转Bt基因玉米(瑞丰125、DBN9936、DBN9978)对亚洲玉米螟的抗虫效果研究
2021-06-29孙丹丹全玉东王月琴王振营何康来
孙丹丹 全玉东 王月琴 王振营 何康来


摘要 :評价转Bt基因玉米对靶标生物亚洲玉米螟的杀虫作用是转基因玉米研发的重要一环。本文采用室内生测法对3种转Bt基因抗虫玉米‘瑞丰125(表达Cry1Ab/Cry2Aj杀虫蛋白),‘DBN9936‘DBN9978(表达Cry1Ab杀虫蛋白)对亚洲玉米螟敏感品系ACB-S及抗Cry1Ab品系ACB-AbR、抗Cry1Ac品系ACB-AcR、抗Cry1F品系ACB-FR、抗Cry1Ah品系ACB-AhR、抗Cry1Ie品系ACB-IeR的杀虫活性进行测定,同时采用心叶期和抽丝期人工接虫法进行田间抗虫效果鉴定。结果表明,取食3种Bt玉米的ACB-S幼虫, 3 d死亡率100%,而取食对照常规玉米3 d存活率100%。取食3种Bt玉米的5个抗性品系幼虫除ACB-AbR和ACB-AcR有2%~6%的个体存活4~5 d, 6 d死亡率也达到了100%,其余品系均在3 d全部死亡,而取食对照玉米5~6 d的死亡率仅为4%~14%,差异显著。田间心叶期食叶级别及穗期活虫数、雌穗被害和茎秆被蛀等为害等级说明3种Bt玉米高抗亚洲玉米螟。明确了‘瑞丰125‘DBN9936和‘DBN9978对亚洲玉米螟有很高的杀虫活性和田间防治效果。5个Bt蛋白抗性亚洲玉米螟品系幼虫在常规玉米上显示一定的适合度劣势。
关键词 :转基因玉米; Bt杀虫蛋白; 亚洲玉米螟; 寄主抗性
中图分类号:
S 435.132
文献标识码: A
DOI: 10.16688/j.zwbh.2020008
Resistance of transgenic Bt maize (Ruifeng 125, DBN9936 & DBN9978) to
Asian corn borer
SUN Dandan, QUAN Yudong, WANG Yueqin, WANG Zhenying, HE Kanglai*
(State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection,Chinese Academy of Agricultural Sciences, Beijing 100193, China)
Abstract
Evaluation for resistance to the targets such as Asian corn borer (ACB), Ostrinia furnacalis, is an important step of research and development novel insect resistant transgenic Bt maize. In present research, three kinds of insect resistant transgenic Bt maize, i.e. ‘Ruifeng 125 expressing Cry1Ab/Cry2Aj protein, ‘DBN9936 and ‘DBN9978 expressing Cry1Ab protein, were evaluated in the laboratory and field. Laboratory bioassays were conducted by exposing neonates of ACB susceptible strain (ACB-S), Cry1Ab resistant strain (ACB-AbR), Cry1Ac resistant strain (ACB-AcR), Cry1F resistant strain (ACB-FR), Cry1Ah resistant strain (ACB-AhR), and Cry1Ie resistant strain (ACB-IeR) to fresh whorl leaves, respectively. Field trials were conducted by artificial infestation of ACB at whorl and silking stages. Mortalities were 100% within 3 days when ACB-S larvae fed on three Bt maize leaf tissues, whereas all larvae survived when they fed on the control of conventional maize leaf tissues. When ACB-AbR and ACB-AcR larvae fed on three Bt maize leaf tissues, 2%-6% of larvae could survival for 4-5 days, but not longer than six days. In contrast, there were 4%-14% of larval mortalities when these larvae fed on control within six days. Leaf feeding ratings from whorl stage infestation, larval survivals and ear and stalk bored and tunnels indicated that three Bt maize varieties were highly resistant to ACB. In conclusion, Bt maize ‘Ruifeng 125 ‘DBN9936 and ‘DBN9978 are highly toxic to ACB and could provide season-long protection against ACB. Laboratory selected Cry1Ab, Cry1Ac, Cry1Ah, Cry1F, and Cry1Ie resistant strains demonstrate certain fitness cost.
Key words
transgenic maize; Bt insecticidal protein; Ostrinia furnacalis; host plant resistance
转Bt基因玉米因具有特定且高效的目标性状而受到种植者的欢迎[13]。据国际农业生物技术应用服务组织(ISAAA)统计, 2018年全球已有14个国家和地区种植转基因玉米5 890万hm2,其中转Bt基因抗虫玉米达到550万 hm2,耐除草剂玉米560万 hm2,聚合抗虫/耐除草剂玉米4 780万hm2 [4]。我国玉米种植面积稳定在4 200万 hm2[5],虫害是影响玉米高产稳产以及优质的重要问题之一。因此,抗虫始终是玉米品种改良的重要研究内容,亦是利用现代生物技术进行作物品种改良的首选目标性状之一[6]。种植转基因抗虫作物可显著减少化学杀虫剂的使用,从而降低环境污染。同时因其特异性的杀虫特点,对非靶标生物安全,可保护生物多样性[23]。
苏云金芽胞杆菌Bacillus thuringiensis,即Bt在芽胞形成的过程中产生的杀虫蛋白晶体(insecticidal crystal protein,ICP)对鳞翅目、鞘翅目、双翅目等多种害虫具有特异的杀虫作用,尤其是cry基因,因其编码的Cry杀虫蛋白生物活性强而被广泛应用[6]。目前,全球应用最广泛的抗虫作物主要为跨国公司研发的表达Cry和Vip 类杀虫蛋白的转基因作物[4]。大面积持续种植转基因玉米的同时,也会引发靶标害虫产生抗性。在南非, 夜蛾科昆虫Busseola fusca 对Bt玉米‘MON810 产生了抗性[7],在波多黎各和巴西,草地贪夜蛾Spodoptera frugiperda 对表达Bt Cry1F的玉米产生了抗性[89],在巴西,草地贪夜蛾对表达Bt Cry1Ab的玉米产生了抗性[10],在加拿大,欧洲玉米螟Ostrinia nubilalis对表达Bt Cry1F的玉米产生了抗性[11]。因此,商业化种植某一转基因抗虫玉米的同时,必须配套实施合理的抗性治理措施,以保障其长期的可持续利用。高剂量庇护所是目前应用最广的能有效延缓靶标害虫产生抗性的策略[1214]。然而,其有效性的条件之一就是“高剂量”,即转基因抗虫作物表达的目的Bt蛋白量能杀死靶标害虫种群中全部抗性隐性纯合(rr)和杂合(Sr)个体[15]。因此,抗虫性评价是转基因玉米研发与应用的重要环节。本文开展了3种国产转基因玉米‘瑞丰125‘DBN9936‘DBN9978对靶标害虫亚洲玉米螟的杀虫效果评价,同时通过测定其对亚洲玉米螟不同Bt蛋白抗性品系的杀虫活性,评估了其对靶标害虫抗性治理的潜力。
1 材料和方法
1.1 供试亚洲玉米螟
亚洲玉米螟敏感品系(ACB-S)为在室内无琼脂半人工饲料[16]上连续饲养种群,饲养过程中未接触过Bt制剂或Bt蛋白。亚洲玉米螟Cry1Ab抗性品系ACB-AbR(RR>190)[17]、Cry1Ac抗性品系ACB-AcR(RR>3 000)[17]、Cry1F抗性品系ACB-FR(RR>1 000)[18]、Cry1Ah抗性品系ACB-AhR(RR>190)[19]、Cry1Ie抗性品系ACB-IeR(RR>850)[20]均以敏感品系为起始虫源,在无琼脂半人工饲料中加入一定量的Cry1Ab、Cry1Ac、Cry1F、Cry1Ah、Cry1Ie蛋白进行幼虫全生育期汰选所得。所有幼虫均在温度(27±1)℃,相对湿度70%~80%,光周期L∥D=16 h∥8 h的条件下饲养。
1.2 供试玉米
试验所用表达Cry1Ab/Cry2Aj杀虫蛋白转Bt基因抗虫玉米‘瑞丰125,由浙江大学提供;表达Cry1Ab杀虫蛋白转Bt基因抗虫玉米‘DBN9936和‘DBN9978,由北京大北农生物技术有限公司提供。同时各单位提供了相应的非转基因受体对照,大北农提供了用于在田间试验的‘DBN567和‘Nonghua101。室内生测试验用苗种植在中国农业科学院植物保护研究所科研温室,盆栽。田间试验包括在海南三亚中国农业科学院棉花研究所大茅基地對‘瑞丰125和‘DBN9936的接虫鉴定,以及在河北玉田对‘DBN9978的接虫鉴定。
1.3 Bt玉米抗螟性离体组织生测
室内生测在玉米生长至7~8叶期时进行。从温室取新鲜植株,将未展开的幼嫩心叶剪成2~3 cm2大小,放在24孔培养板中,每板1个处理,每个处理重复2次。每孔接1头待测试亚洲玉米螟敏感或抗性品系初孵幼虫(孵化时间<12 h),放置在温度(27±1)℃、光周期L∥D=16 h∥8 h,相对湿度70%~80%的人工气候培养箱中。每天调查一次幼虫存活情况,根据叶片被取食消耗情况更换同一植株的新叶片,直至Bt玉米组幼虫全部死亡。最后称量并记录对照组存活幼虫体重。
1.4 田间接虫鉴定
田间鉴定分心叶期接虫和穗期接虫,对应自然发生的心叶期世代和穗期世代。心叶期接虫在玉米植株生长至6叶期,每株接亚洲玉米螟(ACB-S)2日龄幼虫20~30头,1周后再次接虫。穗期接虫在玉米抽丝散粉期,每株接亚洲玉米螟2日龄幼虫20~30头。接虫2周后,调查为害级别。心叶期调查记录食叶级别,穗期调查记录活虫数、雌穗(不包括花丝)受害/隧道长度(cm),同时剖查茎秆,记录单株虫孔数、隧道长度和活虫数等计算被害级别[21]。试验小区为随机区组排列,每个品种每次重复接虫40株以上,重复3次。
1.5 数据分析
亚洲玉米螟幼虫取食不同玉米品种心叶后的存活率、田间心叶期接虫后不同品种的食叶级别及穗期接虫后的被害级别的差异分别进行单因素方差分析。处理间差异显著时,平均数采用LSD测验。死亡率百分数进行反正弦转换。采用SAS Proc ANOVA过程进行分析。
2 结果与分析
2.1 Bt玉米对ACB-S品系的杀虫效果
ACB-S幼虫取食3种Bt玉米心叶2 d后,其死亡率达到89.6%以上,显著高于取食非转基因对照玉米心叶CK(F3,4=53.51, P=0.001)(图1),3 d后幼虫全部死亡。而取食非转基因对照玉米心叶3 d的幼虫存活率仍为100%。说明转Bt基因玉米‘瑞丰125‘DBN9936和‘DBN9978对ACB-S有很高的杀虫效果。
2.2 Bt玉米对亚洲玉米螟Cry1A类杀虫蛋白抗性品系的杀虫效果
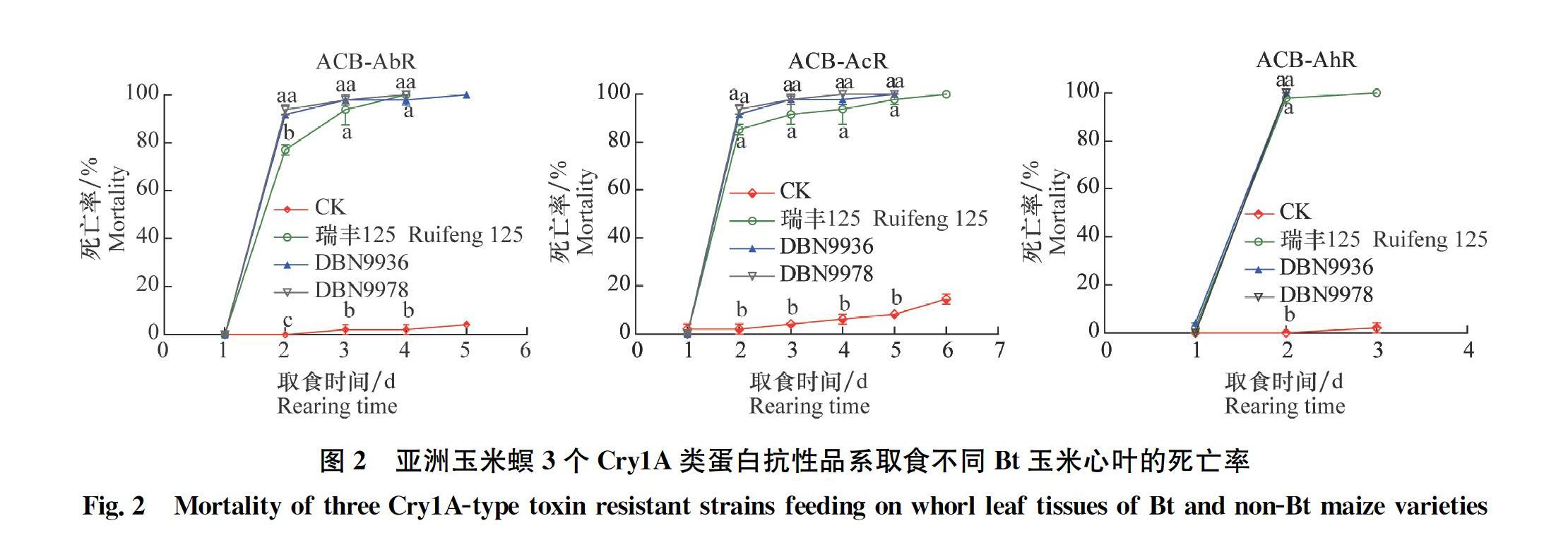
亚洲玉米螟3个Cry1A类蛋白抗性品系ACB-AbR,ACB-AcR,ACB-AhR初孵幼虫分别取食3种Bt玉米心叶2 d 后大量死亡,死亡率达79%以上,随取食时间增加,死亡率继续升高, 3~5 d后全部死亡。而取食非转基因玉米心叶的幼虫在1 d即有出现少量死亡,死亡率约2%,且随时间延长缓慢增加,6 d后的最高死亡率为14%,显著低于Bt玉米处理(图2)。同时,不同抗性品系取食3种Bt玉米心叶2~5 d时的死亡率有显著差异(ACB-AbR:2 d,F3,4=614.58, P<0.000 1; 3 d, F3,4=27.95, P=0.003 8;4 d, F3,4=97.65, P=0.000 3; ACB-AcR: 2 d, F3,4=101.34, P=0.003; 3 d, F3,4=54.18, P=0.001 1; 4 d F3,4=33.86, P=0002 7; 5 d, F3,4=147.19, P=0.000 2; ACB-AhR: 2 d, F3,4=224.18, P<0.000 1),因此,不同处理幼虫全部死亡的时间也有一定差异,即幼虫全部死亡时间为ACB-AhR在2~3 d,ACB-AbR在4~5 d,ACB-AcR在4~6 d。这些结果说明3种Bt玉米对实验室汰选的亚洲玉米螟3个Cry1A类杀虫蛋白抗性品系具有很高的杀虫活性。
2.3 Bt玉米对ACB-FR和ACB-IeR品系的杀虫效果
亚洲玉米螟ACB-FR和ACB-IeR品系初孵幼虫分别取食3种Bt玉米心叶,2~3 d全部死亡,而取食非转基因玉米的初孵幼虫3 d后的死亡率约2%,差异显著(ACB-FR: 2 d, F3,4=62.04, P=0.000 8; ACB-IeR: 2 d, F3,4=108.98, P=0.000 3)说明3种Bt玉米对亚洲玉米螟ACB-FR和ACB-IeR品系具有很好的杀虫效果(图3)。
2.4 Bt玉米田间抗虫性
心叶期接虫鉴定结果表明,Bt玉米与常规玉米对照及当地常规玉米品种‘Nonghua101相比,被害级别差异显著(表1)。Bt玉米叶片仅有针孔状为害状,平均食叶级别≤1.3,即高抗亚洲玉米螟。而常规玉米对照叶片被取食严重,平均食叶级别≥8.5,即高感。
穗期接虫鉴定结果表明,Bt玉米与常规玉米对照及当地常规玉米品种‘Nonghua101相比,存活虫数、被害级别差异显著(表1)。Bt玉米个别植株上有1头发育不良的低龄幼虫,雌穗仅少数花丝被害,籽粒、穗轴等都没有被害,平均被害级别为1.6,即高抗。而常规玉米对照除花丝严重被害外,籽粒、穗轴等都严重被取食,茎秆被蛀,平均被害级别达到7.9以上,即高感。
3 讨论
高剂量表达目标杀虫蛋白的转Bt基因抗虫玉米能够全生育期抵抗欧洲玉米螟和亚洲玉米螟等害虫的为害[2224]。与使用化学杀虫剂一样,大面积长期种植转Bt基因抗虫玉米将不可避免地引起靶标害虫对目标杀虫蛋白产生抗性[2526]。靶标害虫易产生抗性的主要原因之一是转Bt基因抗虫玉米表达的目标Bt杀虫蛋白量低。有报道,由于表达Cry1Ab杀虫蛋白的转基因抗虫玉米‘MON810对草地贪夜蛾表现中等杀虫效果,导致田间很快产生抗性[89];表达Cry1F转基因抗虫玉米防治Striacosta albicosta[27]及表达Cry3Bb1和mCry3A转基因抗虫玉米防治玉米根叶甲Diabrotica virgifera virgifera[28]杀虫效果没有达到高剂量,田间很快产生了抗性。本研究结果明确了表达Cry1Ab的3种转Bt基因抗虫玉米‘瑞丰125‘DBN9936和‘DBN9978對亚洲玉米螟具有高效杀虫活性,田间抗虫性水平达到高抗,杀虫效果达到98%以上。这对于今后商业化种植这些品种时,制定实施高剂量庇护所抗性治理策略,以延缓抗性产生,保障产品长期可持续利用提供了重要的科学依据。
本研究表明,实验室汰选的亚洲玉米螟Cry1Ab抗性品系ACB-AbR对3种表达Cry1Ab杀虫蛋白的Bt玉米有一定的抗性,表现在比敏感品系生存时间长1~3 d,然而最终存活时间没有超过7 d。一方面说明Bt玉米的抗虫性状属功能性显性;另一方面玉米本身的内在抗虫性物质可能与Bt杀虫蛋白互作提高了杀虫效果。这一现象在小菜蛾、欧洲玉米螟等都有报道[2930]。
交互抗性,即由于害虫对某一种胁迫因子(如某一种Bt杀虫蛋白)的汰选产生抗性的同时,对其他胁迫因子(其他Bt杀虫蛋白)也产生了抗性。有报道,对Cry1Ac杀虫蛋白产生抗性的烟芽夜蛾Heliothis virescens (F.)对Cry1Aa, Cry1Ab, Cry1F, Cry1B, Cry1C和Cry1A亦产生了交互抗性[31],对Cry1Ab产生抗性的欧洲玉米螟,对Cry1Ac有高水平的交互抗性,对Cry1F有低水平的交互抗性[32]。前期研究表明,室内用Cry1Ac, Cry1Ah, Cry1F杀虫蛋白汰选的亚洲玉米螟抗性品系,对Cry1Ab蛋白存在一定的交互抗性[1719],Cry1Ie杀虫蛋白汰选的亚洲玉米螟抗性品系, 对Cry1Ab蛋白没有交互抗性[20]。本研究结果显示,ACB-AcR品系取食3种Bt玉米心叶的生存力显著增加,比敏感品系生存时间增加1~4 d,显著长于ACB-Ah, ACB-FR, ACB-Ie品系。说明ACB-AcR品系对3种Bt玉米具有一定交互抗性。
靶标害虫对Bt杀虫蛋白产生抗性是其适应胁迫(选择)的结果。抗性品系(基因型)与敏感品系往往在形态、生物学特征和生理生化反应演化出显著差异。暴露于Bt杀虫蛋白胁迫下,抗性品系适合度通常高于敏感品系。去除胁迫,则抗性品系的适合度会显示一定的劣势。如对Cry1Ac杀虫蛋白产生抗性的棉铃虫Helicoverpa armigera在常规棉花和不添加Cry1Ac蛋白的飼料上幼虫的发育历期延长,净增殖率降低[3]。本研究结果表明,与敏感品系相比,抗性品系在常规玉米对照上的存活率有一定的下降,且下降幅度与抗性倍数高低相关。说明抗性品系适合度下降,即表现出适合度劣势。此外适合度劣势还表现在幼虫体重下降(图4),体重降低幅度与抗性倍数成正相关。在实施高剂量庇护所抗性治理策略条件下,适合度劣势有利于抗性治理,因为抗性个体在庇护所的存活率降低。
参考文献
[1] MILNE A E, BELL J R, HUTCHISON W D, et al. The effect of farmers decisions on pest control with Bt crops: A billion dollar game of strategy [J/OL]. PLoS Computational Biology, 2015, 11(12): e1004483. DOI: 10.1371/journal.pcbi.1004483.
[2] HUTCHISON W D, BURKNESS E C, MITCHELL P D, et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers [J]. Science, 2010, 330(6001): 222225.
[3] TABASHNIK B E. Communal benefits of transgenic corn [J]. Science, 2010, 330(6001): 189190.
[4] ISAAA. Global status of commercialized biotech/GM crops in 2018: Biotech crops continue to help meet the challenges of increased population and climate change [M]. NY: The International Service for the Acquisition of Agri-biotech Applications. 2018: 75.
[5] 国家统计局. 中国统计年鉴[M]. 北京: 国家统计出版社, 2019.
[6] 黎裕,王天宇. 玉米转基因技术研发与应用现状及展望[J]. 玉米科学, 2018, 26(2): 115.
[7] VAN RENSBURG J B J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize [J]. South African Journal of Plant and Soil, 2007, 24(3): 147151.
[8] STORER N P, BABCOCK J M, SCHLENZ M, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico [J]. Journal of Economic Entomology, 2010, 103(4): 10311038.
[9] FARIAS J R, ANDOW D A, HORIKOSHI R J, et al. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil [J]. Crop Protection, 2014, 64: 150158.
[10]OMOTO C, BERNARDI O, SALMERON E, et al. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil [J]. Pest Management Science, 2015, 72(9): 17271736.
[11]SCHAAFSMA A, FARHAN Y, SMITH J. The first case of field failure of Bt corn to control European corn borer Ostrinia nubilalis (Lepidoptera: Crambidae) discovered in Nova Scotia, Canada [C]∥27th IWGO Conference Abstract. 2019, Engelberg, Switzerland.
[12]GOULD F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology [J]. Annual Review of Entomology, 1998, 43: 701726.
[13]TABASHNIK B E, GASSMANN A J, CROWDER D W. Insect resistance to Bt-crops: evidence versus theory [J]. Nature Biotechnology, 2008, 26(2): 199202.
[14]SANAHUJA G, BANAKAR R, TWYMAN R. Bacillus thuringiensis: a century of research, development and commercial applications [J]. Plant Biotechnology Journal, 2011, 9(3): 283300.
[15]ALSTAD D N, ANDOW D A. Managing the evolution of insect resistance to transgenic plants [J]. Science, 1995, 268(5219): 18941896.
[16]宋彥英, 周大荣, 何康来. 亚洲玉米螟无琼脂半人工饲料的研究与应用[J]. 植物保护学报, 1999, 26(4): 324328.
[17]ZHANG Tiantao, HE Mingxia, GATEHOUSE A, et al. Inheritance patterns, dominance and cross-resistance of Cry1Ab-and Cry1Ac-selected Ostrinia furnacalis (Guenée) [J]. Toxins, 2014, 6(9): 26942707.
[18]WANG Yueqin, WANG Yidong, WANG Zhenying, et al. Genetic basis of Cry1F-resistance in a laboratory selected Asian corn borer strain and its cross-resistance to other Bacillus thuringiensis toxins [J/OL]. PLoS ONE, 2016,11(8): e0161189. DOI: 10.1371/journal.pone.0161189.
[19]SHABBIR M Z, QUAN Yudong, WANG Zhenying, et al. Characterization of the Cry1Ah resistance in Asian corn borer and its cross-resistance to other Bacillus thuringiensis toxins [J/OL]. Scientific Reports, 2018, 8: 234. DOI: 10.1038/s41598-017-18586-2.
[20]WANG Yueqin, YANG Jing, QUAN Yudong, et al. Characterization of Asian corn borer resistance to Bt toxin Cry1Ie [J/OL]. Toxins, 2017, 9(6): 186. DOI: 10.3390/toxins9060186.
[21]何康来, 王振营, 周大荣, 等. 玉米抗螟性鉴定方法与评价标准[J]. 沈阳农业大学学报, 2000, 31(5): 5155.
[22]ARCHER T L, SCHUSTER G, PATRICK C, et al. Whorl and stalk damage by European and Southwestern corn borers to four events of Bacillus thuringiensis transgenic maize [J]. Crop Protection, 2000, 19(3): 181190.
[23]ARCHER T L, PATRICK C, SCHUSTER G, et al. Ear and shank damage by corn borers and corn earworms to four events of Bacillus thuringiensis transgenic maize [J]. Crop Protection, 2001, 20(2): 139144.
[24]HE Kanglai, WANG Zhenying, ZHOU Darong, et al. Evaluation of transgenic Bt corn for resistance to the Asian corn borer (Lepidoptera: Pyralidae) [J]. Journal of Economic Entomology, 2003, 96(3): 935940.
[25]MCGAUGHEY W H. Insect resistance to the biological insecticide Bacillus thuringiensis [J]. Science, 1985, 229(4709): 193195.
[26]TABASHNIK B E. Evolution of resistance to Bacillus thuringiensis [J]. Annual Review of Entomology, 1994, 39: 4779.
[27]SMITH J L, LEPPING M D, RULE D M, et al. Evidence for field-evolved resistance of Striacosta albicosta (Lepidoptera: Noctuidae) to Cry1F Bacillus thuringiensis protein and transgenic corn hybrids in Ontario, Canada [J]. Journal of Economic Entomology, 2017, 110(5): 22172228.
[28]GASSMANN A J, PETZOLD-MAXWELL J L, KEWESHAN R S, et al. Field-evolved resistance to Bt maize by western corn rootworm [J/OL]. PLoS ONE, 2011, 6(7): e22629. DOI: 10.1371/journal.pone.022629.
[29]TABASHNIK B E, CARRIERE Y, DENNEHY T J, et al. Insect resistance to transgenic Bt crops: lessons from the laboratory and field [J]. Journal of Economic Entomology, 2003, 96(4): 10311038.
[30]HUANG Fangneng, BUSCHMAN L L, HIGGINS R A, et al. Survival of Kansas Dipel-resistant European corn borer (Lepidoptera: Crambidae) on Bt and non-Bt corn hybirds [J]. Journal of Economic Entomology, 2002, 95(3): 614621.
[31]GOULD F, ANDERSON A, REYNOLDS R, et al. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins [J]. Journal of Economic Entomology, 1995, 88(6): 15451559.
[32]SIQUEIRA H A A, MOELLENBECK D, SPENCER T, et al. Cross-resistance of Cry1Ab-selected Ostrinia nubilalis (Lepidoptera: Crambidae) to Bacillus thuringiensis δ-endotoxins [J]. Journal of Economic Entomology, 2004, 97(3): 10491057.
[33]LIANG Gemei, WU Kongming, YU Hongkun, et al. Changes of inheritance mode and fitness in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin [J]. Journal of Invertebrate Pathology, 2008, 97(2): 142149.
(責任编辑:田 喆)
