卡氏乳香中1个新的降二萜
2023-09-13徐莲莲陈志超何梦丽付剑江陈芳有罗永明
徐莲莲,陈志超,何梦丽,杨 宝,付剑江,陈芳有,罗永明
•化学成分 •
卡氏乳香中1个新的降二萜
徐莲莲,陈志超,何梦丽,杨 宝,付剑江,陈芳有*,罗永明*
江西中医药大学药学院,江西 南昌 330004
研究卡氏乳香中的化学成分及其抗骨质疏松活性。运用硅胶柱色谱、ODS柱色谱和制备高效液相色谱等方法进行化学成分分离,通过波谱数据及文献对比对其进行结构解析。卡氏乳香的95%乙醇提取物中分离得到11个化合物,分别鉴定为 (1,2,8,9)-1,9-环氧-8-乙酰氧基-4,5-二氧代降西松烷型-2-烯(1)、(1,3,7,11,12)-1,12-epoxy-4-methylenecembr-7-ene-3,11-diol(2)、boscartin F(3)、boscartene K(4)、fidmansumbin-13(17)-en-3,16-diene(5)、3α-acetoxyl-7-oxo-tirucalla-8,24-dien-21-oic acid(6)、boscartene N(7)、3β-hydroxy-tirucallic acid(8)、α-boswellic acid(9)、boscarterol O(10)、boscarterol F(11)。化合物1是新化合物,命名为卡氏乳香素,化合物5为首次从乳香中分离得到,化合物2和10首次从该属植物中分离得到。对11个化合物进行抗骨质疏松活性筛选,发现化合物1、3、6和10具有良好的抑制破骨细胞活性。
卡氏乳香;萜类化合物;降二萜;(1,2,8,9)-1,9-环氧-8-乙酰氧基-4,5-二氧代西松烷型-2-烯;卡氏乳香素
乳香是橄榄科植物乳香树Birdw.及同属植物Birdw.树皮渗出的胶状树脂,具有活血定痛、消肿生肌的功效[1]。大量的临床试验结果表明,乳香具有较好的抗炎活性,主要用于治疗类风湿性关节炎、溃疡性结肠炎和骨关节炎[2-3]。乳香属大约包含25种,其中卡氏乳香.Birdw.受到研究人员广泛的关注,研究表明其主要化学成分为三萜类和二萜类[4]。早期文献研究主要集中于乳香酸等三萜类成分,近年来从中报道了一些结构新颖、活性多样的西松烷型二萜,但研究尚不深入[5]。本研究对卡氏乳香95%乙醇提取物的化学成分进行研究,从中分离鉴定出11个化合物,分别为 (1,2,8,9)-1,9-环氧-8-乙酰氧基-4,5-二氧代西松烷型-2-烯[(1,2,8,9)-1,9-epoxy-8-acetoxy-4,5-dioxo-2-cembranene,1]、(1,3,7,11,12)-1,12-epoxy-4- methylenecembr-7-ene-3,11-diol(2)、boscartin F(3)、boscartene K(4)、fidmansumbin-13(17)-en- 3,16-diene(5)、3α-acetoxyl-7-oxo-tirucalla-8,24- dien-21-oic acid(6)、boscartene N(7)、3β-hydroxy- tirucallic acid(8)、α-boswellic acid(9)、boscarterol O(10)、boscarterol F(11)。其中化合物1是新化合物,命名为卡氏乳香素,化合物5为首次从乳香中分离得到,化合物2和10首次从该属植物中分离得到。
1 仪器与材料
Bruker Avance Ⅲ HD 600 MHz型核磁共振波谱仪(Bruker公司,瑞士);AB SCIEX Triple ESI 5600+型高分辨飞行时间质谱联用仪(AB SCIEX公司,美国);Jasco J-1500型圆二色谱仪(Jasco公司,日本);Rulph Autopol IV自动旋光仪(Rudolph Research Analytical公司,美国);Agilent 1260型高效液相色谱仪(Agilent公司,美国);FTIR-650红外光谱仪(天津港东公司);Waters 515 制备型液相色谱仪(Waters公司,美国);C18半制备色谱分析柱(250 mm×10 mm,5 μm,YMC,日本);柱色谱硅胶(200~300目)和薄层色谱硅胶GF254(青岛海洋化工厂);色谱纯甲醇(西陇科学股份有限公司);纯净水(杭州娃哈哈集团有限公司);分析纯石油醚(PE)、二氯甲烷、醋酸乙酯和甲醇(西陇科学股份有限公司)。
卡氏乳香药材(批号20190401)于2019年10月购自于江西继中堂健康科技有限公司,经南昌大学第一附属医院周健副主任药师鉴定为卡氏乳香树Birdw.树皮渗出的胶状树脂。样品(20191020)保存于江西中医药大学药学院中药化学教研室。
2 提取与分离
卡氏乳香树脂10 kg,粉碎后用95%乙醇冷浸提取3次,合并提取液减压浓缩,得到总浸膏6.7 kg,所得浸膏经硅藻土柱色谱依次用石油醚、二氯甲烷、醋酸乙酯和甲醇进行分离,回收有机溶剂,分别得到石油醚部位2.9 kg、二氯甲烷部位1.8 kg、醋酸乙酯部位850.0 g和甲醇部位200.0 g。二氯甲烷部位浸膏经PRP 512A树脂洗脱分离(30%、50%、70%、95%乙醇-水溶液)得到4个部分。
取70%乙醇组分(400.0 g)经硅胶柱色谱分离,以石油醚-醋酸乙酯(30∶1→0∶1)梯度洗脱得到7个组分(Fr. 1~7),其中Fr. 4(184.0 g)经减压柱色谱以二氯甲烷-甲醇(100∶1→0∶1)得到6个组分(Fr. 4-1~4-6)。Fr. 4-1(50.0 g)经MCI柱色谱洗脱(50%→100%甲醇-水溶液)得到6个组分(Fr. 4-1-1~4-1-6),其中Fr. 4-1-6(17.0 g)进行反复硅胶柱色谱分离得到化合物6(600.0 mg)、8(202.0 mg)、9(29.1 mg)和11(33.0 mg),Fr. 4-1-5(30.0 g)经反复硅胶柱色谱和制备液相色谱(80%甲醇-水溶液,体积流量2 mL/min)分离纯化得到化合物4(7.0 mg,R=1.2 min)、5(13.1 mg,R=1.5 min)和7(6.2 mg,R=1.8 min)。对Fr. 4-3进行ODS柱色谱(洗脱梯度为60%~100%的甲醇-水溶液)得到5个组分(Fr. 4-3-1~4-3-5),其中Fr. 4-3-1经反复硅胶柱色谱和制备液相柱色谱(65%甲醇-水溶液,体积流量2 mL/min)分离得到化合物1(5.6 mg,R=2.0 min)、2(11.1 mg,R=2.1 min)和3(9.6 mg,R=1.8 min)。Fr. 4-6(14.0 g)经ODS柱色谱(60%→100%甲醇-水溶液)进行梯度洗脱得到5个组分(Fr. 4-6-1~4-6-5),其中Fr. 4-6-1经制备液相柱色谱(60%甲醇-水溶液,体积流量2 mL/min)得到化合物10(12.2 mg,R=2.2 min)。
3 结构鉴定
通过1H-1H COSY谱和HMBC(图1)可以对以上做进一步证实。1H-1H COSY谱显示H-2/H-3、H-6/H-7/H-8、H-10/H-11和H-12/H3-13/H3-14相关信号。在HMBC谱中,H-2 (H6.75) 与C-1、C-3、C-4和C-11相关,且H-3 (H6.24) 与C-1、C-2、C-4和C-15相关,H-15 (H2.30) 与C-3和C-4相关,由此确定C-2、C-3、C-4和C-15为α, β不饱和酮部分;H-12 (H1.79) 与C-1、C-2、C-11、C-13和C-14相关,H-17 (H1.20) 与C-8、C-9和C-10相关,结合C-1和C-9的化学位移可以确定C-1和C-9之间连有1个五元氧环[6];H-16 (H2.14)与C-5和C-6相关,H-6a (H2.49) 与C-5和C-7相关,可以确定C-5为酮羰基部分;H-13 (H0.91) 和C-1、C-12和C-14相关, H-14 (H0.87) 与C-1、C-12和C-13相关,H-12 (H1.79) 与C-1、C-12、C-13相关,可以确定C-1上连有1个异丙基结构。
在NOESY谱中,观察到H-17与H8/H12的相关信号,表明H-8/H-12/H-17位于同一平面内。化合物1的ECD图谱在234 nm (= −4.70) 处呈现负的Cotton效应,化合物1的计算ECD和实验ECD图谱基本一致(图2),确定该化合物的绝对构型为1,8,9,其结构如图1所示。通过以上信息推测化合物1的结构为 (1,2,8,9)-1,9-环氧-8-乙酰氧基-4,5-二氧代西松烷型-2-烯,命名为卡氏乳香素。波谱数据归属见表1。
化合物2:白色针状结晶(甲醇);HR-ESI-MS/323.255 0 [M+H]+(C20H35O3计算值为323.258 6)。1H-NMR (600 MHz, CDCl3): 5.33 (1H, t,= 7.5 Hz, H-7), 4.99 (1H, s, H-18b), 4.89 (1H, s, H-18a), 4.28 (1H, d,= 9.9 Hz, H-3), 3.38 (1H, d,= 9.5 Hz, H-11), 2.37 (1H, ddd,= 13.1, 9.9, 3.1 Hz, H-5a), 2.28 (1H, m, H-15), 2.23 (1H, ddd,=10.0, 7.3, 3.1 Hz, H-6a), 2.17 (1H, m, H-9a), 2.08 (1H, m, H-5b), 2.04 (1H, m, H-9b), 2.02 (1H, m, H-14a), 2.00 (1H, m, H-13a), 1.91 (1H, m, H-10a), 1.78 (1H, m, H-13b), 1.72 (1H, dd,= 14.6, 9.9 Hz, H-2a), 1.67 (3H, s, H-19), 1.61 (1H, dd,= 14.6, 1.5 Hz, H-2b), 1.50 (1H, m, H-14b), 1.46 (1H, m, H-6b), 1.42 (1H, m, 10a), 1.12 (3H, s, H-20), 0.90 (3H, d,= 2.5 Hz, H-16), 0.89 (3H, d,= 2.6 Hz, H-17);13C-NMR (150 MHz, CDCl3): 88.9 (C-1), 40.6 (C-2), 70.0 (C-3), 155.1 (C-4), 34.1 (C-5), 29.9 (C-6), 126.5 (C-7), 135.0 (C-8), 33.4 (C-9), 31.2 (C-10), 76.2 (C-11), 85.1 (C-12), 36.2 (C-13), 31.8 (C-14), 33.4 (C-15), 18.2 (C-16), 17.4 (C-17), 110.0 (C-18), 18.6 (C-19), 20.2 (C-20)。以上数据与文献对照基本一致[7]。对化合物2进行X-射线单晶衍射分析,确定其绝对构型,见图3。晶体数据:分子式为C20H34O3,=323.499,斜方晶系,晶胞参数=0.646 71 (3) nm,=1.912 80 (9) nm,=0.790 44 (3) nm,=0. 947 83 (7) nm3,=2,=293(2),Kμ (Cu K)=0.577 mm−1,空间集群为P212121(No. 19), 收集衍射点数为7629,独立衍射点数为3193,int=0.044 4,sigma=0.054 1,1=0.059 5,2=0.146 3,Flack常数为−0.1 (2)。因此,化合物1确定为(13,7,11,12)-1,12-epoxy-4- methylenecembr-7-ene-3,11-diol。
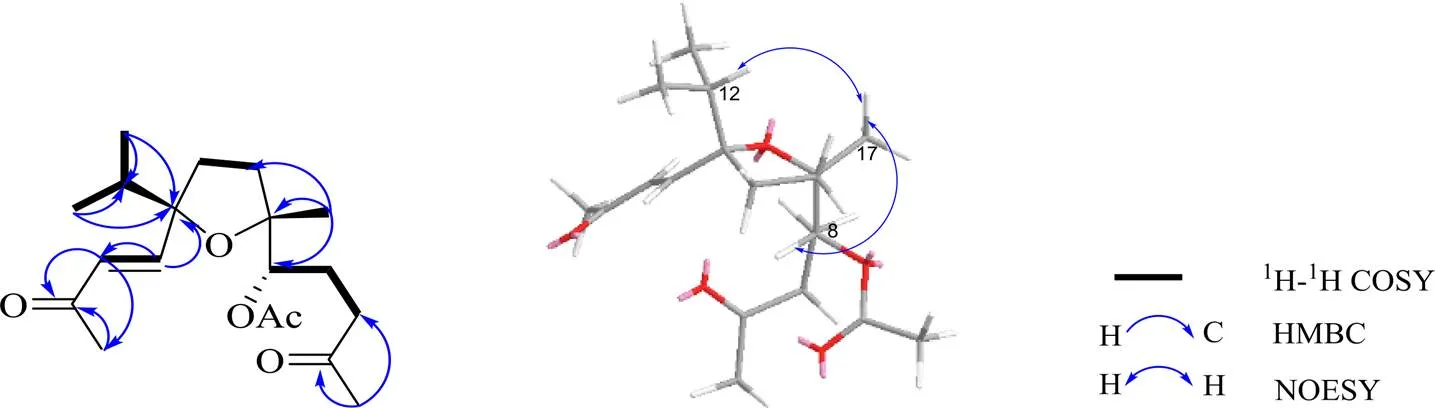
图1 化合物1的主要1H-1H COSY、HMBC和NOESY相关
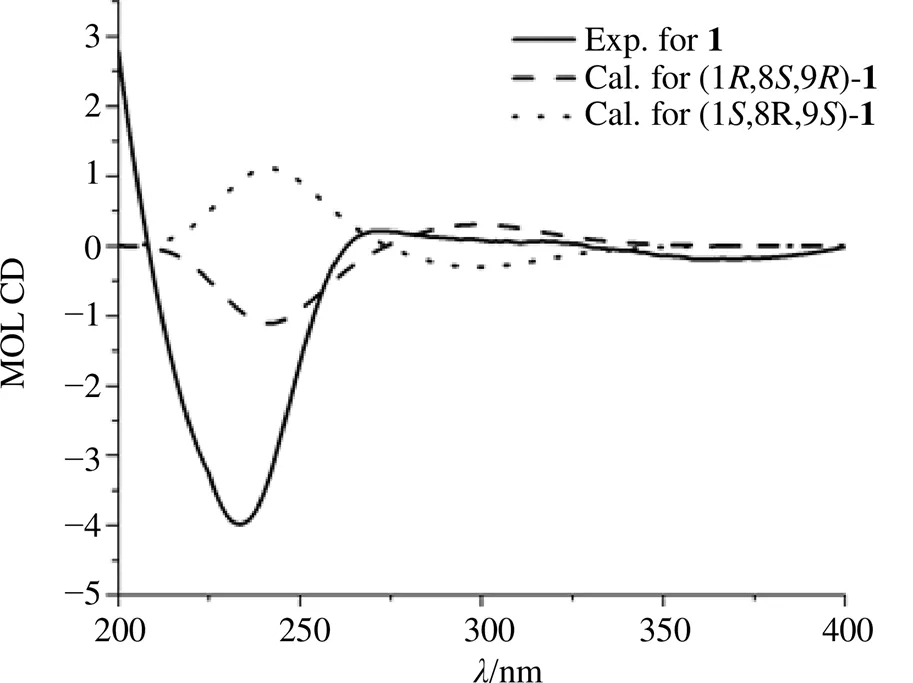
图2 化合物1的实验和计算ECD图
表1 化合物1的1H-NMR和13C-NMR数据(600/150MHz, CDCl3)
Table1 1H-NMR and 13C-NMR data of compound 1 (600/150MHz, CDCl3)
碳位δHδc 1 89.2 26.75 (1H, d, J = 16.0 Hz)149.9 36.24 (1H, d, J = 16.0 Hz)129.3 4 198.7 5 208.0 62.41 (1H, m), 2.49 (1H, m)40.2 71.72 (1H, m), 1.76 (1H, m)24.2 84.79 (1H, dd, J = 10.5, 2.6 Hz)77.9 9 84.6 101.65 (1H, m), 1.76 (1H, m)35.0 111.94 (1H, m), 2.00 (1H, m)33.2 121.79 (1H, dd, J = 6.8, 7.0 Hz)38.3 130.91 (1H, d, J = 7.0 Hz)17.8 140.87 (1H, d, J = 6.8 Hz)18.4 152.30 (3H, s)28.2 162.14 (3H, s)30.2 171.20 (3H, s)22.4 18 171.0 192.02 (3H, s)21.3
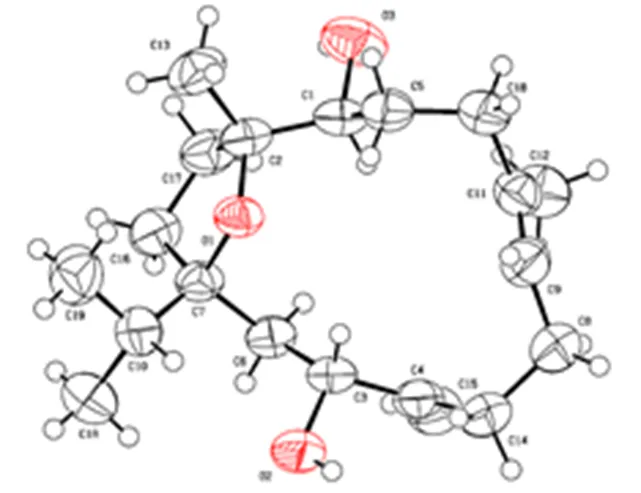
图3 化合物2的椭球图(椭球率为50%)
化合物3:白色针状结晶(甲醇);HR-ESI-MS/337.235 9 [M+H]+(C20H33O4计算值为337.237 9)。1H-NMR (600 MHz, CDCl3): 6.84 (1H, dd,= 6.3, 5.8 Hz, H-7), 3.86 (1H, d,= 12.5 Hz, H-10a), 3.53 (1H, d,= 10.4 Hz, H-11), 3.14 (1H, dd,= 4.7, 2.3 Hz, H-3), 2.46 (1H, m, H-6a), 2.46 (1H, m, H-6b), 2.27 (1H, m, H-5a), 2.21 (1H, sept,= 6.8 Hz, H-15), 2.11 (1H, m, H-13a), 2.05 (1H, dd,= 12.5, 10.4 Hz, H-10b), 2.02 (1H, m, H-14a), 1.87 (1H, m, H-13b), 1.82 (1H, dd,= 15.8, 2.3 Hz, H-2a), 1.78 (3H, s, H-19), 1.62 (1H, dd,= 15.8, 4.7 Hz, H-2b), 1.48 (1H, m, H-14b), 1.36 (1H, m, H-5b), 1.14 (3H, s, H-20), 1.13 (3H, s, H-18), 0.98 (3H, d,= 6.9 Hz, H-17), 0.92 (3H, d,= 6.9 Hz, H-16);13C-NMR (150 MHz, CDCl3): 89.0 (C-1), 37.4 (C-2), 60.8 (C-3), 58.7 (C-4), 37.9 (C-5), 25.6 (C-6), 144.5 (C-7), 137.2(C-8), 203.5 (C-9), 40.9 (C-10), 78.3 (C-11), 84.9 (C-12), 36.8 (C-13), 30.5 (C-14), 33.0 (C-15), 18.8 (C-16), 17.0 (C-17), 16.3 (C-18), 11.4 (C-19), 19.8 (C-20)。以上数据与文献对照基本一致[8],故鉴定该化合物为boscartin F。
化合物4:白色针状结晶(甲醇);HR-ESI-MS/373.272 9 [M+H]+(C24H37O3计算值为373.274 3)。1H-NMR (600 MHz, CDCl3): 5.80 (1H, d,= 1.3 Hz, H-17), 4.68 (1H, t,= 2.6 Hz, H-3), 2.71 (1H, dd,= 14.2, 4.7 Hz, H-12a), 2.56 (1H, d,= 19.1 Hz, H-15a), 2.42 (1H, td,= 13.6, 6.8 Hz, H-12b), 2.10 (3H, s, CH3CO-), 1.94 (1H, m, H-2a), 1.88 (1H, d,= 19.1 Hz, H-15b), 1.84 (1H, m, H-11a), 1.70 (1H, m, H-9), 1.67 (1H, m, H-7a), 1.64 (1H, m, H-2b), 1.54 (1H, m, H-1a), 1.53 (1H, m, H-11b), 1.48 (1H, m, H-6a), 1.40 (1H, m, H-6b), 1.35 (3H, s, H-22), 1.31 (1H, m, H-7b), 1.28 (1H, m, H-1a), 1.28 (1H, m, H-5), 0.89 (3H, s, H-20), 0.88 (3H, s, H-19), 0.87 (3H, s, H-21), 0.80 (3H, s, H-18);13C-NMR (150 MHz, CDCl3): 34.4 (C-1), 23.1 (C-2), 78.1 (C-3), 36.9 (C-4), 50.8 (C-5), 18.2 (C-6), 35.5 (C-7), 41.5 (C-8), 50.2 (C-9), 37.7 (C-10), 22.1 (C-11), 27.5 (C-12), 190.6 (C-13), 51.6 (C-14), 46.2 (C-15), 209.5 (C-16), 126.6 (C-17), 16.1 (C-18), 16.3 (C-19), 22.0 (C-20), 28.0 (C-21), 22.6 (C-22), 170.9 (CH3CO-), 21.6 (CH3CO-)。以上数据与文献对照基本一致[9],故鉴定该化合物为boscartene K。
化合物5:黄色油状物;HR-ESI-MS/329.248 1 [M+H]+(C22H33O2计算值为329.248 1)。1H-NMR (600 MHz, CDCl3): 5.79 (1H, s, H-17), 2.71 (1H, ddd,= 14.1, 5.0, 1.9 Hz, H-12a),2.53 (1H, d,= 19.1 Hz, H-15a), 2.49 (1H, m, H-2a), 2.49 (1H, m, H-2b), 2.43 (1H, m, H-12b), 1.99 (1H, m, H-1a), 1.88 (1H, d,= 19.1 Hz, H-15b), 1.80 (1H, m, H-11a), 1.66 (1H, dd,= 12.7, 3.1 Hz, H-9), 1.66 (1H, m, H-6a), 1.55 (1H, m, H-7a), 1.51 (1H, m, H-1b), 1.46 (1H, m, H-6b), 1.42 (1H, m, H-7b), 1.42 (1H, m, H-11b), 1.31 (3H, s, H-22), 1.27 (1H, m, H-5), 1.11 (3H, s, H-20), 1.05 (3H, s, H-21), 0.96 (3H, s, H-19), 0.84 (3H, d,= 0.8 Hz, H-18);13C-NMR (150 MHz, CDCl3): 39.8 (C-1), 34.1 (C-2), 217.5 (C-3), 47.4 (C-4), 55.2 (C-5), 19.7 (C-6), 50.1 (C-7), 41.1 (C-8), 51.1 (C-9), 37.3 (C-10), 22.4 (C-11), 27.5 (C-12), 187.4 (C-13), 51.5 (C-14), 46.1 (C-15), 209.2 (C-16), 126.9 (C-17), 15.9 (C-18), 16.4 (C-19), 26.9 (C-20), 21.0 (C-21), 22.7 (C-22)。以上数据与文献对照基本一致[10],故鉴定该化合物为fidmansumbin-13(17)-en- 3,16-diene。
化合物6:白色粉末;HR-ESI-MS/513.357 4 [M+H]+(C32H49O5计算值为513.358 0)。1H-NMR (600 MHz, DMSO-6): 11.60 (1H, s, COOH), 5.09 (1H, t,= 6.4 Hz, H-24), 4.57 (1H, s, H-3), 2.39 (1H, m, H-2a), 2.36 (1H, m, H-2b), 2.34 (1H, m, H-6b), 2.32 (1H, m, H-6a), 2.27 (1H, m, H-11a), 2.22 (1H, m, H-11b), 2.18 (1H, m, H-16b), 2.10 (1H, m, H-5), 2.03 (1H, m, H-17), 2.02 (3H, s, CH3CO-), 2.00 (1H, m, H-15a), 1.94 (1H, m, H-16a), 1.94 (1H, m, H-23a), 1.90 (1H, m, H-23b), 1.72 (1H, m, H-1a), 1.59 (3H, s, H-26), 1.54 (3H, s, H-27), 1.45 (1H, m, H-12a), 1.45 (1H, m, H-15b), 1.22 (1H, m, H-22a), 1.22 (1H, m, H-22b), 1.17 (1H, m, H-1b), 1.12 (1H, m, H-12b), 1.06 (1H, m, H-16b), 1.03 (3H, s, H-19), 0.97 (3H, s, H-29), 0.96 (3H, s, H-28), 0.89 (3H, s, H-30), 0.80 (3H, s, H-18);13C-NMR (150 MHz, DMSO-6): 32.2 (C-1), 22.4 (C-2), 76.1 (C-3), 36.2 (C-4), 47.3 (C-5), 34.9 (C-6), 196.6 (C-7), 137.5 (C-8), 165.6 (C-9), 38.7 (C-10), 21.1 (C-11), 27.6 (C-12), 43.1 (C-13), 43.9 (C-14), 29.0 (C-15), 26.9 (C-16), 46.8 (C-17), 15.7 (C-18), 17.9 (C-19), 45.4 (C-20), 177.1 (C-21), 31.0 (C-22), 25.5 (C-23), 123.8 (C-24), 131.2 (C-25), 22.7 (C-26), 17.5 (C-27), 25.5 (C-28), 26.6 (C-29), 24.1 (C-30), 169.9 (CH3CO-), 20.9 (CH3CO-)。以上数据与文献对照基本一致[11],故鉴定该化合物为3α- acetoxyl-7-oxo-tirucalla-8,24- dien-21-oic acid。
化合物7:白色粉末;HR-ESI-MS/467.313 3 [M+H]+(C30H43O4计算值为467.316 7)。1H-NMR (600 MHz, CDCl3): 5.22 (1H, m, H-24), 5.20 (1H, m, H-23), 2.78 (1H, ddd,= 15.4, 13.8, 6.0 Hz, H-2), 2.68 (1H, td,= 8.9, 4.9 Hz, H-20), 2.49 (1H, d,= 13.9 Hz, H-6a), 2.43 (1H, m, H-6b), 2.41 (1H, m, H-2b), 2.40 (1H, m, H-11), 2.38 (1H, m, H-22a), 2.29 (1H, m, H-17), 2.23 (1H, m, H-15a), 2.18 (1H, m, H-1a), 2.13 (1H, dd,= 13.8, 4.3 Hz, H-5), 2.04 (1H, m, H-12a), 1.99 (1H, m, H-16a), 1.78 (1H, m, H-1b), 1.78 (1H, m, H-12b), 1.76 (3H, s, H-26), 1.75 (3H, s, H-27), 1.66 (1H, m, H-15b), 1.46 (1H, m, H-16b), 1.29 (3H, s, H-19), 1.13 (3H, s, H-29), 1.09 (3H, s, H-28), 1.03 (3H, s, H-30), 0.83 (3H, s, H-18);13C-NMR (150 MHz, CDCl3): 35.5 (C-1), 34.6 (C-2), 214.5 (C-3), 47.5 (C-4), 49.5 (C-5), 36.3 (C-6), 197.5 (C-7), 139.1 (C-8), 164.7 (C-9), 39.3 (C-10), 24.1 (C-11), 27.7 (C-12), 47.5 (C-13), 44.9 (C-14), 31.3 (C-15), 24.7 (C-16), 43.3 (C-17), 16.7 (C-18), 18.2 (C-19), 41.8 (C-20), 178.9 (C-21), 35.0 (C-22), 75.3 (C-23), 123.2 (C-24), 139.1 (C-25), 25.9 (C-26), 18.6 (C-27), 24.1 (C-28), 21.6 (C-29), 24.7 (C-30)。以上数据与文献对照基本一致[12],故鉴定该化合物为boscartene N。
化合物8:白色粉末,HR-ESI-MS/457.367 8 [M+H]+(C30H49O3计算值为457.368 2)。1H-NMR (600 MHz, CDCl3): 5.09 (1H, m, H-24), 3.23 (1H, dd,= 11.7, 4.5 Hz, H-3), 2.28 (1H, m, H-17), 2.08 (1H, m, H-20), 2.08 (1H, m, H-11a), 2.05 (1H, m, H-23a), 1.98 (1H, m, H-11b), 1.95 (1H, m, H-24b), 1.76 (1H, m, H-12a), 1.74 (1H, m, H-12b), 1.70 (1H, m, H-2a), 1.68 (3H, s, H-26), 1.66 (1H, m, H-1b), 1.58 (3H, s, H-27), 1.56 (2H, m, H-22), 1.56 (2H, m, H-7), 1.53 (1H, m, H-6a), 1.41 (1H, dd,= 12.4, 6.2 Hz, H-2b), 1.35 (2H, m, H-16), 1.35 (1H, m, H-6b), 1.25 (1H, m, H-15a), 1.22 (1H, m, H-15b), 1.18 (1H, m, H-1a), 1.12 (1H, dd,= 12.4, 1.9 Hz, H-5), 1.00 (3H, s, H-29), 0.93 (3H, s, H-17), 0.88 (3H, s, H-30), 0.82 (3H, s, H-28), 0.74 (3H, s, H-18);13C-NMR (150 MHz, CDCl3): 35.4 (C-1), 27.7 (C-2), 79.1 (C-3), 39.1 (C-4), 51.1 (C-5), 19.0 (C-6), 29.0 (C-7) , 134.2 (C-8), 132.4 (C-9), 37.4 (C-10), 26.1 (C-11), 32.6 (C-12), 44.0 (C-13), 49.7 (C-14), 29.5 (C-15), 28.0 (C-16), 47.1 (C-17), 20.2 (C-18), 15.7 (C-19), 47.6 (C-20), 182.3 (C-21), 21.7 (C-22), 27.0 (C-23), 123.7 (C-24), 133.3 (C-25), 24.6 (C-26), 17.8 (C-27), 28.2 (C-28), 15.9 (C-29), 25.8 (C-30)。以上数据与文献对照基本一致[13],故鉴定该化合物为3βhydroxy-tirucallic acid。
化合物9:白色粉末,HR-ESI-MS/457.367 8 [M+H]+(C30H49O3计算值为457.368 2)。1H-NMR (600 MHz, CDCl3): 5.19 (1H, t,= 3.6 Hz, H-12), 4.08 (1H, t,= 2.8 Hz, H-3), 2.21 (1H, m, H-11a), 1.99 (1H, m, H-11b), 1.95 (1H, dd,= 14.0, 4.8 Hz, H-18), 1.89 (1H, m, H-21), 1.88 (1H, m, H-22), 1.77 (1H, m, H-21b), 1.71 (1H, m, H-2a), 1.68 (1H, m, H-22b), 1.64 (1H, m, H-6a), 1.59 (1H, m, H-1a), 1.52 (1H, m, H-7a), 1.49 (1H, m, H-2b), 1.44 (1H, m, H-19a), 1.43 (1H, m, H-7b), 1.40 (1H, m, H-6b), 1.37 (1H, m, H-5), 1.35 (3H, s, H-23), 1.32 (1H, m, H-1b), 1.27 (1H, m, H-15a), 1.23 (1H, m, H-16a), 1.21 (1H, m, H-19b), 1.15 (3H, s, H-27), 1.10 (1H, m, H-15b), 1.03 (1H, m, H-9), 1.00 (3H, s, H-26), 0.98 (1H, m, H-16b), 0.88 (3H, s, H-25), 0.87 (3H, s, H-29), 0.87 (3H, s, H-30), 0.83 (3H, s, H-28);13C-NMR (150 MHz, CDCl3): 33.5 (C-1), 26.1 (C-2), 70.6 (C-3), 47.3 (C-4), 49.0 (C-5), 19.6 (C-6), 32.7 (C-7), 39.7 (C-8), 46.7 (C-9), 37.5 (C-10), 23.6 (C-11), 121.6 (C-12), 145.0 (C-13), 41.8 (C-14), 26.1 (C-15), 26.8 (C-16), 32.7 (C-17), 47.3 (C-18), 46.7 (C-19), 31.0 (C-20), 34.6 (C-21), 37.5 (C-22), 24.0 (C-23), 183.2 (C-24), 13.0 (C-25), 16.6 (C-26), 26.1 (C-27), 28.3 (C-28), 33.5 (C-29), 23.6 (C-30)。以上数据与文献对照基本一致[14],故鉴定该化合物为α-boswellic acid。
化合物10:黄色油状物,HR-ESI-MS/339.190 9 [M+Na]+(C20H28NaO3计算值为339.193 6)。1H-NMR (600 MHz, CDCl3): 7.1 (1H, m, H-17), 4.82 (1H, d,= 2.1 Hz, H-16), 4.71 (1H, s, H-12b), 4.69 (1H, s, H-12a), 2.45 (1H, dd,= 13.3, 6.3 Hz, H-9a), 2.27 (1H, td,= 9.6, 6.6 Hz, H-1), 2.16 (1H, m, H-9b), 2.04 (1H, m, H-8a), 1.91 (1H, m, H-2a), 1.77 (3H, s, H-20), 1.75 (1H, m, H-3a), 1.68 (1H, m, H-3b), 1.65 (1H, m, H-2b), 1.65 (1H, m, H-15), 1.37 (1H, d,= 10.8 Hz, H-5), 1.34 (3H, s, H-11), 1.29 (1H, m, H-7), 1.07 (1H, m, H-8b), 1.04 (1H, m, H-6), 0.93 (3H, s, H-14);13C-NMR (150 MHz, CDCl3): 51.7 (C-1), 25.9 (C-2), 41.6 (C-3), 81.2 (C-4), 53.3 (C-5), 25.9 (C-6), 24.4 (C-7), 25.0 (C-8), 38.7 (C-9), 153.6 (C-10), 25.2 (C-11), 106.7 (C-12), 23.3 (C-13), 12.0 (C-14), 65.8 (C-15), 72.5 (C-16), 155.6 (C-17), 143.6 (C-18), 207.1 (C-19), 10.2 (C-20)。以上数据与文献对照基本一致[15],故鉴定该化合物为boscarterol O。
化合物11:白色油状物,HR-ESI-MS/301.212 1 [M+H]+(C20H29O2计算值为301.216 8)。1H-NMR (600 MHz, CDCl3): 9.39 (1H, s, H-19), 6.81 (1H, d,= 11.2 Hz, H-15), 6.46 (1H, dd,=15.1, 11.2 Hz, H-16), 5.82 (1H, d,= 15.1 Hz, H-17), 4.75 (1H, s, H-12a), 4.73 (1H, s, H-12b), 2.48 (1H, dd,= 13.6, 6.2 Hz, H-9a), 2.26 (1H, td,= 10.6, 6.1 Hz, H-1), 2.07 (1H, t,= 1.8 Hz, H-9b), 1.95 (1H, dq,= 11.6, 6.4 Hz, H-2a), 1.95 (1H, m, H-8a), 1.85 (3H, s, H-20), 1.79 (1H, td,= 13.1, 6.3, 2.4 Hz, H-3a), 1.67 (1H, m, H-2b), 1.61 (1H, td,= 12.8, 11.5, 6.2 Hz, H-3b), 1.52 (1H, t,= 10.8 Hz, H-5), 1.29 (3H, s, H-14), 1.26 (1H, m, H-7), 1.24 (3H, s, H-11), 1.20 (1H, m, H-8b), 1.03 (1H, t,= 11.2, 9.7 Hz, H-6);13C-NMR (150 MHz, CDCl3): 52.9 (C-1), 26.8 (C-2), 42.1 (C-3), 80.9 (C-4), 53.5 (C-5), 33.0 (C-6), 30.7 (C-7), 24.4 (C-8), 38.3 (C-9), 152.6 (C-10), 26.3 (C-11), 107.4 (C-12), 28.4 (C-13), 12.3 (C-14), 156.0 (C-15), 120.3 (C-16), 150.1 (C-17), 135.1 (C-18), 195.1 (C-19), 9.6 (C-20)。以上数据与文献对照基本一致[16],故鉴定该化合物为boscarterol F。
4 抗骨质疏松活性筛选
本实验对卡氏乳香中分离得到的11个化合物进行了抗骨质疏松活性筛选,采用体外核因子-κB配体受体致活剂(receptor activator of nuclear factor kappa-B ligand,RANKL)诱导小鼠骨髓原代巨噬细胞(bone marrow-derived macrophages,BMMs)形成成熟破骨细胞模型,进行抗骨质疏松活性评估。将细胞接种于96孔板中,接种12 h,整板更换为新的α-MEM完全培养基,实验设对照组、模型组和给药组(浓度分别为1、10 μmol/L)。对照组加入巨噬细胞集落刺激因子(macrophage colony-stimulating factor,MCSF)的原代小鼠巨噬细胞,模型组加入诱导因子MCSF以及RANKL的小鼠原代巨噬细胞,给药组在模型组的基础上分别加入1、10 μmol/L化合物溶液。每48小时换药1次,直至模型组出现明显的细胞融合为止,即得破骨细胞,弃去培养基,加入500 μL预冷的固定剂在冰上静置10 min,加入2 mL PBS 稀释固定剂,再除去PBS,加入500 μL乙醇-丙酮(50︰50)溶液,在−30~−20 ℃下培养1 min。用双蒸水洗3次,加入TRAP染色,在37 ℃培养箱内反应15~45 min至显色,在显微镜下观察,结果见图4。由此可知,化合物1、3、6和10在1 μmol/L浓度时具有一定的破骨细胞抑制活性,化合物1在10 μmol/L浓度时具有良好的破骨细胞抑制活性。
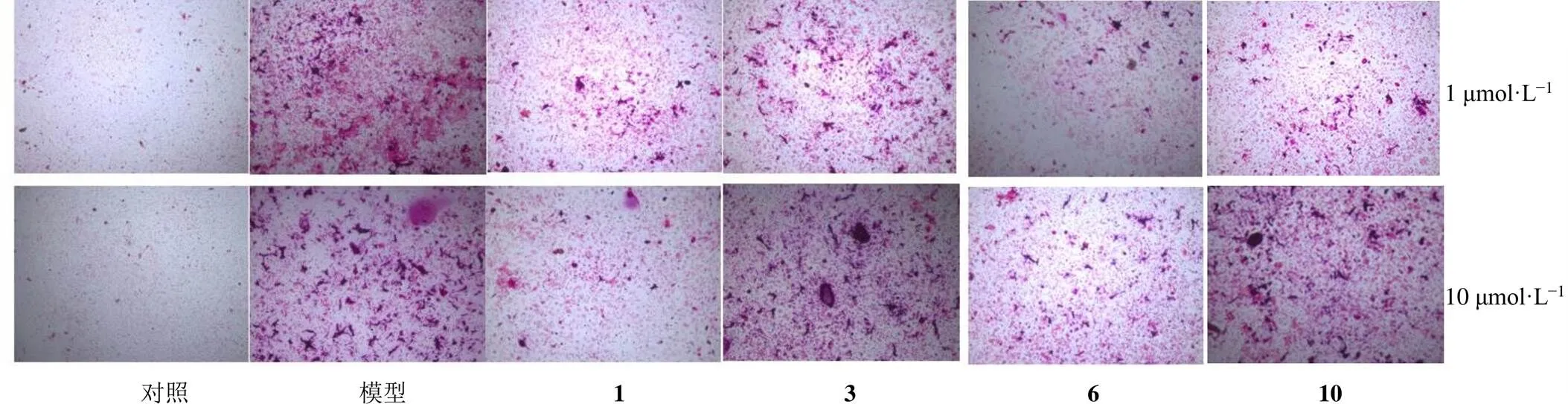
图4 化合物1、3、6和10抑制破骨细胞活性
5 讨论
本实验对乳香乙醇提取物中二氯甲烷部分进行研究,从中分离得到11个化合物,其中化合物1是新化合物,化合物5为首次从乳香中分离得到,化合物2和10为首次从该属植物中分离得到。对11个化合物进行抗骨质疏松活性筛选,结果显示化合物1、3、6和10在1 μmol/L浓度时具有一定的破骨细胞抑制活性,化合物1在10 μmol/L浓度时具有良好的破骨细胞抑制活性。本实验丰富了乳香化学成分和生物活性研究,阐释了其发挥抗骨质疏松活性的物质基础,为其进一步开发提供了依据。
利益冲突 所有作者均声明不存在利益冲突
[1] 中国药典 [S]. 一部. 2020: 233.
[2] Moussaieff A, Mechoulam R.resin: From religious ceremonies to medical uses; A review of,and clinical trials [J]., 2009, 61(10): 1281-1293.
[3] Ammon H P T. Boswellic acids in chronic inflammatory diseases [J]., 2006, 72(12): 1100-1116.
[4] Woolley C L, Suhail M M, Smith B L,. Chemical differentiation ofandessential oils by gas chromatography and chiral gas chromatography-mass spectrometry [J]., 2012, 1261: 158-163.
[5] 徐莲莲, 陈芳有, 罗永明, 等. 乳香属中西松烷型二萜类化合物研究进展 [J]. 中药材, 2023, doi: 10.13863/ j.issn1001-4454.2023.03.042.
[6] Yu Q H, Sura M B, Wang D W,. Isolation of boswelliains A—E, cembrane-type diterpenoids fromand an evaluation of their wound healing properties [J]., 2021, 39(9): 2451-2459.
[7] Greve H L, Kaiser M, Brun R,. Erratum: Terpenoids from the oleo-gum-resin ofand their antiplasmodial effects[J]., 2017, 83(14/15): E4.
[8] Ren J, Wang Y G, Wang A G,. Cembranoids from the gum resin ofas potential antiulcerative colitis agents [J]., 2015, 78(10): 2322-2331.
[9] Wang Y G, Ma Q G, Tian J,. Hepatoprotective triterpenes from the gum resin of[J]., 2016, 109: 266-273.
[10] Provan G J, Gray A I, Waterman P G. Mansumbinane derivatives from stem bark of[J]., 1992, 31(6): 2065-2068.
[11] Yu J Q, Zhao H W, Wang D J,. Extraction and purification of five terpenoids from olibanum by ultrahigh pressure technique and high-speed countercurrent chromatography [J]., 2017, 40(13): 2732-2740.
[12] Yang J B, Ren J, Wang A G. Isolation, characterization, and hepatoprotective activities of terpenes from the gum resin ofBirdw [J]., 2018, 23: 73-77.
[13] Badria F A, Mikhaeil B R, Maatooq G T,. Immunomodulatory triterpenoids from the oleogum resin ofBirdwood [J]., 2003, 58(7/8): 505-516.
[14] Culioli G, Mathe C, Archier P,. A lupane triterpene from frankincense (sp., Burseraceae) [J]., 2003, 62(4): 537-541.
[15] Wang J J, Suo X Y, Sun H R,. Prenylaromadendrane-type diterpenoids from the gum resin offlueck and their cytotoxic effects [J]., 2022, 36(21): 5400-5406.
[16] Wang Y, Ren J, Wang A,Hepatoprotective prenylaromadendrane-type diterpenes from the gum resin of[J]., 2013, 76(11): 2074-2079.
A new norditerpenoid from gum resin of
XU Lian-lian, CHEN Zhi-chao, HE Meng-li, YANG Bao, FU Jian-jiang, CHEN Fang-you, LUO Yong-ming
School of Pharmacy, Jiangxi University of Chinese Medicine, Nanchang 330004, China
To study the chemical compositions ofand their anti-osteoporotic activities.The chemical components were separated by silica gel column chromatography, ODS column chromatography and preparative HPLC, and their structures were analyzed by comparison of spectral data and literature.Eleven compounds were isolated from 95% ethanol extract ofand identified as (1,2,8,9)-1,9-epoxy-8-acetoxy-4,5-dioxo-2-cembranene (1),(1,3,7,11,12)-1,12-epoxy-4- methylenecembr-7-ene-3,11-diol (2), boscartin F (3), boscartene K (4), fidmansumbin-13(17)-en-3,16-diene (5), 3α-acetoxyl-7- oxo-tirucalla-8,24-dien-21-oic acid (6), boscartene N (7), 3β-hydroxy-tirucallic acid (8), α-boswellic acid (9), boscarterol O (10), boscarterol F (11).Compound 1 is a new compound, named boscartenoid A, compound 5 is the first time isolated from the species, and compounds 2 and 10 are the fiist time isolated from this genus. Eleven compounds were screened for anti-osteoporotic activity and compounds 1, 3, 6 and 10 were found to have good inhibitory activity against osteoclasts.
Birdw.;terpenoids; norditerpenoids; (1,2,8,9)-1,9-epoxy-8-acetoxy-4,5-dioxo-2-cembranene; boscartenoid A
R284.1
A
0253 - 2670(2023)18 - 5833 - 07
10.7501/j.issn.0253-2670.2023.18.001
2023-05-03
国家自然科学基金项目(82060697);江西中医药大学校级科技创新团队发展计划(CXTD22015);学校博士科研启动基金项目(2018BSZR003)
徐莲莲,硕士研究生在读。E-mail: xulianliana@163.com
陈芳有,副教授,从事中药物质基础研究。Tel: 13037218802 E-mail: tedchenfy@163.com
罗永明,教授,从事中药物质基础研究。Tel: 13970058758 E-mail: loym999@163.com
[责任编辑 王文倩]
