WO3/Al2O3-TiO2催化剂的制备及其在甘油气相脱水制备丙烯醛中的应用
2013-11-19王奂祎贺站锋
刘 涛, 王奂祎, 贺站锋, 王 丹, 蒋 毅
(1. 中国科学院 成都有机化学研究所,四川 成都 610041; 2. 中国科学院大学,北京 100049)
近年来,生物柴油在全球的迅猛发展[1]致使副产物甘油的大量过剩,如何有效利用过剩的甘油成为一个重要的研究课题。比较有效的解决方式是将甘油通过化学法,如选择性氧化、脱水、氢解、卤化、酯化和醚化、蒸汽重整等途径[2,3]转化成附加值更高的化工产品。在这些转化途径中,甘油脱水制备丙烯醛[4]具有较大的发展潜力。
目前,用于甘油脱水制备丙烯醛的固体酸催化剂主要有四大类:氧化物[5~8]、杂多酸[9~11]、分子筛[12~14]和磷酸盐[15~17]。其中含有WO3的催化剂具有较大的应用潜力。Arda Ulgen等报道WO3/ZrO2[5]或WO3/TiO2[8]催化甘油脱水制备丙烯醛,收率均在70%以上;Lauriol-Garbey P等[7]用SiO2修饰WO3/ZrO2为催化剂,反应150 h,转化率达80%,丙烯醛选择性>70%;Kraleva E等[18]报道W-SBA-15催化剂在280 ℃以下反应,转化率达90%,丙烯醛选择性>70%;Suprun W等[17]使用过渡金属修饰铝磷酸盐为催化剂,其对丙烯醛的选择性顺序为:W>Mo>Cu>V~Fe>Cr>Mn>Ce。以上催化剂,虽然在甘油脱水制备丙烯醛的反应中,具有较佳的催化活性,但还难以实现商业化应用,新催化剂的开发仍是甘油脱水研究的热点。工业Al2O3(含5 wt%TiO2)是一种广泛使用的载体,而WO3/Al2O3-TiO2催化剂应用于甘油脱水高选择性制备丙烯醛的相关研究还未见文献报道。

Scheme1
1 实验部分
1.1 仪器与试剂
NOVA 2200e型氮吸附仪;Rigaku D/max-2500/PC型X-射线衍射仪(XRD);SC-200型和SC3000-B型气相色谱仪[HP-INNOWAX毛细管柱(30 m×0.32 mm×0.25 μm);载气:高纯N2;FID检测器;1,2-丙二醇为内标];STA449C型热重分析仪(TGA);自行组装的TCD-GC装置(内径9 mm,长度35.0 cm)。
钨酸铵(H40N10O41W12·xH2O),分析纯,国药集团化学试剂有限公司;30%过氧化氢,分析纯,广东光华化学厂有限公司;工业Al2O3(含5 wt%TiO2),江苏三剂实业有限公司,使用前于750 ℃焙烧2 h,研磨至40 目~60 目后备用。
在反应瓶中加入钨酸铵2.94 g(0.97 mmol)和水10 mL,搅拌使其溶解;加入Al2O3载体10 g,搅拌均匀,于室温浸渍24 h;于110 ℃干燥10 h制得Cat20[w(WO3)=20%]。
改变钨酸铵用量,用类似方法制得Catw(表1)。

表 1 制备的实验条件Table 1 Conditions of preparing
*w(WO3)=m(WO3)/[m(WO3)+m(Al2O3-TiO2)]×100%; T:焙烧温度
1.3 甘油脱水制备丙烯醛反应

2 结果与讨论
2.1 表征

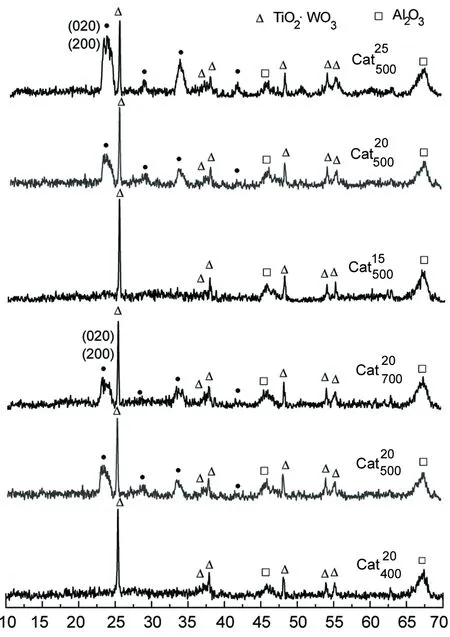
2θ/(°)图的XRD谱图

Temperature/℃图2 Cat20的TGA曲线Figure 2 TGA curvesl of Cat20
2.2 载体与催化剂的织构性质


表 2 载体和催化剂的织构性质Table 2 Textural properties of the support and catalysts

w/%图3 w对脱水反应的影响*Figure 3 Effect of w on dehydration of glycerol over

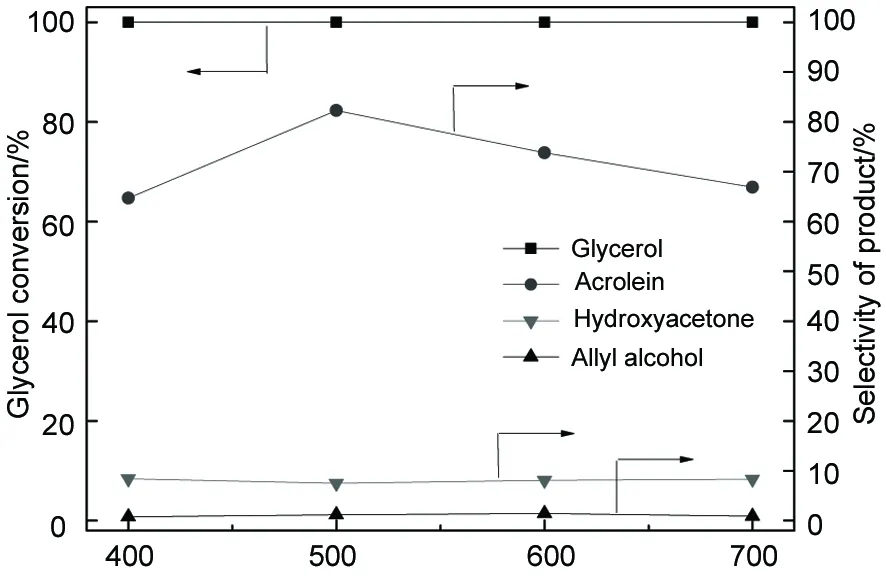
Calcination temperature/℃图4 焙烧温度对脱水反应的影响Figure 4 Effect of calcination temperature on dehydration of glycerol over
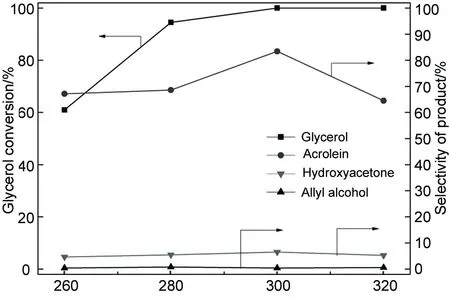
Reaction temperature/℃图5 反应温度对脱水反应的影响Figure 5 Effect of reaction temperature on dehydration of glycerol over
(1)w
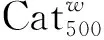
(2)焙烧温度

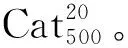
2.4 反应温度对反应的影响

2.5 催化剂的NH3-TPD

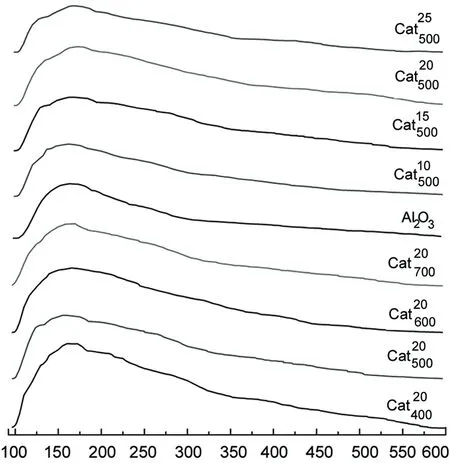
Temperature/℃图的NH3-TPDFigure 6 NH3-TPD prfiles of
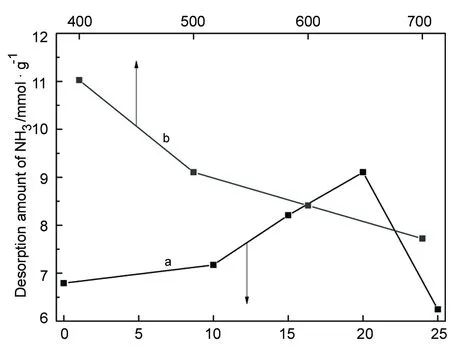
w/%图总酸量与w(a)或焙烧温度(b)的关系Figure 7 Effect of w(a) or calcination temperature(b) on the total amount of acid of

[1] Rahmat N, Abdullah A Z, Mohamed A R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives:A critical review[J].Renew Sust Energ Rev,2010,14(3):987-1000.
[2] Yuguo Zheng, Xiaolong Chen, Yinchu Shen. Commodity chemicals derived from glycerol,an important biorefinery feedstock[J].Chem Rev,2008,108(12):5253-5277.
[3] Zhou Chun-Hui, Beltramini J N, Fan Yong-Xian,etal. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals[J].Chem Soc Rev,2008,37(3):527-549.
[4] Kim Y T, Jung K D, Park E D. Gas-phase dehydration of glycerol over ZSM-5 catalysts[J].Micropor Mesopor Mat,2010,131(1-3):28-36.
[5] Ulgen A, Hoelderich W. Conversion of glycerol to acrolein in the presence of WO3/ZrO2catalysts[J].Catal Lett,2009,131(1):122-128.
[6] Lauriol-Garbay P, Millet J M M, Loridant S,etal. New efficient and long-life catalyst for gas-phase glycerol dehydration to acrolein[J].J Catal,2011,280(1):68-76.
[7] Lauriol-Garbey P, Loridant S, Bellière-Baca V,etal. Gas phase dehydration of glycerol to acrolein over WO3/ZrO2catalysts:Improvement of selectivity and stability by doping with SiO2[J].Catal Commun,2011,16(1):170-174.
[8] Ulgen A, Hoelderich W F. Conversion of glycerol to acrolein in the presence of WO3/TiO2catalysts[J].App Catal A-Gen,2011,400(1-2):34-38.
[9] Atia H, Armbruster U, Martin A. Dehydration of glycerol in gas phase using heteropoly acid catalysts as active compounds[J].J Catal,2008,258(1):71-82.
[10] Alhanash A, Kozhevnikova Elena F, Kozhevnikov Ivan V. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt[J].App Catal A-Gen,2010,378(1):11-18.
[11] Atia H, Armbruster U, Martin A. Influence of alkaline metal on performance of supported silicotungstic acid catalysts in glycerol dehydration towards acrolein[J].App Catal A-Gen,2011,393(1-2):331-339.
[12] Schuth F, Jia C J, Liu Y,etal. Small-sized HZSM-5 zeolite as highly active catalyst for gas phase dehydration of glycerol to acrolein[J].J Catal,2010,269(1):71-79.
[13] Kim Y T, Jung K D, Park E D. A comparative study for gas-phase dehydration of glycerol over H-zeolites[J].App Catal A-Gen,2011,393(1-2):275-287.
[14] Lourenco J P, Macedo M I, Fernandes A. Sulfonic-functionalized SBA-15 as an active catalyst for the gas-phase dehydration of glycerol[J].Catal Commun,2012,19(0):105-109.
[15] Qingbo Liu, Zhen Zhang, Ying Du,etal. Rare earth pyrophosphates:Effective catalysts for the production of acrolein from vapor-phase dehydration of glycerol[J].Catal Lett,2009,127(3-4):419-428.
[16] Wang Feng, Dubois J L, Ueda W. Catalytic dehydration of glycerol over vanadium phosphate oxides in the presence of molecular oxygen[J].J Catal,2009,268(2):260-267.

[18] Kraleva E, Palcheva R, Dimitrov L,etal. Solid acid catalysts for dehydration of glycerol to acrolein in gas phase[J].J Mat Sci,2011,46(22):7160-7168.
[19] Song-Hai Chai, Hao-Peng Wang, Yu Liang,etal. Sustainable production of acrolein:Investigation of solid acid-base catalysts for gas-phase dehydration of glycerol[J].Green Chem,2007,9(10):1130-1136.
[20] Benitez V M, Querini C A, Figoli N S,etal. Skeletal isomerization of 1-butene on WOx/γ-Al2O3[J].App Catal A- Gen,1999,178(2):205-218.
