Mechanism of artesunate inhibits gastric cancer cells
2021-09-02SHIKeLIUDongyangFUMingshiCHENFeng
SHI Ke, LIU Dongyang, FU Mingshi, CHEN Feng*
(1. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China;2. Department of General Surgery, Shanghai Sixth People’s Hospital East Campus Affiliated to Shanghai University of Medicine & Health Sciences, Shanghai 201306, China)
[Abstract] Objective: To investigated the biological effects of artesunate (ART) on gastric cancer cells and explored the underlying molecular mechanisms to understand its potential therapeutic role in gastric cancer. Methods:Cell Count Kit-8 (CCK-8) assay was performed to evaluate the effect of ART on cell viability. Apoptosis and cell cycle were analyzed by flow cytometry. Transwell assays were used to assess its effects on metastasis. Western blot analysis was conducted to determine the levels of cell cycle and epithelial-mesenchymal transition (EMT)-related proteins, and proteins involved in the JAK2/STAT3 signaling pathway. Results: ART inhibited cell proliferation (P<0.05) and induced apoptosis (P<0.05) in a dose-dependent manner in gastric cancer cells. It also induced cell cycle arrest at the G2/M phase (P<0.01) in HGC-27 cells, but in SGC-7901 cells at S phase (P<0.05), and the cell cycle-related proteins CDK2, CyclinE1, CyclinD1 were downregulated. With the increasing concentration of ART, the inhibitory effect on migration and invasion was enhanced, as well as the EMT-related proteins N-Cadherin, Snail and Slug decreased. Mechanically, JAK2/STAT3 signaling was blocked upon ART treatment. Conclusion: ART is endowed with anti-gastric cancer activity and may contribute to gastric cancer treatment.
[Key words] artesunate; gastric cancer; metastasis; cell cycle; JAK2/STAT3 signal pathway
1 Introduction
Gastric cancer is a very common malignant tumor of the digestive tract. Most patients with early gastric cancer present no obvious symptoms, which cannot be detected and should be paid attention to as early as possible. Therefore, most patients present advanced stage disease at the time of diagnosis. Gastric cancer ranks among the highest in morbidity and mortality worldwide[1]. With more than 1,000,000 new cases diagnosed and estimated 783,000 deaths in 2018[2], the 5-year survival rate of gastric cancer patients is only 27.4%[3].
In the 1970s, artemisinin was first extracted fromArtemisiaannuaby Tu Youyou[4-5], who saved numerous lives and won the Nobel prize. Artesunate (ART) is one of its derivatives and a hemisuccinate ester of dihydroartemisinin[6], which possesses better water solubility and exerts anti-malaria effects. In recent years, ART has been found to display potential clinical benefits in treating non-malaria diseases, such as those caused by resistant parasites, viruses, fungi, bacteria, and non-infectious diseases, exhibiting inflammatory and anti-fibrotic (e.g., inhibition of knee intraarticular adhesion)[7]as well as anti-tumor[8]effects. Its potential anti-tumor roles have been shown in breast[9]and esophageal[10]among others, over the last few years.
Generally, gastric cancer metastasis indicates a poor prognosis in clinics[11], liver and peritoneal metastases are the major causes of death in patients with gastric cancer[12]. Biomechanical studies show that, ART (30 mg/L) inhibits metastasis by changing the biomechanical properties in esophageal squamous cell carcinoma, according to observations using atomic force microscopy. Increased adhesive force, cytomembrane roughness, and reduced elasticity were found in the drug-treated group compared with the control[10]. Changes in the cell membrane structure can directly affect the cytoskeleton, whose changes affect chromosome stability and cell proliferation. Meanwhile, cell elasticity is associated with cell deformation, which can also influence cell migration and invasion[13]. Additionally, ART inhibits cell invasion andinvivometastasis in non-small cell lung cancer by targeting the transcription of extracellular proteases, such as u-PA, MMP2, and MMP7[14]. Similar attenuating effects have also been shown in metastatic renal cell carcinoma[15]. Noteworthy, no studies on the effect of ART on gastric cancer metastasis were conducted.
The Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway is a crucial signal-transducing mechanism implicated in cell transformation and tumorigenicity[16]. Blocking their phosphorylation inhibits gastric cancer growth, inflammation, and angiogenesis and induces apoptosis in vivo and in vitro[17]. Some genes, drugs, and proteins with anti- or pro-tumor effects function by inhibiting or activating the JAK2/STAT3 signaling pathway[18-20]. However, whether ART exerts its anti-tumor role via this signaling pathway has never been determined in cancer-related studies. Indeed, only two studies reported its inhibitory or activating function in JAK2/STAT3 signaling in the treatment of rosacea[21]and follicular helper T cells, which play a central role in systemic lupus erythematosus[22].
In this study, we concisely explored the anti-tumor effects of ART on SGC-7901 and HGC-27 cell lines, which have not been explored in this context in previous studies. Apoptosis and cell cycle were assessed to elucidate the growth suppressive effect of this drug. Furthermore, Transwell assays and EMT-marker determination were performed to understand the role of ART in preventing cancer metastasis. Finally, we estimated the molecules in the JAK2/STAT3 signaling pathway affected by ART, which has never been researched in ART-related anti-cancer studies.
2 Materials and Methods
2.1 Cell lines and cell culture
Human gastric cancer cell lines SGC-7901 and HGC-27 were obtained from the Chinese Academy of Sciences, Shanghai Cell Institution(Shanghai, China). Both cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Gibco, USA) in an atmosphere of 5% CO2at 37 ℃. ART was purchased from Guilin Pharmaceutical Co., Ltd (Guangxi, China).
2.2 CCK-8 assay
SGC-7901 and HGC-27 cells were cultured in a 96-well culture plate at 5000 cells/well. After overnight incubation, the medium was replaced with fresh medium containing different concentrations of ART (0, 30, 60, and 90 μmol/L) for an additional 0, 24, and 48 h. Cells were washed with 1× PBS, and then 10 μL CCK-8 reagent (Hanbio, Shanghai, China) and 100 μL RPMI-1640 medium were added per well, after which cells were incubated at 37 ℃ for 1 h. The optical density was measured using a microplate reader (EL×800; BioTek Instruments, Inc., Winooski, VT, USA) at a wavelength of 450 nm.
2.3 Annexin V-FITC/PI staining experiment
Cell apoptosis was evaluated using an Annexin V-FITC/PI detection kit (556547; BD, USA). SGC-7901 and HGC-27 cells were treated with different concentrations of ART (0, 30, 60, and 90 μmol/L) for 24 h at 37 ℃. Cells were collected, washed twice with cold PBS, and then treated according to the manufacturer’s instructions. The evaluation was performed using a FACSCalibur instrument (Becton Dickinson, USA).
2.4 Cell cycle assay
SGC-7901 and HGC-27 cells were seeded in 6-well plates and treated with ART (0, 30, 60, or 90 μmol/L) for 24 h. Cells were harvested and washed with cold PBS, followed by the manufacturer’s instructions. Flow cytometric analysis was performed using a FACSCalibur instrument (Becton Dickinson, USA).
2.5 Cell migration and invasion assay
The cell invasion and migration assays were conducted in Transwell chambers (Corning, USA) with 8-μm pores, with or without Matrigel (Corning), respectively. Briefly, SGC-7901 and HGC-27 cells were treated with ART (0, 30, 60, and 90 μmol/L) for 24 h, trypsinized, and resuspended in serum-free RPMI-1640 medium. Cells were added to the upper chamber at 2×105or 2×104cells/well in 200 μL serum-free RPMI-1640 medium. RPMI-1640 medium containing 10% FBS (600 μL) was added to the lower chamber. After incubation for 24 h, the chamber was scraped off with a sterile cotton swab. Cells in the chambers were washed 3 times with PBS, fixed with 4% formaldehyde for 20 min, and stained with crystal violet for 20 min. Chambers were photographed using an inverted microscope.
2.6 Western blot assay
After treatment with ART for 24 h, cells were suspended in 100 μL ice-cold RIPA lysis buffer(Beyotime, China) and incubated at 4 ℃ for 1 h. Cell extracts were centrifuged at 11 200 r/min, 20 min, 4 ℃, and the protein content was quantified using a bicinchoninic acid protein assay kit (IL, Pierce, Rockford, USA) according to the manufacturer’s instructions. 20 μg protein was separated on a 10% SDS-PAGE gel and transferred to polyvinylidene difluoride(PVDF) membranes. Membranes were blocked with 5% skim milk, incubated with primary antibodies N-Cadherin, Snail+Slug, GAPDH, CDK2, CyclinD1, CyclinE1, JAK2,p-JAK2, STAT3, p-STAT3(all antibodies were purchased from Abcam, UK) overnight at 4 ℃, and then incubated with Horseradish peroxidase (HRP)-conjugated secondary antibodies(Solarbio, China) for 1 h at 25 ℃. Immunoreactive proteins were detected by enhanced chemiluminescence (Vazyme, China).
2.7 Statistical analysis
All experiments were independently performed at least 3 times, and datas were expressed as the mean ± SD. Differences between ART-treated and control groups were analyzed with the t-test using GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). Differences were considered statistically significant atP<0.05.
3 Results
3.1 ART inhibited the proliferation and induced apoptosis of gastric cancer cells
ART inhibited SGC-7901 and HGC-27 cell proliferation in a dose-dependent manner, as determined by the CCK-8 assay (Fig. 1A, B). The efficacy of ART in inducing apoptosis was evaluated using flow cytometry, and the most significant apoptosis-promoting effect of ART in both cell lines was observed when its concentration reached 90 μmol/L (Fig. 1C, D).
3.2 ART induced the cell cycle arrest in gastric cancer cells
The cell cycle distribution in both cell lines after treatment with different concentrations of ART for 24 h using flow cytometry were determined. The results indicate that in HGC-27 cells, the cell cycle was arrested at the G2/M phase, with a decreased proportion of cells in the G1/G0 and S phases generally increasing with the concentration of ART (Fig. 2A). Meanwhile, the effect of ART on SGC-7901 cells was not obvious, with cells in the S phase significantly increasing at 90 μmol/L (Fig. 2B). The levels of cell cycle-related proteins, such as CDK2, cyclin D1, and cyclin E1, were evaluated, showing an ART concentration-dependent decrease (Fig. 2C).

The proliferation of SGC-7901 (A) and HGC-27 (B) cells treated with ART. The apoptosis of SGC-7901 (C) and HGC-27 (D) cells which were treated with ART. Compared with Group 0 μmol/L, 1)P<0.05, 2)P<0.01

Cell cycle distribution was determined using flow cytometry, the cell proportion of SGC-7901 (A) and HGC-27 (B) cells which were treated by ART. (C) Western blot showed that the CDK2, cyclin E1, cyclin D1 levels in SGC-7901 and HGC-27 cells which were treated with ART. Compared with Group 0 μmol/L, 1)P<0.05, 2)P<0.01.
3.3 ART inhibited migration, invasion and EMT in gastric cancer cell lines
Transwell assays were performed to determine the ability of invasion and migration, with or without Matrigel, respectively. The number of cells crossed the chamber gradually decreased as the ART dose increased in SGC-7901 and HGC-27 cell lines, which means the migration and invasion abilities were suppressed by ART (Fig.3A, 3B). Subsequently, the expression of EMT markers was determined, and the levels of N-cadherin, Snail, and Slug decreased after ART treatments (Fig. 3C), indicating that ART may exert a metastasis-inhibiting effect on gastric cancer cells.
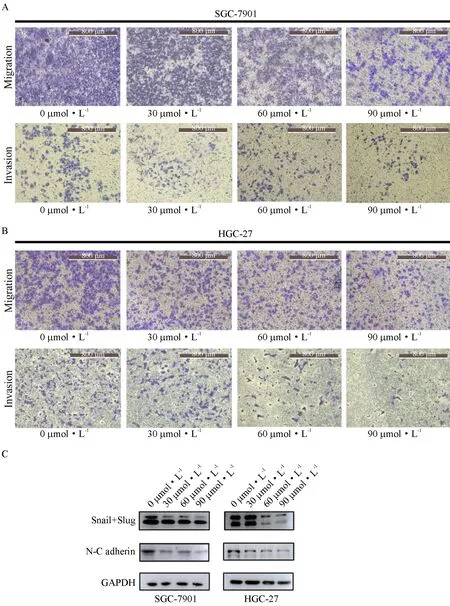
The ability of migration and invasion was measured in (A) SGC-7901 and (B) HGC-27 cells using Transwell plates (×100). (C) The levels of EMT-related proteins.
3.4 ART inhibited the JAK2/STAT3 signaling pathway
The JAK2/STAT3 signaling pathway is a classic pathway in cancers, but it has never been researched in the context of ART treatment for cancers. After ART treatment for 24 h, the JAK2/STAT3 signaling pathway was inhibited both at the level of protein phosphorylation and whole protein expression in HGC-27 cells, while only protein phosphorylation was attenuated in SGC-7901 cells (Fig.4). The inhibiting function of ART occurred in a dose-dependent manner in both cell lines.

JAK2/STAT3 signaling was determined by western blot.
4 Discussion
In this study, we found that ART significantly inhibited cell proliferation at low concentrations, while induced apoptosis and cell cycle arrest in the two analyzed cancer cell lines only at high concentrations. Hence, the mechanism through which gastric cancer cell growth is inhibited at low ART concentrations deserves further study. At present, few studies on the effect of ART on gastric cancer have been carried out. A study showed that ART inhibited the proliferation and promoted apoptosis of gastric cancer cells by reducing the expression of COX-2[23-24], and research on theinvivotherapeutic efficacy of ART also verified its anti-tumor function. Zhou et al.[25]reported that ART plays an inhibitory effect by inducing cell oncosis, a recently discovered cell death mechanism that differs from apoptosis. Moreover, a previous study demonstrated that ART had a potential role in preventingHelicobacterpylori-induced gastric carcinogenesis via inhibition of NF-κB signaling[26].
ART has already been demonstrated to induce reactive oxygen species (ROS)-mediated apoptosis by mediating p38/MAPK signaling, arrest the cell cycle at the S phase, and inhibit mitosis in embryonal rhabdomyosarcoma cells[27]. G2/M phase arrest, a ROS-dependent process[28], is also raised by ART by upregulating Beclin-2 and p21[29]and phosphorylating ATM, which in turn lies at the center of the DNA damage response system, whose activation eventually induces checkpoint activation and cell cycle arrest[30]. Nevertheless, the role of ART in this process has not been studied in gastric cancer, and here, we also found the effects of ART in the two kinds of gastric cancer cells.
In our study, we conducted Transwell assays and EMT marker expression determination, and the results indicate the inhibitory effects of ART on metastasis in a dose-dependent manner. EMT reflects an increased mobility of cancer cells, and we found that the expression of interstitial markers, such as N-cadherin, Snail, and Slug, decreased significantly after ART treatment. And the same effects also have been found in non-small cell lung cancer[31]. Overall, our findings suggest that the metastatic ability of gastric cancer cells was reduced by ART, and the mechanism through which this occurs needs further exploration.
JAK2/STAT3 signaling is a classic signal transduction pathway in cancer. In gastric cancer, inhibition of this pathway promotes apoptosis and autophagy[32], attenuates tumor angiogenesis[33], and inhibits metastasis, EMT[20, 34]and growth[35]. Importantly, our study demonstrated for the first time that ART inhibits the JAK2/STAT3 signaling pathway in gastric cancer cells. Of note, regulation of cancer cells by ART was shown to occur through other molecular signaling pathways, including the Wnt/β-catenin[36]and NF-κB[37]pathways.
In recent years, studies on the anti-tumor effects and related molecular mechanisms of ART have gradually revealed its potential application in the clinical treatment of tumors. However, the side effects of ART are equally worth considering. In fact, one study showed that ART induces G0/G1 cell cycle arrest and apoptosis by increasing intracellular ROS levels in normal liver cells[38]. Hence, the possible side effects of drugs on healthy normal cells cannot be neglected when developing therapies.
Acknowledgement
This work was supported by the National Cultivation Project of Shanghai University of Medicine & Health Sciences (Grant No. SFP-18-20-14-005) and the Technology Development Foundation of Pudong District (Grant No. PKJ2016 -Y54), China.
作者贡献声明
史珂:设计与进行主要实验、统计分析数据,撰写论文;刘东洋:部分实验操作;符明诗:部分实验操作;陈风:提出研究思路和框架,修改论文.
利益冲突声明
本研究未受到企业、公司等第三方资助且不存在潜在利益冲突.
