-11]。已有研究表明,无水运输方法已应用于史氏鲟[11]、鲫鱼(Carassius auratus)[12]、虹鳟鱼(Oncorhynchus mykiss)[13]等冷水鱼类的活体运输。低温和保持鱼的平静是提高无水运输效率的有效手段,它可以通过减缓新陈代谢和减少应激来增加鱼体堆积密度和存活率[8-9,12]。
然而,低温也是一种应激源,不恰当的低温会增加运输时的应激,加速内环境稳态的失调。在鱼类中,低温、空气暴露和运输胁迫导致组织损伤[14]、生存能力降低[15]、改變生理反应[16],从而影响鱼体能量代谢[17-18]、导致免疫力低下[19]并增加氧化应激[20]。本研究采用MS-222麻醉史氏鲟,测定不同无水保活运输温度史氏鲟的氧化应激水平,以确保合适的无水保活条件,减少运输过程中对鱼类造成的应激。
1 材料与方法
1.1 材料与试剂
史氏鲟购买自湖北洪湖当地渔场,体长(54±6) cm,体质量(700±77) g。实验用鱼被转移到4 个塑料桶(容积700 L)中进行为期2 周的适应,所有塑料桶都处于同一个循环水系统中。每桶放置相同数量的鱼,以保持一致的生活条件。在适应期,每天喂鱼2 次。养殖的水体参数为:水温(23.0±0.6) ℃、溶解氧(6.80±0.14) mg/L、pH(7.15±0.07)、明暗交替周期为12 h/12 h。实验前24 h暂停投喂,清空鱼消化道。
MS-222(使用时现用现配,并用碳酸氢钠调节溶液pH值至7.34) 上海晶纯生化科技股份有限公司;超氧化物歧化酶(superoxide dismutase,SOD)、过氧化氢酶(catalase,CAT)、谷胱甘肽过氧化物酶(glutathione peroxidase,GPX)、丙二醛(malondialdehyde,MDA)、皮质醇、血糖含量测定试剂盒 南京建成生物科技有限公司。
1.2 仪器与设备
3K15离心机 德国Sigma公司;SPARK酶标仪 瑞士Tecan公司;ME235S电子分析天平 德国Sartorius公司;VORTEX 3涡旋振荡器 德国IKA公司;Step One PlusTM荧光定量仪 美国Applied Biosystems公司。
1.3 方法
1.3.1 MS-222麻醉实验
测定不同质量浓度(70、80、90、100、110、120 mg/L)MS-222溶液对史氏鲟的麻醉效果,每条鱼只用于1 个质量浓度的测试,设置6 组平行。实验鱼放于90 L玻璃容器中,含有MS-222溶液40 L,持续曝气。每条鱼单独观察10 min,在观察时间内不能进入更深层次麻醉期或者呼吸微弱则被放入清水中复苏。记录各阶段时间,MS-222溶液麻醉的最佳标准为3 min内达到麻醉状态,5 min内复苏[21-22]。所有经过麻醉的鱼放在清水中暂养7 d,记录死亡率。史氏鲟麻醉分期见表1。
1.3.2 模拟运输
根据1.3.1节结果,用110 mg/L MS-222溶液(23 ℃)麻醉史氏鲟,模拟无水运输。120 条鱼被分成60 袋(每袋2 条鱼),袋中无水、充满纯氧。在运输12 h和24 h过程中,分别设3 个温度处理组(4、8、12 ℃),每个处理组5 个运输袋(n=10),以驯养鱼为对照组。运输12、24 h后立即取样,敲击鱼头部致死,然后用医用纱布擦净尾部,断尾取血。收集的血液样本在室温下静置1 h,3 000×g离心15 min,分离血浆、收集上清液,置于-20 ℃冰箱中保存。
1.3.3 生化指标测定
采用试剂盒进行生化指标测定,分别分析应激指标皮质醇含量、血糖浓度及抗氧化相关指标SOD、CAT、GPX活性、MDA含量等。
1.4 数据处理
用Microsoft Excel 2019和SPSS 19.0软件对实验结果进行统计分析,用Graphpad prism 7软件作图,实验结果以平均值±标准差表示,并在单因素方差分析基础上采用Duncans多重比较法进行差异显著性分析,显著性水平为P<0.05。
2 结果与分析
2.1 MS-222麻醉史氏鲟的较佳质量浓度确定
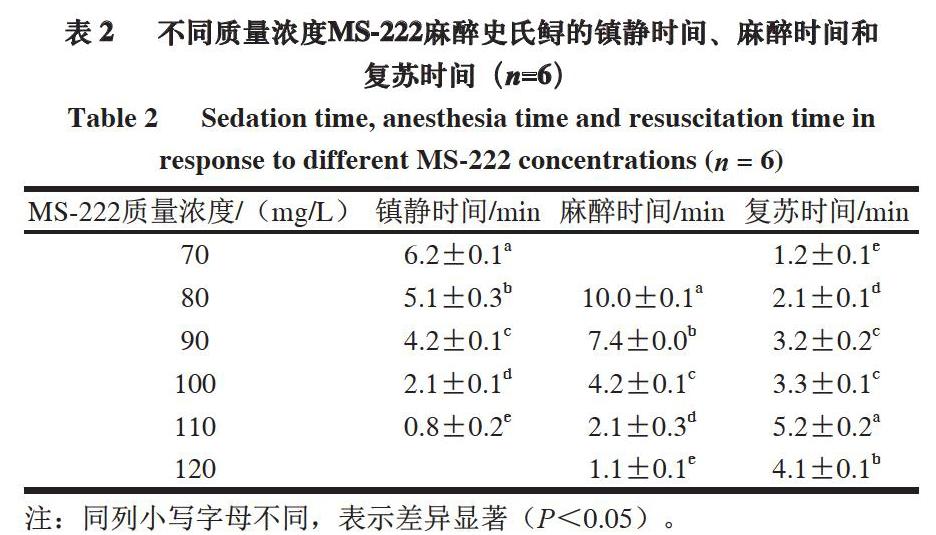
由表2可知,随着MS-222质量浓度增加,史氏鲟进入相同麻醉时期的时间缩短,完全复苏时间延长。
MS-222质量浓度70 mg/L时不能使史氏鲟达到麻醉状态,而质量浓度为120 mg/L时几乎不能观察到镇静期。
MS-222质量浓度70~110 mg/L时都能使史氏鲟进入深度镇静状态,80~120 mg/L时能使史氏鲟达到麻醉状态。本研究中史氏鲟麻醉的有效MS-222质量浓度为70 mg/L,这比花鲈(Lateolabrax maculatus)(40 mg/L)[14]、金头鲷(Spams aurata)(30 mg/L)[23]高,比鲫鱼(150 mg/L)[24]、波斯鲟(Acipenser persicus)(75 mg/L)[25]低。Mirghaed等[26]发现,鱼类麻醉剂麻醉使用的疗效标准为3 min内达到麻醉状态,5 min内复苏。因此,MS-222用于史氏鲟麻醉,合适的镇静质量浓度为100~110 mg/L,合适的麻醉质量浓度为110~120 mg/L。本研究的最佳麻醉质量浓度为110 mg/L,和鲫鱼[26]及孔雀鱼(Poecilia vivipara)[27]相同。
2.2 温度和保活运输时间对史氏鲟存活率的影响
小写字母不同,表示同一保活运输时间下不同温度差异显著(P<0.05)。
由图1可知,随着保活运输时间延长,各个温度条件下存活率均降低。保活运输12 h时,12 ℃条件下存活率最高,为85%;保活运输24 h时,4 ℃条件下存活率最高,为62.5%,但和12 ℃无明显差异;保活运输12 h和24 h时,8 ℃条件下存活率均最低。因此,保活运输12 h时,12 ℃是史氏鲟麻醉保活运输的适宜温度;保活运输24 h时,4 ℃是史氏鲟麻醉保活运输的适宜温度。另外,12 ℃条件下,12~24 h的保活运输过程中存活率下降最快。
温度过高或过低均不利于鱼体运输,其原因是温度较高时鱼体新陈代谢旺盛,但水温过低也会导致鱼体遭受冷应激,长期低温会产生严重的损伤和死亡,从而导致存活率下降[4,28]。Zeng Peng等[15]研究温水性鱼类鲤鱼麻醉休眠的较佳保活温度时发现,4 ℃条件下完全失去运动能力与平衡能力时10 min进入休眠状态,但仅能维持5 h,而在14 ℃条件下,鱼体的运动与平衡能力正常,在呼吸较弱的镇静或半休眠状态下,能维持24 h。本研究中驯化温度(23 ℃)和运输温度(12 ℃)温差较小,在短时间(12 h)运输时能更好维持史氏鲟状态;4 ℃和8 ℃温差较大,产生了冷应激,降低了存活率,但4 ℃条件下鱼体代谢相对较慢,因此存活率较8 ℃高;随着保活运输时间延长(24 h),12 ℃条件下新陈代谢较快,逐渐脱离最佳的麻醉状态,使得存活率相较于4 ℃下降更快。
2.3 温度和保活运输时间对史氏鲟应激指标的影响
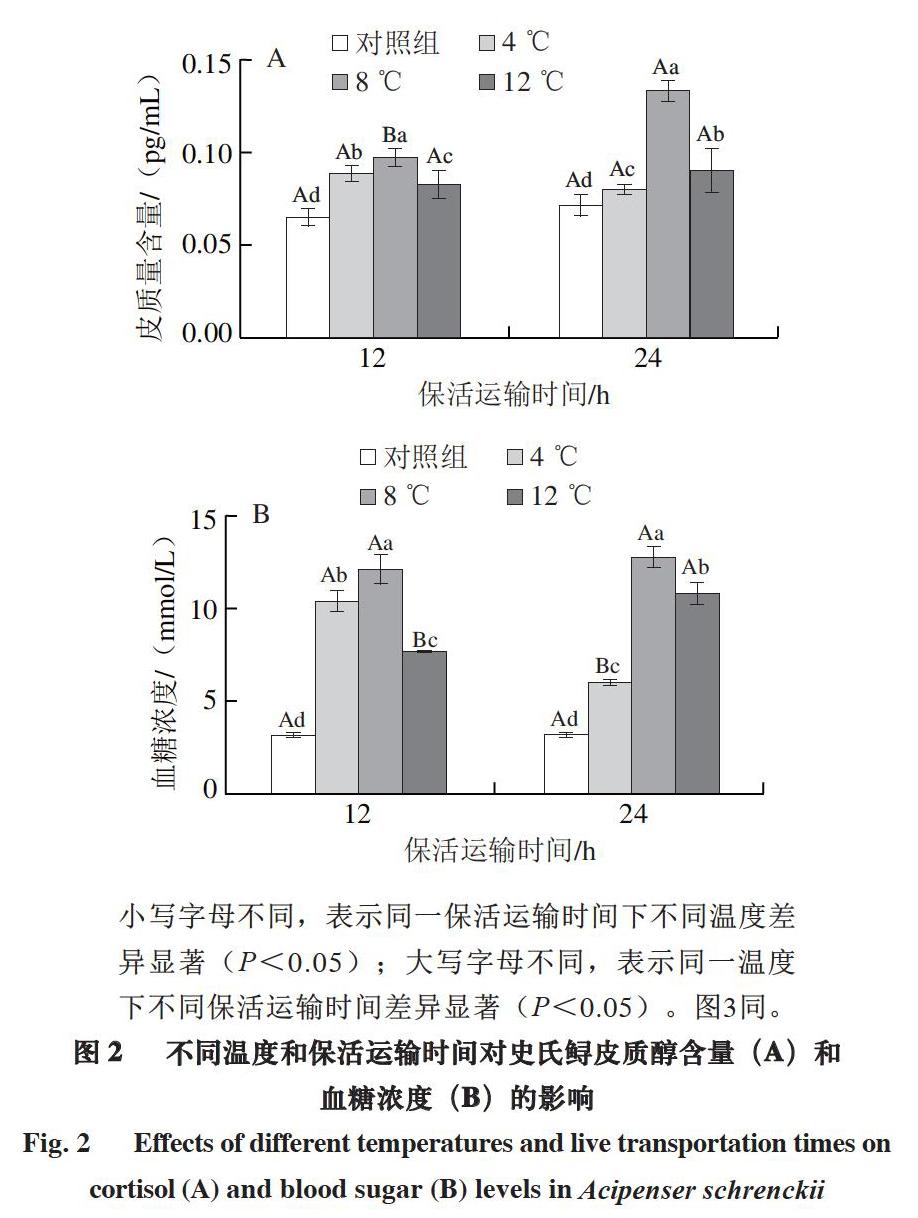
小写字母不同,表示同一保活运输时间下不同温度差异显著(P<0.05);大写字母不同,表示同一温度下不同保活运输时间差异显著(P<0.05)。图3同。
皮质醇含量、血糖浓度常被作为应激反应的敏感指标,可以反映鱼类应激反应的强弱[29]。由图2可知:與对照组相比,保活运输后所有温度处理组皮质醇含量和血糖浓度均显著升高(P<0.05);随着保活运输时间延长,8 ℃条件下皮质醇含量显著升高(P<0.05),但是4 ℃和12 ℃条件下皮质醇含量变化不明显,而4 ℃条件下血糖浓度显著降低,12 ℃条件下显著升高。保活运输12 h时,12 ℃条件下皮质醇含量和血糖浓度最低;而保活运输24 h时,4 ℃条件下皮质醇含量和血糖浓度最低。
当鱼类受到应激后,下丘脑-垂体-肾上腺轴迅速作用,促进促肾上腺皮质激素的释放,从而导致肾上腺皮质醇激素的合成与释放。Mirghaed等[30]研究表明,鲤鱼暴露于丁香酚中,皮质醇和血糖水平显著升高后下降。本研究中,史氏鲟麻醉休眠后在4 ℃条件下保活运输24 h后的血清皮质醇含量降低,但与保活运输12 h差异不显著,血糖浓度显著降低,表明低温麻醉能减缓保活运输后的应激反应。而12 ℃条件下保活运输24 h后皮质醇含量升高,和保活运输12 h差异不显著,但血糖浓度显著升高,表明12 ℃运输鱼体的应激持续增强。本研究中血糖浓度的变化比皮质醇含量的变化更加显著,说明皮质醇恢复较快,血糖恢复较慢。
2.4 温度和保活运输时间对史氏鲟氧化应激指标的影响
由图3可知:保活运输12 h时,12 ℃条件下SOD、CAT、GPX活性及MDA含量处于较低水平;保活运输24 h时,4 ℃条件下GPX活性处于较低水平,CAT活性和其他组无显著性差异,MDA含量较低。说明保活运输12 h时12 ℃是较佳的保活温度,保活运输24 h时4 ℃是较佳的保活温度。
通常,在正常的環境条件下,鱼类的抗氧化防御系统可提供针对活性氧(reactive oxygen species,ROS)的充分保护[31]。然而,当暴露于非生物和/或生物胁迫时,鱼类产生的ROS会增加,并且ROS产生与抗氧化剂清除细胞自由基之间的平衡可能会改变,进而导致氧化应激[32],从而刺激各种防御机制,如GPX循环,以清除ROS和ROS产生的有毒代谢产物[33]。还原型谷胱甘肽通常被认为是调节氧化还原平衡的中间物质,GPX利用SOD还原的H2O2与还原型谷胱甘肽反应生成氧化型谷胱甘肽和H2O,以达到清除自由基的目的。随着保活运输时间的延长,保活运输24 h时,12 ℃条件下GPX活性与对照组相比显著升高,这可能是由于大量的自由基积累,产生剧烈氧化应激,使得还原型谷胱甘肽不足以保持氧化还原状态平衡,机体产生大量GPX。保活运输24 h后存活率大幅度下降可能是由于GPX循环适应性失败导致。在保活运输12 h时,4 ℃条件下CAT活性显著高于其余组,可能是由于氧化应激产生,从而需要补充SOD和CAT对其进行消除。此外,4 ℃条件下保活运输12 h和24 h,GPX活性和对照组无明显差异,表明4 ℃运输时不会超出GPX循环适应能力。
3 结 论
本研究中MS-222较佳的麻醉质量浓度为110 mg/L。保活运输12 h时,4、8、12 ℃条件下的存活率分别为75.3%、50.4%、85%,保活运输24 h时,4、8、12 ℃条件下的存活率分别为62.5%、37.2%、62.4%。因此,保活运输12 h时,12 ℃是史氏鲟麻醉保活运输的适宜温度;保活运输24 h时,4 ℃是史氏鲟麻醉保活运输的适宜温度。
与保活前相比,各温度组史氏鲟随着保活运输时间的延长,皮质醇含量和血糖浓度显著升高,保活运输12 h和24 h时分别在12 ℃和4 ℃保持最低水平。保活运输12 h时,SOD、GPX、CAT活性及MDA含量在12 ℃较低。保活运输24 h时,各温度组CAT活性无明显差异,4 ℃条件下GPX活性较低,同时MDA含量显著低于其他组。因此保活运输12 h时,12 ℃条件下存活率最高,应激最小,鱼体受到氧化损伤最小,是较佳的运输温度;保活运输24 h时,4 ℃条件下存活率最高,应激最小,鱼体受到氧化损伤最小,是较佳的运输温度。
参考文献:
[1] BRONZI P, CHEBANOV M, MICHAELS J T, et al. Sturgeon meat and caviar production: global update 2017[J]. Journal of Applied Ichthyology, 2019, 35(1): 257-266. DOI:10.1111/jai.13870.
[2] ZHANG Yongjun, WANG Wensheng, LIU Yan, et al. Development and evaluation of an intelligent traceability system for waterless live fish transportation[J]. Food Control, 2019, 95: 283-297. DOI:10.1016/j.foodcont.2018.08.018.
[3] NIE Xiaobao, LEI Jilin, CHEN Shixi, et al. Physiological, proteomic, and gene expression analysis of turbot (Scophthalmus maximus) in response to cold acclimation[J]. Aquaculture, 2018, 495: 281-287. DOI:10.1016/j.aquaculture.2018.05.054.
[4] ZHANG Yongjun, XIAO Xinqing, LIU Yan, et al. Survival prediction system for waterless live Chinese sturgeon transportation based on temperature related glucose changes[J]. Journal of Food Process Engineering, 2017, 41(2): e12646. DOI:10.1111/jfpe.12646.
[5] SUMMERFELT S T, Z?HLKE A, KOLAREVIC J, et al. Effects of alkalinity on ammonia removal, carbon dioxide stripping, and system pH in semi-commercial scale water recirculating aquaculture systems operated with moving bed bioreactors[J]. Aquacultural Engineering, 2015, 65: 46-54. DOI:10.1016/j.aquaeng.2014.11.002.
[6] SINGH R K, VARTAK V R, BALANGE A K, et al. Water quality management during transportation of fry of Indian major carps, Catla catla (Hamilton), Labeo rohita (Hamilton) and Cirrhinus mrigala (Hamilton)[J]. Aquaculture, 2004, 235(1/4): 297-302. DOI:10.1016/j.aquaculture.2003.12.011.
[7] WANG Wensheng, ZHANG Yongjun, LIU Yan, et al. Effects of waterless live transportation on survivability, physiological responses and flesh quality in Chinese farmed sturgeon (Acipenser schrenckii)[J]. Aquaculture, 2020, 518: 734834. DOI:10.1016/j.aquaculture.2019.734834.
[8] REFAEY M M, TIAN X, TANG R, et al. Changes in physiological responses, muscular composition and flesh quality of channel catfish Ictalurus punctatus suffering from transport stress[J]. Aquaculture, 2017, 478: 9-15. DOI:10.1016/j.aquaculture.2017.01.026.
[9] HARMON T S. Methods for reducing stressors and maintaining water quality associated with live fish transport in tanks: a review of the basics[J]. Reviews in Aquaculture, 2009, 1(1): 58-66. DOI:10.1111/j.1753-5131.2008.01003.x.
[10] REFAEY M M, LI D, TIAN X, et al. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus[J]. Aquaculture, 2018, 492: 73-81. DOI:10.1016/j.aquaculture.2018.04.003.
[11] ZHANG Yongjun, ZHANG Xiaoshuan, NGA M T T, et al. Development and evaluation of key ambient factors online monitoring system in live Urechis unicinctus transportation strategies[J]. Computers and Electronics in Agriculture, 2018, 145: 43-52. DOI:10.1016/j.compag.2017.12.017.
[12] MI Hongbo, QIAN Chunlu, MAO Linchun, et al. Quality and biochemical properties of artificially hibernated crucian carp for waterless preservation[J]. Fish Physiology and Biochemistry, 2012, 38: 1721-1728. DOI:10.1007/s10695-012-9669-2.
[13] SHABANI F, ERIKSON U, BELI E, et al. Live transport of rainbow trout (Onchorhynchus mykiss) and subsequent live storage in market: water quality, stress and welfare considerations[J]. Aquaculture, 2016, 453: 110-115. DOI:10.1016/j.aquaculture.2015.11.040.
[14] DONG Hongbiao, WANG Wenhao, DUAN Yafei, et al. Transcriptomic analysis of juvenile Chinese sea bass (Lateolabrax maculatus) anesthetized by MS-222 (tricaine methanesulfonate) and eugenol[J]. Fish Physiology and Biochemistry, 2020, 46: 909-920. DOI:10.1007/s10695-019-00755-x.
[15] ZENG Peng, CHEN Tianji, SHEN Jiang. Effects of cold acclimation and storage temperature on crucian carp (Carassius auratus gibelio) in a waterless preservation[J]. Fish Physiology and Biochemistry, 2014, 40(3): 973-982. DOI:10.1007/s10695-013-9898-z.
[16] XAVIER B, MEGARAJAN S, RANJAN R, et al. Effect of packing density on selected tissue biochemical parameters of hatchery produced fingerlings of orange spotted grouper Epinephelus coioides (Hamilton, 1822) during transportaion[J]. Fish, 2018, 65(2): 138-143. DOI:10.21077/ijf.2018.65.2.75268-19.
[17] COSTAS B, ARAG?O C, RUIZ-JARABO I, et al. Different environmental temperatures affect amino acid metabolism in the eurytherm teleost Senegalese sole (Solea senegalensis Kaup, 1858) as indicated by changes in plasma metabolites[J]. Amino Acids, 2012, 43(1): 327-335. DOI:10.1007/s00726-011-1082-0.
[18] SUN Zhenzhu, TAN Xiaohong, LIU Qingying, et al. Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress[J]. Aquaculture, 2019, 498: 545-555. DOI:10.1016/j.aquaculture.2018.08.051.
[19] CHENG Changhong, YE Chaoxia, GUO Zhixun, et al. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress[J]. Fish and Shellfish Immunology, 2017, 64: 137-145. DOI:10.1016/j.fsi.2017.03.003.
[20] SANTOS P P, RAFACHO B, ARDISSON L, et al. Evaluation of oxidative stress and energy metabolism in the heart of rats supplemented with different vitamin D doses[J]. The FASEB Journal, 2012, 26(3): 828-837. DOI:10.1096/fasebj.26.1_supplement.385.8.
[21] MYLONAS C C, CARDINALETTI G, SIGELAKI I, et al. Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) at different temperatures[J]. Aquaculture, 2005, 246(1/4): 467-481. DOI:10.1016/j.aquaculture.2005.02.046.
[22] HE Ruipeng, LEI Bo, SU Yuepeng, et al. Effectiveness of eugenol as an anesthetic for adult spotted sea bass (Lateolabrax maculatus)[J]. Aquaculture, 2020, 523: 735180. DOI:10.1016/j.aquaculture.2020.735180.
[23] TELES M, OLIVEIRA M, JEREZ-CEPA I, et al. Transport and recovery of gilthead sea bream (Sparus aurata L.) sedated with clove oil and MS222: effects on oxidative stress status[J]. Frontiers in Physiology, 2019, 10: 1-10. DOI:10.3389/fphys.2019.00612.
[24] LE Qijun, HU Jiabiao, CAO Xiaohuan, et al. Transcriptomic and cortisol analysis reveals differences in stress alleviation by different methods of anesthesia in crucian carp (Carassius auratus)[J]. Fish and Shellfish Immunology, 2018, 84: 1170-1179. DOI:10.1016/j.fsi.2018.10.061.
[25] FALAHATKAR B, POURSAEID S. Anaesthetic efficacy of tricaine methanesulfonate for Persian sturgeon larvae[J]. Aquaculture Research, 2016, 48(8): 4578-4581. DOI:10.1111/are.13084.
[26] MIRGHAED A T, GHELICHPOUR M, HOSEINI S M. Myrcene and linalool as new anesthetic and sedative agents in common carp, Cyprinus carpio: comparison with eugenol[J]. Aquaculture, 2016, 464: 165-170. DOI:10.101.2016.06.028.
[27] N?STOR S, AZEVEDO A D, PETRY A C. Comparative efficacy of benzocaine, tricaine methane sulfonate and eugenol as anesthetic agents in the guppy Poecilia vivipara[J]. Aquaculture Reports, 2017, 6: 56-60. DOI:10.1016/j.aqrep.2017.04.002.
[28] STOCKMAN J, WEBER E, KASS P H, et al. Physiologic and biochemical measurements and response to noxious stimulation at various concentrations of MS-222 in Koi (Cyprinus carpio)[J]. Veterinary Anaesthesia and Analgesia, 2013, 40(1): 35-47. DOI:10.1111/j.1467-2995.2012.00739.x.
[29] SEMENKOVA T B, BAYUNOVA L V, BOEV A A, et al. Effects of stress on serum cortisol levels of sturgeon in aquaculture[J]. Journal of Applied Ichthyology, 2010, 15(4/5): 270-272. DOI:10.1111/j.1439-0426.1999.tb00249.x.
[30] MIRGHAED A T, YASARI M, MIRZARGAR S S, et al. Rainbow trout (Oncorhynchus mykiss) anesthesia with myrcene: efficacy and physiological responses in comparison with eugenol[J]. Fish Physiology and Biochemistry, 2018, 44(3): 919-926. DOI:10.1007/s10695-018-0481-5.
[31] ZHOU Chuifan, HUANG Meiying, LI Ying, et al. Changes in subcellular distribution and antioxidant compounds involved in Pb accumulation and detoxification in Neyraudia reynaudiana[J]. Environmental Science and Pollution Research, 2016, 23(21): 21794-21804. DOI:10.1007/s11356-016-7362-1.
[32] WANG Mei, ZHANG Chao, ZHANG Zhuo, et al. Distribution and integrated assessment of lead in an abandoned lead-acid battery site in Southwest China before redevelopment[J]. Ecotoxicology and Environmental Safety, 2016, 128: 126-132. DOI:10.1016/j.ecoenv.2016.02.017.
[33] LIN Haiying, SUN Tao, ZHOU Yi, et al. Anti-oxidative feedback and biomarkers in the intertidal seagrass Zostera japonica induced by exposure to copper, lead and cadmium[J]. Marine Pollution Bulletin, 2016, 109(1): 325-333. DOI:10.1016/j.marpolbul.2016.05.062.
