基于新型材料的柔性生物电干电极的研究进展
2021-08-09马美静刘丽妍高新华刘皓
马美静 刘丽妍 高新华 刘皓

摘 要:柔性生物电干电极是可穿戴健康监控系统的重要组成部分,近几年,研究领域对于生物电干电极的关注逐渐增多。为探讨基于柔性材料的生物电干电极的研究进展,首先对干电极的几个基本性能进行了介绍,生物电干电极常用的性能表征有导电性能、界面阻抗性能、运动伪影和信号噪声,并与湿电极性能进行对比。然后从界面材料方面对柔性生物电干电极进行了分类总结,银纳米线、PEDOT:PSS、聚吡咯、碳纳米管和石墨烯等新型材料的应用,为柔性生物电干电极的长期使用和规模化生产提供了可能。最后对柔性生物电干电极的研究进展进行了总结和展望。
关键词:干电极;长期监测;生物电信号;智能可穿戴;导电材料
中图分类号: TB333
文献标志码:A
文章编号:1009-265X(2021)04-0018-09
Abstract: Flexible biopotential dry electrode is an important part of wearable health monitoring system. In recent years, biopotential dry electrodes have received more and more attention in the research field. In order to discuss the research progress of biopotential dry electrodes based on flexible materials, the basic properties of dry electrodes are introduced first. The common properties of biopotential dry electrodes are characterized by conductivity, interfacial impedance, motion artifacts and signal noise, and are compared with the properties of wet electrodes. Then, the flexible biopotential dry electrodes are classified and summarized in terms of interface materials. The application of new electrode materials such as silver nanowires, PEDOT: PSS, polypyrrole, carbon nanotubes and graphene provides the possibility for the long-term use and large-scale production of flexible biopotential dry electrodes. In the end, the research progress of flexible biopotential dry electrodes is summarized and prospected.
Key words: dry electrode; long-term monitoring; biopotential signal; smart wearable; conductive material
心血管疾病(CVD)作為一种在高龄人群中具有高患病率的疾病,及早预防和治疗具有重要意义。《中国心血管病报告》(2018)的数据显示,中国CVD患病率处于持续上升阶段,推算CVD现患人数2.9亿,心血管病死亡占城乡居民总死亡原因的首位[1]。通过便携式可穿戴式远程生物电监护系统[2],可以降低医院护理费用,实现对生物电信号的日常采集[3]。生物电信号主要有心电信号(ECG),脑电信号(EEG),肌电信号(EMG)和眼电信号(EOG)等[4]。
生物电信号医疗检测用电极多为一次性Ag/AgCl凝胶湿电极,易对皮肤造成刺激,长时间使用时凝胶变干还会影响到信号质量[5],不适合集成到可穿戴设备上使用。传统干电极采用硬质金属板[6-8]、硅片[9-13]、陶瓷[14]和印刷电路板(PCB)[15]等为基底,并通过刻蚀、溅射等方法制作,这些电极通常舒适性较差,也不适合在服装上集成。柔性生物电干电极[16]具有良好的柔韧性和生物相容性,不使用凝胶、不需要皮肤准备,容易和服装相结合等特点,适合长期监测使用。为探讨基于柔性材料的生物电干电极的研究进展,本文从干电极的基本性能出发,介绍柔性生物电干电极的常用表征方法,并对柔性生物电干电极的新型界面材料和制备方法进行分类,最后进行总结和展望。
1 柔性干电极的基本性能
1.1 导电性能
导电性能是电极材料的一项基本性能,生物电干电极的界面材料通常要求具有优良的导电性能[17],通过测量干电极材料的电导率、电阻率和表面方阻等可以表征干电极的导电性能。干电极的导电性能与电极的材料有关。金(Au)、银(Ag)、铜(Cu)等是导电性能优异的金属材料,但是Au、Ag作为贵金属价格昂贵,Ag和Cu长期接触电解质容易氧化[18]。一些导电高聚物和碳基材料具有良好的生物相容性、导电性和环境稳定性,在生物电干电极领域具有广泛应用[19-20]。通过测试电极受到折叠、压缩和拉伸前后的电阻变化,可以评价电极长期的机械稳定性和柔韧性。Choi等[21]讨论了银纳米线(AgNW)电极折叠前后的电阻变化,电极的电阻在对折时增加,释放时减小,但是,电阻变化的幅度很小,即使折叠后,电极的电阻也仅比折叠前的样品高3 %。Del等[22]讨论了聚(3,4-乙撑二氧噻吩):聚苯乙烯磺酸(PEDOT:PSS)涂层纺织品在空气和水中拉伸时的电阻变化,通过使用二乙烯基砜(DVS)交联剂可以改善PEDOT:PSS涂层织物电阻的稳定性。为了提高聚吡咯棉织物的导电性,Zhou等[23]通过先化学聚合然后再次电化学聚合的方法,将织物电阻从1297 Ω降到了325 Ω,以用于生物电信号的采集。
1.2 界面阻抗性能
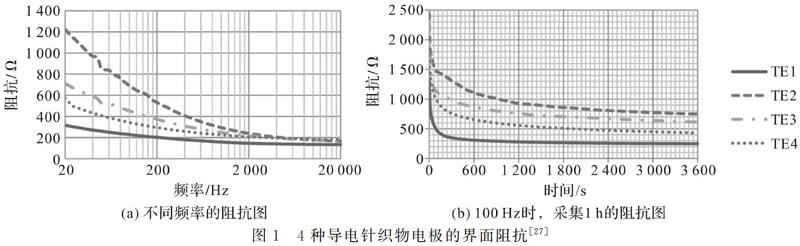
生物电电极是将人体中的离子电流转换为电极中的电子电流的换能器,而电极—皮肤界面阻抗是电荷转移效率的一种指标[24]。电极的界面阻抗性能对采集的生物电信号的质量至关重要,通常阻抗越低生物电位越精确[18]。生物电信号被认为是一种低频信号,不同生物电信号的频率分布不同[25]。为了降低阻抗失配,生物电干电极应在低频区域具有较低的阻抗值,湿电极阻抗值一般分布在180 kΩ(20 Hz)左右[26]。界面阻抗性能的评价主要有两种方式,如图1[27]所示,一种是表征不同频率下的界面阻抗,另一种是表征特定频率下界面阻抗随时间的变化。电极和人体皮肤界面的阻抗性能除了与电极材料本身的性质有关外,还易受电极与皮肤接触状态的影响,主要影响因素有以下几种:
a)皮肤表面角质层的影响:不同部位或不同人体的皮肤角质层厚度具有明显差异,会显著影响界面阻抗[27]。
b)干电极结构和尺寸的影响:表皮接触式电极会受到角质层高阻抗的影响,而穿透式微针电极因为穿透角质层,会显著降低界面阻抗[28]。电极与皮肤之间较大接触面积将导致较低的接触阻抗,但是太大的接触面积会影响舒适性并限制记录的分辨率[29]。
c)汗液等电解质的影响:干电极与皮肤刚接触时,界面阻抗较高,接触一段时间皮肤表面出汗后,界面阻抗会显著降低,皮肤表面汗液会充当电解质[30]。
d)压力和固定方式的影响:电极的固定方式会影响干电极与皮肤的接触压力,一定范围内电极—皮肤界面压力的增加会使阻抗降低[31],压力变化还会影响电极—皮肤界面的有效接触面积[32]和皮肤表面的水分变化[18]。
1.3 运动伪影和信号噪声性能
运动伪影和信号噪声是评价生物电电极在动静态采集信号质量的重要指标。湿电极通过凝胶和皮肤稳定接触,受到的环境噪声和产生的运动伪影较少,干电极和皮肤之间无法良好接触不可避免地存在气泡或缝隙,受到的环境噪声和产生的运动伪影较大[33]。实验时干电极的伪影水平在开始时明显高于湿电极,但随着测试时间的延长,汗液会在界面聚集,导致伪影水平降低[34]。而湿电极随着试验时间延长,凝胶电解质变干,信号质量会有下降的趋势[35]。干电极噪声可分为环境噪声和接触噪声[36]。环境噪声有采集电路的噪声和周围用电器50/60 Hz工频干扰[37],这些噪声可以通过开发的屏蔽技术降低。接触噪声主要由皮肤和干电极表面之间的最小运动产生的电荷扰动造成,电荷的重新分布会引入影响ECG信号的电位变化[18]。为进一步分析信号质量,通常需要对采集的信号进行频谱分析,信号能量可以通过功率谱密度(PSD)[38-39]来表示,因此可以通过计算信号的功率谱密度来表示噪声水平和运动伪影,并与湿电极进行比较。信号噪声和有效信号的关系可以通过信噪比(SNR)来表示,具体见式(1)[40-41]:
SNR=PsignalPnoise(1)
式中:Psignal為有效信号的总功率,W;Pnoise为噪声的总功率,W。
干电极信号质量的评价方法目前没有统一的规定,除了分析运动伪影和信号噪声外,还会与湿电极的性能进行比较,湿电极的性能被认为是参考标准[42],通过分析两种电极的信号相关度来评价干电极。
1.4 其他性能
除了上述性能,一些其他性能也被用于表征生物电干电极。根据一次性凝胶湿电极的标准ANSI/AAMI EC12—2000,直流偏置电压是评价一对电极化学性能的重要指标,干电极可以通过测量开路电压进行表征,开路电压表示两个测试端之间的电位总和,与生物电信号的运动伪影和噪声相关[43-44],对生物电信号采集的准确性具有影响。为了满足集成可穿戴设备长期监测或循环使用的需求,柔性生物电干电极还要满足耐水洗和可重复使用等性能。材料上还要无毒,生物相容。更进一步的电极评价还应该考虑电极的临床应用可靠性,作为湿电极的替代品,通过临床应用的检验将更具说服力。
2 柔性生物电干电极的新型界面材料和制备方法
柔性生物电干电极由基底材料和界面材料两部分组成,基底材料主要提供良好的力学和机械性能,用于支撑界面材料,而界面材料用于和皮肤接触,采集表皮生物电信号,需要具有良好的生物相容性和电荷传输能力。为了减小干电极的运动伪影,延长干电极的有效监测时间,提高干电极的生物相容性和信号采集质量,一些新型界面材料的应用取得了很大进展,如银纳米线等金属纳米材料、聚(3,4-乙撑二氧噻吩):聚苯乙烯磺酸(PEDOT:PSS)和聚吡咯等导电高聚物、碳纳米管和石墨烯等碳基材料,下面对基于这几类材料的柔性干电极进行详细介绍。
2.1 AgNW柔性干电极
AgNW不仅具有金属银的优良导电性,还具有透光性和可挠性,在柔性传感器[45-48]方面具有广泛应用。因为银离子的抗菌性能,AgNW材料也被用于研究抗菌产品。AgNW分散液通过表面涂层或沉积,可以附着在柔性基底上,实现对生物电信号的采集。
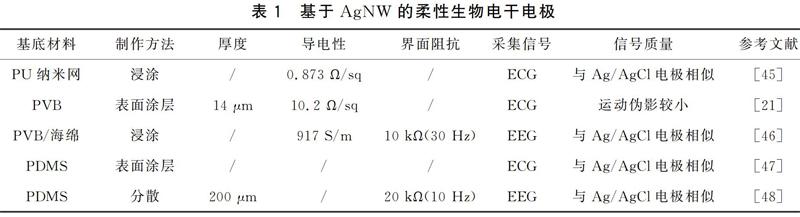
Lee等[45]将AgNW溶液通过涂层工艺赋予聚氨酯纳米网导电性,方阻为0.873 Ω/sq,干燥后制成AgNW干电极,成功采集到ECG信号,并与湿电极的心电图波形进行对比没有显著差异,但该电极的耐久性和耐洗性需要进一步研究。Qin等[47]将AgNW/乙醇溶液固化到聚二甲基硅氧烷(PDMS)柔性基底上,制备了用于非侵入式和可穿戴式的ECG电极,与织物电极相比该电极具有一定的附着力,可以部分减少电极在皮肤上的运动,使用AgNW和Ag/AgCl电极同时采集动静态ECG信号,分析结果显示高度相关(静态ρ=1.000 0,动态ρ=0.999 8)。AgNW也被用于改善弹性体复合材料的导电性,Lee等[48]通过向碳纳米管(CNT)中添加适量AgNW制成AgNW/CNT/PDMS弹性导电纳米复合材料,与只有相同含量的CNT的弹性体的值相比,其阻抗降低了3个数量级。表1对文献中提到的AgNW柔性干电极进行了归纳整理。
2.2 PEDOT:PSS柔性干电极
PEDOT:PSS柔性干电极具有高导电性、亲水性、低杨氏模量和生物相容性,适合作为生物医学电极材料使用[49-51]。基于PEDOT:PSS的柔性生物电干电极的研究报道较多,这种电极的制备方法相对简单,多采用丝网印刷[19]、浸涂[52]等方法将PEDOT:PSS溶液涂覆或填充到柔性基底上,然后晾干制成柔性干电极,非常适合在可穿戴服装上的集成,其制造成本低,有利于大规模生产。
Bihar等[53]在商业用纸上印刷PEDOT:PSS涂层制备出一种心电图纸,将两个手指放在心电图纸上就可以采集ECG,并对比了印刷层数(1层,2层和3层)对信号采集的影响,持续3个月的心电测试(每周采集1次)结果显示印刷3层的电极平均信噪比可达(11.01±0.41) dB。通过对PEDOT:PSS的掺杂和改性可以实现更高的导电性和稳定性。Leleux等[51]探讨了不同第二掺杂物(山梨醇,乙二醇,丙三醇)的PEDOT:PSS对棉织物和涤纶织物导电性的影响,根据测试结果丙三醇掺杂的涤纶织物的电导率最高(575±70 mS/cm,厚度为400 μm)。表2对文献中提到的PEDOT:PSS柔性干电极进行了归纳。
2.3 聚吡咯柔性干电极
聚吡咯具有良好的环境稳定性、生物相容性[54]、导电性和高比电容特性,被用于柔性传感器和柔性电容器的研究。
Zhou等[23]讨论了化学聚合和电化学聚合吡咯两种方法,提出了两步聚合工艺,在棉织物上先化学聚合,然后再电化学聚合吡咯,结果表明经电化学聚合后,电极的导电性提高(3.55 kPa压强下,样品电阻由1 297 Ω下降到325Ω),电信号的传输质量得到改善。Jiang等[55]使用聚吡咯无纺布制作了表面肌电图(sEMG)传感器,将PPy电极缝在松紧带上,以确保与皮肤紧密接触,帮助残疾人控制假肢,与湿式Ag/AgCl电极进行比较表明,信号质量非常相似。除了用于界面导电材料,Abu-Saude等[56]還将聚吡咯涂层用于改善CNT与不锈钢基板结合的机械稳定性,制备了基板上垂直排列的碳纳米管(pvCNT)电极。Zhang等[57]采用化学聚合的方法,以三氯化铁为掺杂剂,对甲苯磺酸为掺杂剂在山羊皮上原位聚合了聚吡咯,制备了一种抗菌、适合长期使用的ECG电极。表3对PPy柔性干电极进行了总结。
2.4 碳纳米管柔性干电极
碳纳米管(CNT)具有良好的导电性能、柔韧性和出色的机械稳定性[58-60]。CNT缠结并随机组装,这使得它们在聚合物弯曲或拉伸时能够更好地相互接触[61]。因为碳纳米管的毒性在医疗领域一直具有争议,所以一些文献对碳纳米管的毒性进行了体内研究[62-65],但是需要更深入的了解。
碳纳米材料可以通过简单的“浸渍和干燥”工艺将导电涂料用于制造导电织物。Zhao等[66]将棉纱浸入单壁碳纳米管(SWCNT)分散液中,干燥后制得SWCNT包覆的棉纱(SWCNT-Cys),并在SWCNT-Cys上覆盖生物纤维涂层,防止CNT直接与人体皮肤接触,而不影响材料的导电性。由于强烈的范德华相互作用,MWCNT易于形成聚集体,Chi等[67]开发了一种平行的溶剂辅助超声波分散方法,首先将MWCNT(质量分数0.5 %)和PDMS预聚物(质量分数20 %)分别分散在正己烷中,随后将MWCNT分散体添加到PDMS分散体中,超声处理5 h,然后将混合物置于75 °C的磁力搅拌下的水中,直到正己烷完全挥发,最终,将固化剂(10:1)添加到由MWCNT/PDMS制成的混合物中,室温搅拌固化。表4对文献中的CNT柔性干电极进行了归纳。
2.5 石墨烯柔性干电极
石墨烯具有优异的导电性、热传导性和稳定性,是制作柔性电子和耐磨传感器的良好材料[68]。与金属包覆聚合物电极相比,石墨烯的生物相容性更好,比金属电极/皮肤界面更友好[69]。
Liu等[69]通过聚合物渗透的方法以PDMS为支撑层,石墨烯为界面导电层,制备了用于生物电采集的柔性干电极。尼龙织物具有表面光滑、耐磨、易吸湿和质量轻等特点,在织物电极方面具有较多应用。Das等[39]和Hallfors等[70]以尼龙织物为基底,采用化学还原氧化石墨烯的方法,制备了具有高导电性且耐用的ECG传感器。Golparvar等[71]首次报道了石墨烯涂层导电纺织电极在EOG采集中的使用和表征,与常规Ag/AgCl湿电极的信号相关性最高达87 %。为避免AgNW氧化使电极电性能降低,Xu等[72-73]用氧化石墨烯(GO)包覆AgNW,并采用丝网印刷等技术沉积在PET基底上,制造了AgNW/GO复合透明电极,表现出优异的光学和电学性能(在550 nm波长下的透光率为83.5 %,表面电阻为11.9 Ω/sq)。表5归纳了文献中基于GO的柔性生物电干电极。
3 总结与展望
3.1 总 结
电极采集的生物电信号的信号质量是评价电极性能的关键,生物电信号质量表征的参数有信噪比、运动伪影和与标准信号的相关度等。电极的导电性能和阻抗性能与生物电信号质量相关。因为研究中采用的测量方法不同,无法简单地从数值上进行比较,但均以湿电极性能作为参考标准,具有广泛的参考价值。由于可穿戴健康监控系统面临的是长期监测,生物相容性和用户穿戴的舒适性也是需要考虑的重要指标。银纳米线、PEDOT:PSS、聚吡咯、碳纳米管和石墨烯等新型电极材料,具有优良的导电性能和一定的柔韧性,可以通过丝网印刷、浸涂或填充等较为简单的方法赋予柔性基底导电性,在控制成本和大规模生产方面具有很大优势。
3.2 展 望
随着智能可穿戴产品和通信技术的发展,可穿戴传感器在健康、运动、时尚等方面的应用快速增长,柔性生物电干电极逐渐被用于集成到各种可穿戴设备中。对于柔性生物电干电极的未来研究,除了研究电极本身的抗噪性能外,还应该考虑电极的临床应用可靠性。作为湿电极的替代品,通过临床应用的检验将更具说服力和可信度。为了适应集成可穿戴设备长期监测或循环使用的需求,柔性生物电干电极还要满足可清洁和重复使用等日常应用方面的要求。
参考文献:
[1]胡盛寿,高润霖,刘力生,等.《中国心血管病报告2018》概要[J].中国循环杂志,2019,34(3):209-220.
[2]OZKAN H, OZHAN O, KARADANA Y, et al. A portable wearable tele-ecg monitoring system[J]. IEEE Transactions on Instrumentation and Measurement,2020,69(1):173-182.
[3]PERIYASWAMY T, BALASUBRAMANIAN M. Ambulatory cardiac bio-signals: From mirage to clinical reality through a decade of progress[J]. International Journal of Medical Informatics,2019,130:103928.
[4]周伟,刘伟,邱清富,等.生物医用電极制造技术及应用研究进展[J].科学通报,2015(15):1352-1360.
[5]RAMASAMY S, BALAN A. Wearable sensors for ECG measurement: a review[J]. Sensor Review,2018,38(4):412-419.
[6]HUANG Y, WU C, WONG A M, et al. Novel active comb-shaped dry electrode for EEG measurement in hairy site[J]. IEEE Transactions on Biomedical Engineering,2015,62(1):256-263.
[7]FIEDLER P, GRIEBEL S, PEDROSA P, et al. Multichannel EEG with novel Ti/TiN dry electrodes[J]. Sensors and Actuators A-Physical,2015,221:139-147.
[8]CELIK N, MANIVANNAN N, STRUDWICK A, et al. Graphene-enabled electrodes for electrocardiogram monitoring[J]. Nanomaterials,2016,6(9):156.
[9]GRISS P, ENOKSSON P, TOLVANEN-LAAKSO H K, et al. Micromachined electrodes for biopotential measurements[J]. Journal of Microelectromechanical Systems,2001,10(1):10-16.
[10]YU L M, TAY F E H, GUO D G, et al. A microfabricated electrode with hollow microneedles for ECG measurement[J]. Sensors and Actuators A-Physical,2009,151(1):17-22.
[11]DIAS N S, CARMO J P, DA SILVA A F, et al. New dry electrodes based on iridium oxide (IrO) for non-invasive biopotential recordings and stimulation[J]. Sensors and Actuators A-Physical,2010,164(1-2):28-34.
[12]OMAHONY C, PINI F, BLAKE A, et al. Microneedle-based electrodes with integrated through-silicon via for biopotential recording[J]. Sensors and Actuators A-Physical,2012,186(SI):130-136.
[13]PEI W, ZHANG H, WANG Y, et al. Skin-potential variation insensitive dry electrodes for ecg recording[J]. IEEE Transactions on Biomedical Engineering,2017,64(2):463-470.
[14]LI G, ZHANG D, WANG S, et al. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp[J]. Sensors and Actuators B-Chemical,2016,237:167-178.
[15]PRATS-BOLUDA G, GARCIA-CASADO J, MARTINEZ-DE-JUAN J L, et al. Active concentric ring electrode for non-invasive detection of intestinal myoelectric signals[J]. Medical Engineering & Physics,2011,33(4):446-455.
[16]高久偉,卢乾波,郑璐,等.柔性生物电传感技术[J].材料导报,2020(1):1095-1106.
[17]JIN G J, UDDIN M J, SHIM J S. Biomimetic cilia-patterned rubber electrode using ultra conductive polydimethylsiloxane[J]. Advanced Functional Materials,2018,28(50):1804351.1-1804351.7.
[18]DANILO P, ANDREA A, ANNALISA B. Survey on textile electrode technologies for electrocardiographic (ECG) monitoring, from metal wires to polymers[J]. Advanced Materials Technologies,2018,3(10):1800008.
[19]PANI D, ACHILLI A, SPANU A, et al. Validation of polymer-based screen-printed textile electrodes for surface EMG detection[J]. IEEE Transactions on Neural Systems and Rehabilitation Engineering,2019,27(7):1370-1377.
[20]PENG H, LIU J, TIAN H, et al. Flexible dry electrode based on carbon nanotube/polymer hybrid micropillars for biopotential recording[J]. Sensors and Actuators A-Physical,2015,235:48-56.
[21]CHOI S B, OH M S, HAN C J, et al. Conformable, thin, and dry electrode for electrocardiography using composite of silver nanowires and polyvinyl butyral[J]. Electronic Materials Letters,2019,15(3):267-277.
[22]DEL AGUA I, MANTIONE D, ISMAILOV U, et al. DVS-Crosslinked PEDOT:PSS free-standing and textile electrodes toward wearable health monitoring[J]. Advanced Materials Technologies,2018,3(10):1700322.
[23]ZHOU Y, DING X, ZHANG J, et al. Fabrication of conductive fabric as textile electrode for ECG monitoring[J]. Fibers and Polymers,2014,15(11):2260-2264.
[24]GRISS P, TOLVANEN-LAAKSO H K, MERILAINEN P, et al. Characterization of micromachined spiked biopotential electrodes[J]. Trans Biomadical Engineering, 2002, 49(6):597-604.
[25]JUNG J, SHIN S, KIM Y T. Dry electrode made from carbon nanotubes for continuous recording of bio-signals[J]. Microelectronic Engineering,2019,203:25-30.
[26]SUN Y, REN L, JIANG L, et al. Fabrication of composite microneedle array electrode for temperature and bio-signal monitoring[J]. Sensors,2018,18(4):1193.
[27]AN X, STYLIOS G K. A hybrid textile electrode for electrocardiogram (ECG) measurement and motion tracking[J]. Materials,2018,11(10):1887.
[28]CARDU R, LEONG P H W, JIN C T, et al. Electrode contact impedance sensitivity to variations in geometry[J]. Physiological Measurement,2012,33(5):817-830.
[29]CHEN Y, OP DE BEECK M, VANDERHEYDEN L, et al. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording[J]. Sensors,2014,14(12):23758-23780.
[30]SHU L, XU T, XU X. Multilayer sweat-absorbable textile electrode for EEG measurement in forehead site[J]. IEEE Sensors Journal,2019,19(15):5995-6005.
[31]COMERT A, HONKALA M, HYTTINEN J. Effect of pressure and padding on motion artifact of textile electrodes[J]. Biomedical Engineering Online,2013,12(1):26.
[32]TAJI B, CHAN A D C, SHIRMOHAMMADI S. Effect of pressure on skin-electrode impedance in wearable biomedical measurement devices[J]. IEEE Transactions on Instrumentation and Measurement,2018,67(8):1900-1912.
[33]LI G, WANG S, DUAN Y Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting[J]. Sensors and Actuators B-Chemical,2018,277:250-260.
[34]MEZIANE N, WEBSTER J G, ATTARI M, et al. Dry electrodes for electrocardiography[J]. Physiological Measurement,2013,34(9):47-69.
[35]ACAR G, OZTURK O, GOLPARVAR A J, et al. Wearable and flexible textile electrodes for biopotential signal monitoring: A review[J]. Electronics,2019,8(5):479.
[36]YAO S, ZHU Y. Nanomaterial-enabled dry electrodes for electrophysiological sensing: a review[J]. Jom,2016,68(4):1145-1155.
[37]劉澄玉,杨美程,邸佳楠,等.穿戴式心电:发展历程、核心技术与未来挑战[J].中国生物医学工程学报,2019(6):641-652.
[38]SCILINGO E P, GEMIGNANI A, PARADISO R, et al. Performance evaluation of sensing fabrics for monitoring physiological and biomechanical variables[J]. IEEE Transactions on Information Technology in Biomedicine,2005,9(3):345-352.
[39]DAS P S, HOSSAIN M F, PARK J Y. Chemically reduced graphene oxide-based dry electrodes as touch sensor for electrocardiograph measurement[J]. Microelectronic Engineering,2017,180:45-51.
[40]YOKUS M A, JUR J S. Fabric-based wearable dry electrodes for body surface biopotential recording[J]. IEEE Transactions on Biomedical Engineering,2016,63(2):423-430.
[41]STAUFFER F, THIELEN M, SAUTER C, et al. Skin conformal polymer electrodes for clinical ECG and EEG recordings[J]. Advanced Healthcare Materials,2018,7(7):1700994.
[42]LOPEZ-GORDO M A, SANCHEZ-MORILLO D, PELAYO VALLE F. Dry EEG electrodes[J]. Sensors,2014,14(7):12847-12870.
[43]MOTA A R, DUARTE L, RODRIGUES D, et al. Development of a quasi-dry electrode for EEG recording[J]. Sensors and Actuators A-Physical,2013,199:310-317.
[44]LIU H, TAO X, XU P, et al. A dynamic measurement system for evaluating dry bio-potential surface electrodes[J]. Measurement,2013,46(6):1904-1913.
[45]LEE E, KIM I, LIU H, et al. Exploration of AgNW/PU nanoweb as ECG textile electrodes and comparison with Ag/AgCl electrodes[J]. Fibers and Polymers,2017,18(9):1749-1753.
[46]LIN S, LIU J, LI W, et al. A flexible, robust, and gel-free electroencephalogram electrode for noninvasive brain-computer interfaces[J]. Nano Letters,2019,19(10):6853-6861.
[47]QIN Q, LI J, YAO S, et al. Electrocardiogram of a silver nanowire based dry electrode: quantitative comparison with the standard Ag/AgCl gel electrode[J]. IEEE Access,2019,7:20789-20800.
[48]LEE J H, HWANG J, ZHU J, et al. Flexible conductive composite integrated with personal earphone for wireless, real-time monitoring of electrophysiological signs[J]. ACS Applied Materials & Interfaces,2018,10(25):21184-21190.
[49]TSUKADA S, NAKASHIMA H, TORIMITSU K. Conductive polymer combined silk fiber bundle for bioelectrical signal recording[J]. PLoS One,2012,7(4):1-10.
[50]CHEN Y, PEI W, CHEN S, et al. Poly(3,4-ethylenedioxythiophene) (PEDOT) as interface material for improving electrochemical performance of microneedles array-based dry electrode[J]. Sensors and Actuators B-Chemical,2013,188:747-756.
[51]LELEUX P, BADIER J, RIVNAY J, et al. Conducting polymer electrodes for electroencephalography[J]. Advanced Healthcare Materials,2014,3(4):490-493.
[52]PANI D, DESSI A, SAENZ-COGOLLO J F, et al. Fully textile, pedot:pss based electrodes for wearable ecg monitoring systems[J]. IEEE Transactions on Biomedical Engineering,2016,63(3):540-549.
[53]BIHAR E, ROBERTS T, SAADAOUI M, et al. Inkjet-printed PEDOT:PSS electrodes on paper for electrocardiography[J]. Advanced Healthcare Materials,2017,6(6):1601167.
[54]張驰,魏德健,曹慧.用于心电信号采集的织物电极技术的研究进展[J].生物医学工程学杂志,2018(5):811-816.
[55]JIANG Y, TOGANE M, LU B, et al. sEMG sensor using polypyrrole-coated nonwoven fabric sheet for practical control of prosthetic hand[J]. Frontiers in Neuroscience,2017,11(93):33.
[56]ABU-SAUDE M, MORSHED B. Characterization of a novel polypyrrole (PPy) conductive polymer coated patterned vertical CNT (pvCNT) dry ECG electrode[J]. Chemosensors, 2018,6(3):27.
[57]ZHANG K, KANG N, ZHANG B, et al. Skin conformal and antibacterial PPy-leather electrode for ECG monitoring[J]. Advanced Electronic Materials,2020,6(8):2000259.
[58]JUNG H, MOON J, BAEK D, et al. CNT/PDMS composite flexible dry electrodes for long-term ecg monitoring[J]. IEEE Transactions on Biomedical Engineering,2012,59(5):1472-1479.
[59]LEE J H, LEE S M, BYEON H J, et al. CNT/PDMS-based canal-typed ear electrodes for inconspicuous EEG recording[J]. Journal of Neural Engineering,2014,11(4):046014.
[60]KANG B, HA T. Wearable carbon nanotube based dry-electrodes for electrophysiological sensors[J]. Japanese Journal of Applied Physics,2018,57(5):05GD02
[61] LIU B, LUO Z, ZHANG W, et al. Silver nanowire-composite electrodes for long-term electrocardiogram measurements[J]. Sensors and Actuators A-Physical,2016,247:459-464.
[62]JUNG H, KWON D, LEE S, et al. Carbon nanofiber-based wearable patches for bio-potential monitoring[J]. Journal of Medical and Biological Engineering,2019,39(6):892-900.
[63]JAFAR A, ALSHATTI Y, AHMAD A. Carbon nanotube toxicity: The smallest biggest debate in medical care[J]. Cogent Medicine,2016,3(1):1217970.
[64] FIRME C P, BANDARU P R. Toxicity issues in the application of carbon nanotubes to biological systems[J]. Nanomedicine: Nanotechnology, Biology and Medicine,2010,6(2):245-256.
[65]MADANI S Y, MANDEL A, SEIFALIAN A M. A concise review of carbon nanotubes toxicology[J]. Nano Rev,2013,4(1):24319547.
[66]ZHAO Y, CAO Y, LIU J, et al. Single-wall carbon nanotube-coated cotton yarn for electrocardiography transmission[J]. Micromachines,2018,9(3):132.
[67]CHI M, ZHAO J, DONG Y, et al. Flexible carbon nanotube-based polymer electrode for long-term electrocardiographic recording[J]. Materials,2019,12(6):971.
[68]YAPICI M K, ALKHIDIR T, SAMAD Y A, et al. Graphene-clad textile electrodes for electrocardiogram monitoring[J]. Sensors and Actuators B-Chemical,2015,221:1469-1474.
[69]LIU B Y, LUO Z Y, ZHANG W Z, et al. A simple method of fabricating graphene-polymer conductive films[J]. International Polymer Processing,2018,33(1):135-138.
[70]HALLFORS N G, ALHAWARI M, JAOUDE M A, et al. Graphene oxide: Nylon ECG sensors for wearable IoT healthcare-nanomaterial and SoC interface[J]. Analog Integrated Circuits and Signal Processing,2018,96(2):253-260.
[71]GOLPARVAR A J, YAPICI M K. Electrooculography by wearable graphene textiles[J]. IEEE Sensors Journal,2018,18(21):8971-8978.
[72]XU X, LUO M, HE P, et al. Screen printed graphene electrodes on textile for wearable electrocardiogram monitoring[J]. Applied Physics A-Materials Science & Processing,2019,125(714):1-7.
[73]XU X, LIU Z, HE P, et al. Screen printed silver nanowire and graphene oxide hybrid transparent electrodes for long-term electrocardiography monitoring[J]. Journal of Physics D-Applied Physics,2019,52(45):455401.
