Significant Association of CD40-1C>T Polymorphism and Stroke in the Han Chinese Population
2018-09-12,,,,,,
, , , , , ,
(Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders(Ministry of Education),Bio-X Institutes, Shanghai Jiao Tong University, Shanghai 200030, China)
Abstract: Stroke is one of the most common diseases in the older population. CD40 receptor plays a key role in antigen presentation and T-cell secondary activation. This study evaluated the association between CD40-1C>T single nucleotide polymorphism and total stroke, ischemic stroke and hemorrhagic stroke in Han Chinese Population. A total of 996 subjects were recruited, including 498 healthy controls and 498 patients (338 ischemic stroke and 160 hemorrhagic stroke). CD40-1C>T single nucleotide polymorphism was genotyped for statistical analysis. Meta-analysis of ischemic stroke was used to contextualize our studies with three previous studies. CD40-1C>T single nucleotide polymorphism was significantly associated with total stroke (Padjusted=0.001, OR=1.41[95% CI=1.15-1.74]), ischemic stroke (Padjusted=0.022, OR=1.33 [95% CI =1.04-1.69]) and hemorrhagic stroke (Padjusted=0.017, OR=1.40 [95% CI =1.06-1.86]). In meta-analysis, there was a significant association between CD40-1C>T SNP and ischemic stroke (P<0.00001, OR=1.39 [95% CI=1.25-1.56]) yielding 1.39 per T allele copy. The results of our study are consistent with previous reports. Our findings indicate that CD40-1C>T polymorphisms may be a risk factor of stroke and its two subtypes.
Keywords: CD40-1C>T; rs1883832; stroke; meta-analysis
Stroke is one of the most common diseases in the older population. In 2013, approximately 6.9 million people had an ischemic stroke and 3.4 million people had a hemorrhagic stroke world wild[1]. As a common disease with high incidence, high morbidity and high mortality, stroke has a significant impact on healthcare expenditures and the worldwide economy[2]. A stroke is a sudden loss of brain function. It is caused by the interruption of flow of blood to the brain (ischemic stroke, IS) or the rupture of blood vessels in the brain (hemorrhagic stroke, CH)[3]. Heterogeneity of stroke pathophysiology is characterized by its various subtypes[3]and the inconsistency in susceptible gene study[4-12]in different populations of stroke.
Tumor necrosis factor (TNF) receptor superfamily member 5 (CD40) is a member of the TNF receptor superfamily. CD40/CD40L is a pair of complementary transmembrane glycoproteins, expressed on immune cells, endothelial cells, smooth muscle cells, platelets and other cells[13]. Increasing evidence supports the role of CD40-mediated platelet activation in inflammation, thrombosis, and atherosclerosis[14].CD40-1C>T single nucleotide polymorphism (rs1883832) is a common polymorphism inCD40 gene Kozak sequence (-1C/T). Evidence suggests that rs1883832 is a novel regulator of CD40 expression[15].
The association ofCD40 SNPs with infection-and autoimmunity-associated diseases were widely studied, such as acute coronary syndrome[15-16]and Graves’ disease[17]. Rs1883832 allele T or carried T genotypes played a risk role in breast cancer[18]and nonHodgkin lymphoma[19]. Del Río-Espínola et al.[20]reported that rs1883832 is associated with brain vessel reocclusion after fibrinolysis in IS patients. And in Korean population, Cho J. M. et al.[21]identified two promoter SNPs ofCD40 gene which were associated with the development of IS. Only a few studies have investigated the association between rs1883832 and risk of stroke in Han Chinese population. Ma et al.[22], Chen et al.[23]and Huang et al.[24]reported that the T allele of rs1883832 was associated with the risk of IS. However, there is no report of the relation between total stroke, CH and rs1883832 till now. To confirm whetherCD40-1C>T is a risk factor for stroke, we conducted a case-control association study in Han Chinese population (498 cases and 498 controls) to evaluate the possible association between rs1883832 and total stroke and its subtypes.
1 Methods
1.1 Subjects
498 unrelated patients (328 with IS and 170 with CH) and 498 healthy individuals were enrolled from Beijing Tiantan Hospital. Clinical diagnoses were carried out by professional physicians and technologists. The subjects were recruited from Han Chinese population and were unrelated to one another. Hemorrhagic stroke included subarachnoid hemorrhage and hypertensive cerebral hemorrhage. Patients with hemorrhage due to trauma, tumor, vascular malformation and coagulopathy were excluded from the study. Ischemic stroke was defined as a sudden onset of focal or global neurologic deficit with signs and symptoms persisting for more than 24h. Patients with a history of cerebral embolism, trauma, transient ischemic attack, cerebrovascular malformations, coagulation disorders, autoimmune diseases, tumors, peripheral vascular disease, or chronic infection diseases were excluded.
Control subjects were also recruited from Beijing Tiantan Hospital. These subjects had no clinical or radiological evidence of stroke and other neurological diseases. They were also free from autoimmune disease, liver disease, nephrosis, and hematological disorders.
Sex, age, total plasma cholesterol (TC), triglycerides (TG), high-density lipoprotein(HDL), low-density lipoprotein(LDL) cholesterol, hypertension and diabetes mellitus were documented on entry into the study.
This study obtained the consent of the Ethics Committee of the Beijing Tiantan Hospital. Written informed consent was obtained from all participants prior to entering the study.
1.2 Genotyping
The rs1883832 polymorphism was genotyped using the Sequenom Mass ARRAY platform (Sequenom, San Diego, CA) according to the iPLEX Gold Application Guide available at (http:∥www.sequenom. com/sites/genetic-analysis/applications/snp-genotyping). The Genotyping Tools and MassARRAY Assay Design software of Sequenom was used to design PCR primer (forward 5’GGACCTGGGGGCAAAGAAGA and reverse: 5’CCCACTCCCAACTCCCGTCT) and iPLEX reaction primers (5’TTTTTTTTTTTTGCAGAGG CAGACGAACCAT).
The genotyping experiment was undertaken according to the manufacturer’s protocol, using recommended reagents in the iPLEX Gold SNP genotyping kit.
1.3 Statistical analysis
Single locus analysis was calculated by a powerful online software SHEsisPlus (http:∥shesisplus.bio-x.cn/SHEsis.html)[25]. Hardy-Weinberg equilibrium tests (HWE) was performed for rs1883832 in control cases. Single site association tests between rs1883832 and total stroke, IS and CH were performed. Logistic regression was used for risk stratification with or without covariate adjustments determined by significant differences between patients and controls (i.e. age, HDL, and hypertension). Inverse variance meta-analysis (RevMan 5.3 software) was used to contextualize our studies with three previous studies, using date from PIMD: 23954880, PIMD: 26957242 and PIMD: 28590502, which also studied the association between rs1883832 and IS. The effect size was represented by an odds ratio (OR) with 95% confidence interval (CI) and I2test was used to assess heterogeneity in combined studies. The random effect model was used to calculate pooled OR.Pvalues of overall OR were generated using the Z-score test.
2 Results
2.1 Clinical characteristics of total stroke patients and controls
Demographic characteristics and clinical vascular variables of control and total stroke patients were showed in Tab.1. There were no significant differences in levels of TC, TG and LDL between the controls and total stroke cases. However, HDL levels were significantly lower in stroke cases than in controls and mean age and incidence of hypertension were significantly higher.
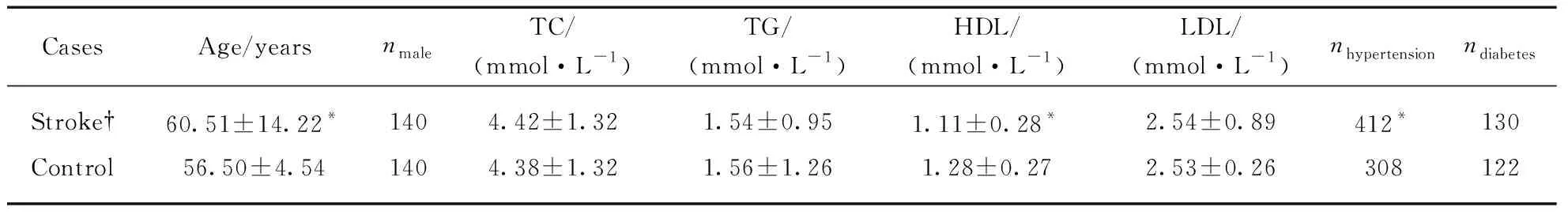
Tab.1 Comparison of clinical variables between total strokes and control subjects
Notes: †Ischemic stroke,n=338, Hemorrhagic stroke,n=160. Data are shown as mean±standard deviation (SD) or asn. *Significant differences between cases and controls.
2.2 Single locus association
No significant deviation from Hardy-Weinberg equilibrium in controls was found for rs1883832 (P=0.6099). Results of single site association tests between rs1883832 and total stroke and its subtypes were shown in Tab.2.
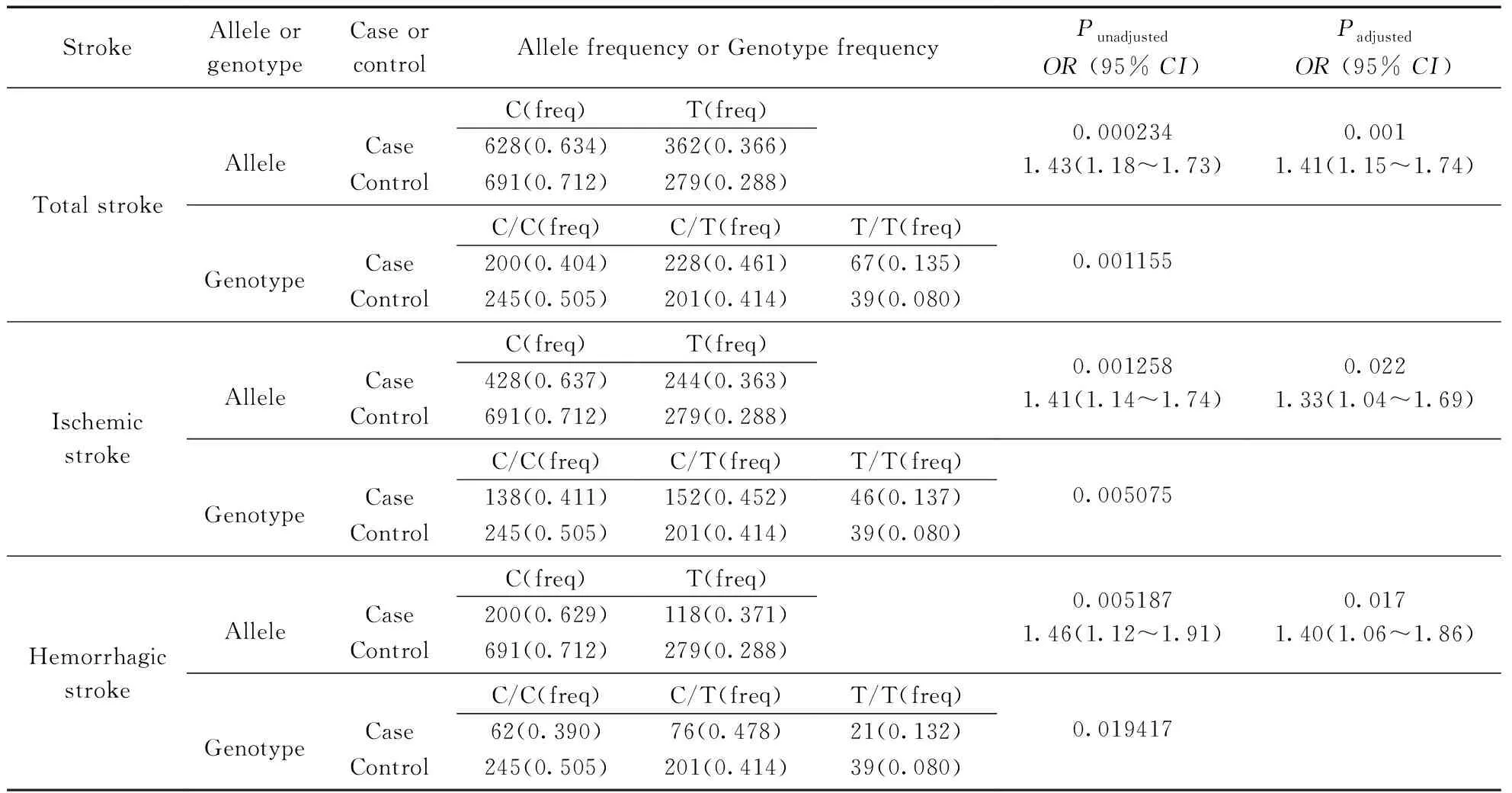
Tab.2 Results of single site association tests between CD40-1C>T SNP and total stroke and its subtypes
Abbreviations:OR, odds ratio, the contrast allele refers to the minor allele;CI, confidence interval;Punadjusted: unadjustedP-value fromt-test;Padjusted:P-value adjusted using logistic regression analysis with age, HD and hypertension as covariates. Case and Control represent the frequency of minor allele in patients and controls respectively.
Rs1883832 was significantly associated with total stroke for both allelic (Padjusted=0.001,OR=1.41[95%CI=1.15-1.74]) and genotypic (P=0.00115) analyzes. Rs1883832 also showed significant differences between IS cases and controls for both allele frequency (Padjusted=0.022,OR=1.33[95%CI=1.04-1.69]) and genotype frequency (P=0.00507). Furthermore, rs1883832 showed significant differences between CH cases and controls in both allele frequency (Padjusted=0.017,OR=1.40[95%CI=1. 06-1.86]) and genotype frequency (P=0.01936) too.
2.3 Meta-analysis
By using the combined keywords “CD40”, “polymorphism”, “stroke”, “association” searching in the PubMed database dating up to 21 October 2017, we found 7 studies evaluating the association between polymorphisms ofCD40 and stroke. After further scrutinize for studies focusing on stroke in which SNPs examined overlapped with ours, only 3 studies were finally recruited in the meta-analysis. These studies all focused on IS, as a result, our meta-analysis only considered the association between IS andCD40-1C>T. The comparison of our study and previous studies were shown in Tab.3. Forests plot for rs1883832 from 4 studies including our own were shown in Fig.1. There was a significant association between rs1883832 and IS (P<0.00001,OR=1.39 [95%CI=1.25-1.56]) yielding 1.39 per T allele copy, with no evidence for statistical heterogeneity (P=0.74,I2=0%) between studies. This meta-analysis indicated that the T allele was associated with increased risk of IS, which was consistent with our study.
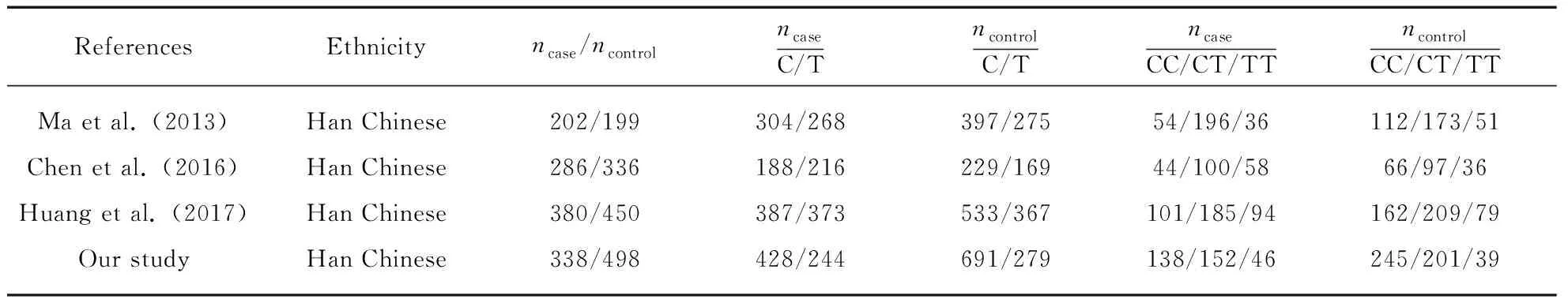
Tab.3 Comparison of data of our study and three previous studies
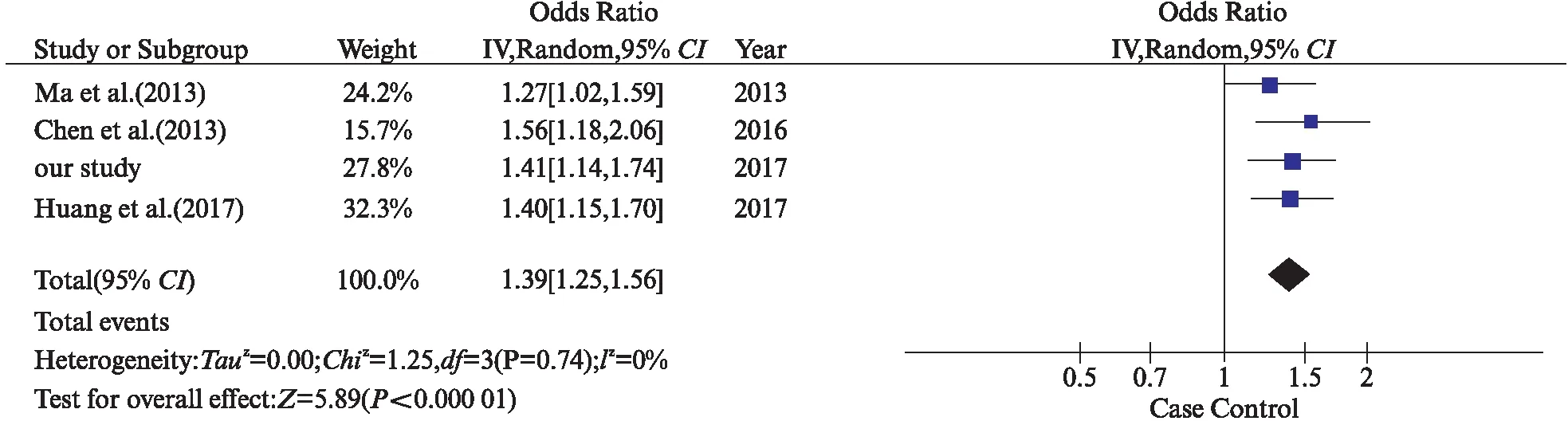
Fig.1 Meta-analysis of studies investigating the association of CD40-1C>T SNP with ischemic stroke using a random effects model. The point estimate of the OR (square proportional to the weight of each study) and 95% CI for the OR (extending lines) for each study. The summary OR and 95% CIs by random effects calculations are depicted as a diamond. Values higher than 1 indicate that the T allele is associated with increased risk of ischemic stroke.
3 Discussion
CD40 is a 48-kDa phosphorylated transmembrane glycoprotein and cell surface receptor. CD40 ligand (CD154) is a major ligand of CD40. CD40/CD40L induces interleukin 10 (IL10) , TNF expression in T cell[26]and IL1A, IL1B, TNF, IL6, IL8 expression in monocytes[27]. Garlichs et al[28]reported that patients with acute cerebral ischemia showed a significant increase of CD40L on platelets and CD40 on monocytes. Wang et al[29]reported that acute coronary syndrome patients showed a significant increase of CD40 and sCD40L co-expression compared with controls (P<0.05). Del Río-Espínola et al[20]reported thatCD40-1C>T polymorphism is associated with brain vessel reocclusion after fibrinolysis in IS patients. And in Korean population, Cho et al[21]identified two promoter SNPs ofCD40 gene which were associated with the development of IS. The increased expression of CD40/CD40L may be a risk factor of IS.
In this study, we demonstrated that rs1883832 was significantly associated with ischemic stroke(Padjusted=0.022,OR=1.33[95%CI=1.04-1.69]), which was consistent with meta-analysis(P<0.00001,OR=1.39 [95%CI=1.25-1.56]). Our findings suggest that the rs1883832 polymorphism T allele may enhance the risk of IS. In previous studies, Chen et al[23]and Huang et al[24]found that individuals carrying the rs1883832TT or rs1883832CT genotypes showed significantly higher soluble CD40 levels compared with the rs1883832CC genotype in the IS group. What’s more, Tian et al[15]demonstrated that patients with acute coronary syndrome showed a significant increase of CD40 expression compared with controls. According to these findings, we suppose that the rs1883832 polymorphism T allele may enhance the capacity of CD40 production and induces excessive immune reaction and thus increase the development of ischemic stroke.
In addition, we also proved the association between rs1883832 and hemorrhagic stroke(Padjusted=0.017,OR=1.40[95%CI=1.06-1.86]). We suggest that T allele of rs1883832 may also increase the risk of CH. The nosogenesis and pathology of CH are different from IS. But in our study they shared a similar risk of rs1883832. As we know, atherothrombotic ischemic stroke is a main kind of IS. On the other side, arteriolosclerosis with hyalinosis can lead to subsequent intracerebral hemorrhage[30]. Atherosclerotic vascular risk factors such as hypertension or diabetes are associated with IS and CH[31]. These findings means that atherosclerosis is a shared risk factor of IS and CH. Compared to our findings, as a result, the express level of CD40 in blood may also increase the risk of CH. These findings indicate that the immunologic mechanism of CD40/CD40L may effect both IS and CH. We also proved the association between rs1883832 and total stroke(Padjusted=0.001,OR=1.41[95%CI=1.15-1.74]). As we know, this is the first study of an association betweenCD40-1C>T SNP and total stroke and hemorrhagic stroke in Han Chinese population. However, the sample size of hemorrhagic stroke cases was relatively small.
4 Conclusions
The study identifiedCD40-1C>T SNP as being significantly associated with total stroke, ischemic stroke and hemorrhagic stroke in Han Chinese population. These findings indicate thatCD40-1C>T SNP may be a risk factor of stroke, which may be meaningful for the diagnosis and treatment of stroke.
