淫羊藿苷对SAMP8小鼠皮层β淀粉样蛋白代谢途径相关蛋白的影响
2017-12-29吕凌丽徐云燕石京山
吕凌丽,黄 娟,刘 静,陈 洪,徐云燕,吴 芹,石京山
(遵义医学院 基础药理教育部重点实验室暨特色民族药教育部国际合作联合实验室,贵州 遵义 563099)
基础医学研究
淫羊藿苷对SAMP8小鼠皮层β淀粉样蛋白代谢途径相关蛋白的影响
吕凌丽,黄 娟,刘 静,陈 洪,徐云燕,吴 芹,石京山
(遵义医学院 基础药理教育部重点实验室暨特色民族药教育部国际合作联合实验室,贵州 遵义 563099)
目的观察淫羊藿苷(ICA)对SAMP8小鼠皮层β淀粉样蛋白代谢途径中Aβ、APP及BACE1、PS1蛋白的影响。方法8月龄雄性SAMP8小鼠随机分为模型、ICA低剂量组(20 mg/kg)、ICA中剂量组(40 mg/kg)、ICA高剂量组(80 mg/kg),同月龄雄性SAMR1小鼠作为空白对照组,每组12只,5月龄开始灌胃,1次/d,连续给药3个月,模型SAMP8组和正常对照SAMR1组给予同等体积的蒸馏水。HE和尼氏染色观察皮层神经元形态学变化,Western blot检测各组小鼠皮层Aβ1-42、APP、PS1、BACE1蛋白水平。结果与同月龄的SAMR1小鼠相比,8月龄的SAMP8小鼠皮层正常神经元数量减少;Western blot检测到Aβ1-42、PS1蛋白在8月龄的SAMP8小鼠皮层中表达升高(P<0.05),APP、BACE1蛋白表达上升无明显差异,给予ICA后4种蛋白水平均降低。结论淫羊藿苷能够减轻神经元损伤,降低皮层Aβ1-42、APP、PS1 、BACE1蛋白表达。
淫羊藿苷;快速衰老小鼠;β淀粉样蛋白;淀粉样前体蛋白
Alzheimer's disease (AD) is a progressive neuro-degenerative disease associated with irreversible memory loss and cognitive dysfunction[1],which is characterisedby Aβ (β-Amyloid,Aβ) deposition,Tau protein phosphorylation,oxygen free radical damage and other factors.Aβ1-42is thought to be a fundamental pathogenesis in AD[2],the accumulation of Aβ triggers a series of reactions[3],such as synaptic dysfunction,synaptic loss,and neuronal death[4].APP (β-amyloidprotein precursor,APP) is a precursor protein of amyloid protein,production comes from the hydrolysis of APP by β-Secretase and γ-Secretase[5-7].Studies have shown that improving BACE1 and PS1 proteins can inhibit β-Secretase and γ-Secretase activity and reduce Aβ production[8].
Senescence accelerated mouse (SAM) were generated by Takeda et al,in 2005 at Kyoto University in Japan[9].This model includes nine major senescence-accelerated mouse-prone (SAMP) substrains and three major senescence-accelerated mouse-resistant (SAMR) substrains[9].SAMP8 mice showed aging-related learning and memory defects,brain aging,AD and other diseases[10],with life expectancy about 12 months,shorter than SAMR1 mice ( about 24 months),SAMR1 mice had physiological indicators like normal animals.The SAMP8 mouse development and occurrence of aging are very similar to dementia,especially increasing Aβ deposition with age in the brain[11].
Epimedium is a berberidaceaeplant,Icariin (ICA) is one of the main active ingredients.Modern pharmacological studies have showed that ICA regulates the immune system,the cardiovascular system,and the nervous system[12].ICA can protect the learning and memory defects of Alzheimer's disease (AD) rats by inhibiting the levels of Aβ and tau hyperphosphorylation[13].In addition,ICA improved the learning and memory of Tg-2576 mice,decreased the levels of APP and Aβ in the brain,and reduced neuronal cell activity[14],it also inhibited the expression of APP cleavage enzyme 1 (BACE1)[15].In this study,SAMP8 mice were used as AD model to study the effect of ICA on neuronal injury in SAMP8 mice and Aβ deposition in brain of SAMP8 mice with dementia.
1 Materials and Methods
1.1 Animals SAMP8 mice were purchased from Hua Fu Kang Biological Technology Company,Beijing,China(Specific pathogen-free[SPF],Certificate no.:SCXK2014-0004).SAMR1 mice purchased from Peking University Medical Department,Beijing,China(Specific pathogen-free [SPF],Certificate no.:SCXK2011-0012).Animals were housed in SPF grade animal room with free access to food and water,under standard temperature conditions (22 ± 2 ℃) and a 12h light-dark cycle.All animals studies were in compliance with Animal Care and Use Guidelines in China and were approved by Animal Use and Care Committee of Zunyi Medical University
1.2 Drugs and Chemicals ICA was obtained from Zelang Pharmaceutical Technology Company,Nanjing,China.The purity of ICA was greater or equal to 98%; BCA protein concentration assay kit was bought from JieRui Biological company,Shanghai,China; Tris,SDS and Glycine was purchased from Solaibo Technology Company,Beijing,China; Marker was purchased from ThermoFisher Scientific,Massachusetts,USA; PVDF membranes was bought from MerkKgaA,Darmstadt,Germany.
1.3 Experimental designs 8-month-old male SAMP8 mice were randomly divided into 4 groups:model and ICA-low,ICA-medium,ICA-high dose group,male SAMR1 mice were used as normal group and 12 mice in each group.SAMP8 mice were administered with ICA at the doses of 20,40 and 80 mg/(kg·d)since 5 months age,once per day for 3 months.Model of SAMP8 and normal of SAMR1 mice were given the same volume distilled water
1.4 Morphometric analysis Three mice of each group were perfused with 0.1M PBS and pre-cooled 4% paraformaldehyde (0.1M PBS configuration) through the brain perfusion,Tissues were fixed in 4% paraformaldehyde solution,embedded in paraffin and cut into 5μm continuous sections,H&E,Nissl staining,and optical microscope was used to observe the cortex histo-morphological changes.
1.5 Western blotting The cortical tissues were homogenized and collected in RIPA lysis buffer (RIPA lysis:protease inhibitor:phosphatase inhibitor=100∶1∶10).Protein was quantified by BCA protein assay and total protein of 30 μg was used.Proteins were separated in 8% SDS-PAGE,70V about for 1h,then increase voltage to 110V; after electrophoresis,bands were transferred to PVDF membranes at a constant voltage of 25V,1.0A for 30 min.The membranes were blocked with 5% nonfat milk in TBST buffer for 2h.After washing with TBST 3 × 10min (TBST∶10 mMTris-HCl,100 mM NaCl,and 0.05% Tween-20),membrane was incubated with rabbitanti-Aβ1-42(1∶1 000,Abcam,Cambridge,MA,USA; ab201060),APP (1∶1 000,Sanggon Biotech,Shanghai,China; D260097),PS1 (1∶1 000,Abcam,Cambridge,MA,USA; ab76083),BACE1 (1∶500,Sanggon Biotech,Shanghai,China; D260097),mouse anti-GAPDH (1∶2 000,Proteintech,Wuhan,China,10494-1-AP)at 4 ℃ overnight.After washing with TBST 3×10 min,membranes was incubated with secondary antibodies for 1 h,after TBST wash,blots were detected using ECL Western blotting detection kit,and scanned to BIO-RAD Gel Imaging and the results were analyzed using Quantity One software v4.52 analysis.
1.6 Statistical Analysis All statistical analyses were performed with SPSS software version 18.0,and date was expressed as mean ± SD.The results were assessed using one-way ANOVA to verify the significance between means,followed by a Least Significance Difference Method (LSD) test for chosen group comparisons.A value ofPless than 0.05 was considered statistically significant.
2 Results
2.1 Effect of ICA in cortexhistomorphology H&E staining was performed to observe the histomorphology changes of cortex tissues in mouse brain.The results showed that SAMR1 mice cortex neuronal morphology was normal; the cells were round and large,with clear nuclei.But the deformation of the most neurons of SAMP8 mice cortex,was observed,nuclear deep staining and the structure is not clear.ICA improved these pathological lesions(Fig 1).
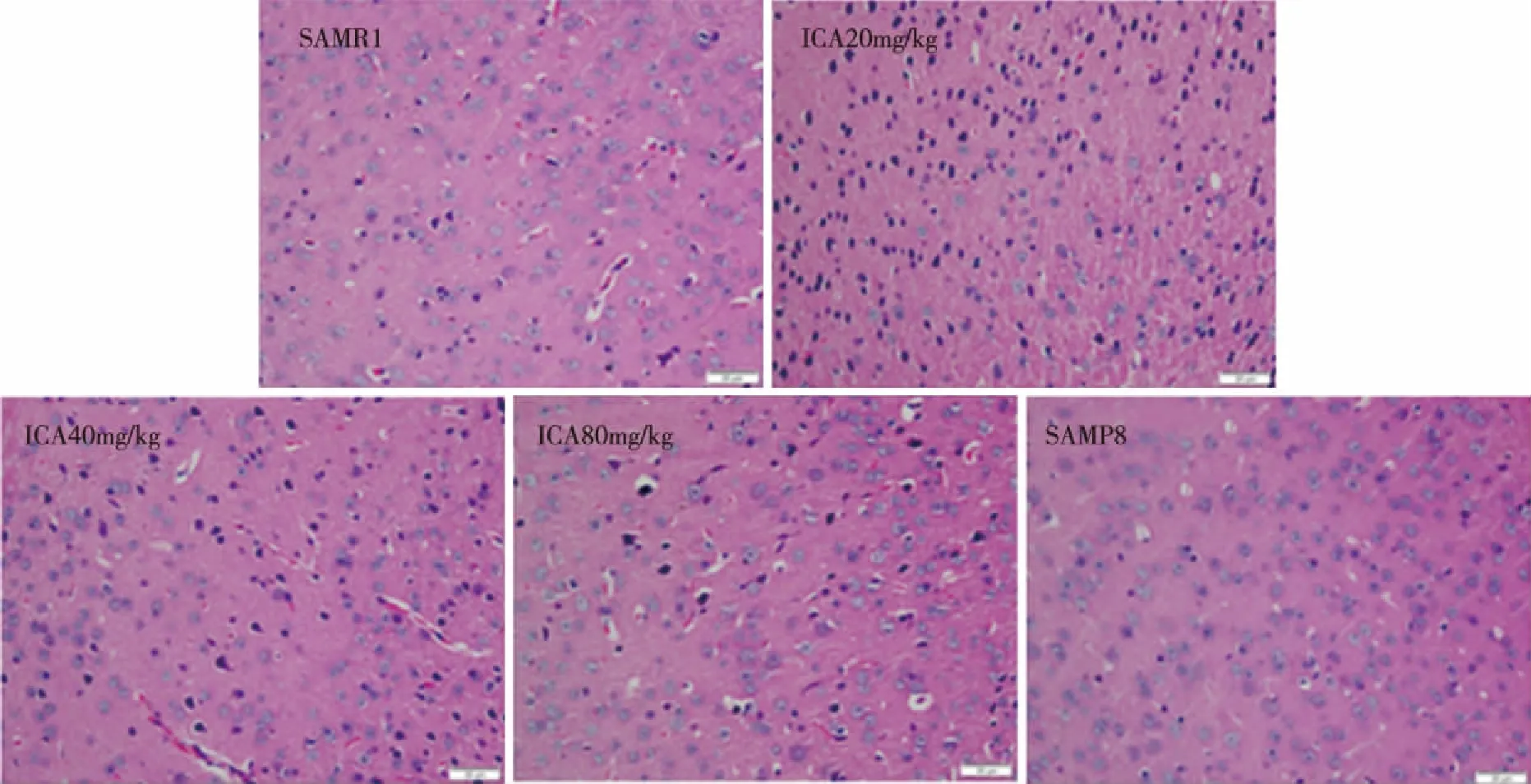
Fig 1 Effects of ICA on pyramidal neurons’ morphology in the cortex (H&E staining,magnification,400×)
2.2 Changes of Nissl bodies in cortex tissues The normal neurons and Nissl bodies in the cortex were observed by Nissl staining.It could be seen from the Fig 2 that SAMR1 mice nerve cells are normal and Nissl body rich.However,SAMP8 mice cortex nerve cells arranged loosely,Nissl bodies were lost,and the emergence of a large number of abnormal cells,but after treatment with ICA normal neurons and Nissl bodies increased(P<0.05).
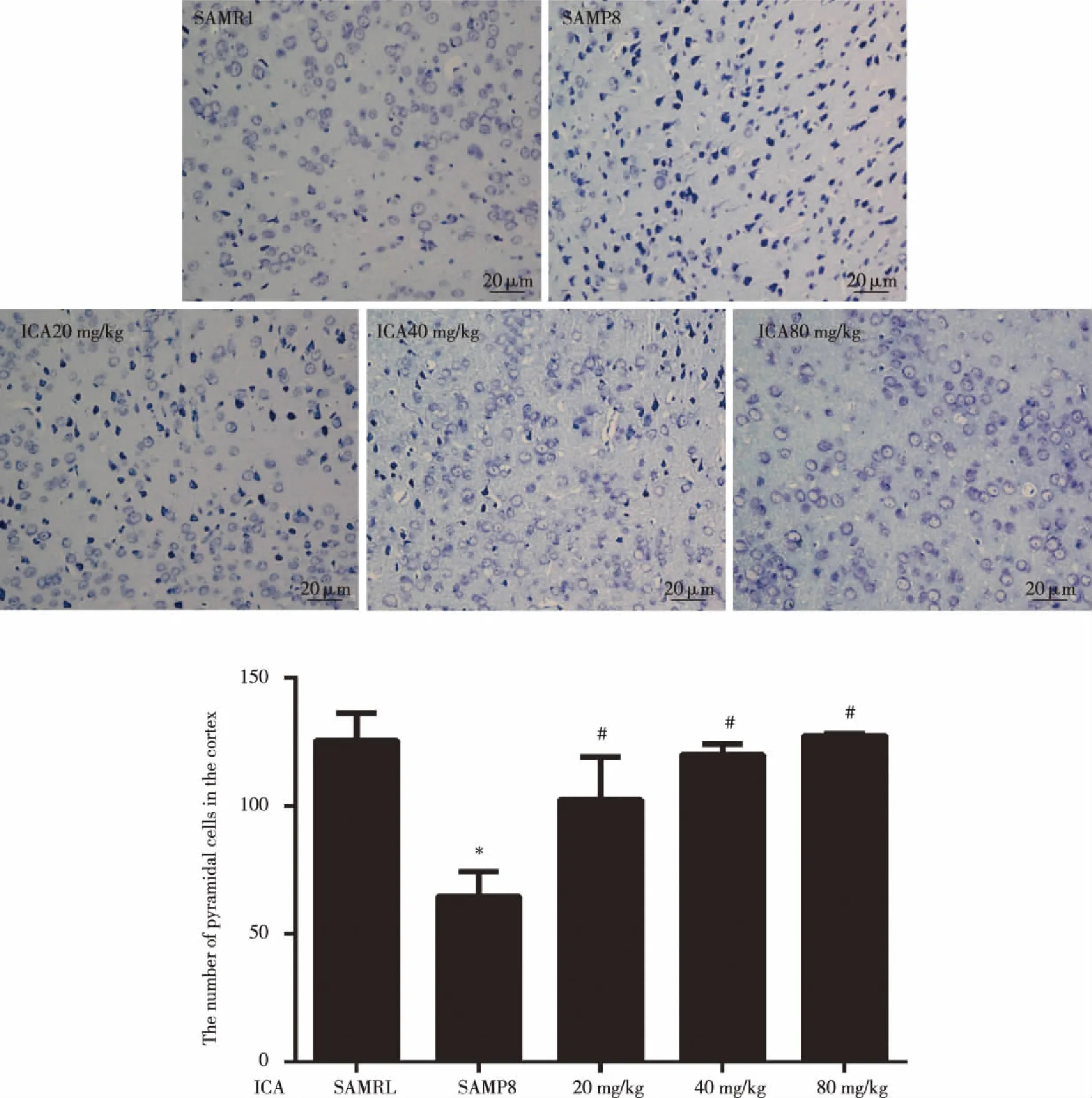
Data were expressed as mean±SD (n=3).Significance *P<0.05 compared to the normal group,#P<0.05 compared to the model group.Fig 2 Effects of ICA on neuronal Nissl bodies in the cortex (Nissl staining,magnification,400×)
2.3 Effects of ICA on the levels of Aβ1-42,APP,and PS1,BACE1 in SAMP8 mice Western blot was used to measure Aβ1-42,APP,BACE1 and PS1 protein in SAMP8 mice.As shown in Fig 3A ,the expression of Aβ1-42protein in model SAMP8 mice was higher than that in blank control mice SAMR1(P<0.05),while the treatment with ICA (20,40 and 80 mg/kg) for 3 months obviously reversed the Aβ1-42in the cortex of SAMP8 mice(P<0.05).It is suggested that ICA can reduce the formation and precipitation of Aβ.In Fig 3B,the treatment with ICA (40 and 80 mg/kg) for 3months obviously reversed the APP in the cortex of SAMP8 mice(P<0.05).In Fig 3C,the expression of BACE1 protein was decreased in ICA-treated mice (20,40 and 80 mg/kg) (P<0.05).Our data indicated that ICA down-regulated the expression of BACE1 in the cortex of SAMP8 mice.In Fig 3D,the expression of PS1 protein in SAMP8 mice was higher than that in SAMR1 mice(P<0.05),the treatment with ICA (20,40 and 80 mg/kg) for 3 months obviously reversed the PS1 in the cortex of SAMP8 mice(P<0.05).
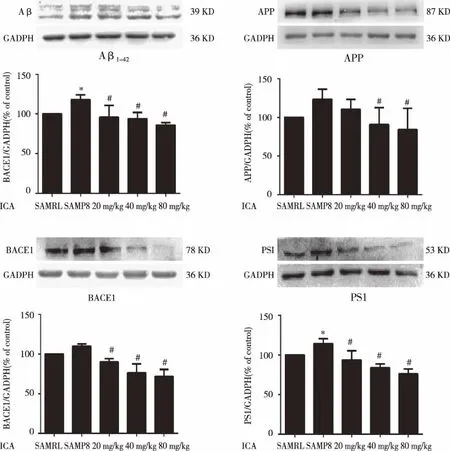
The relative optical density was normalized to GAPDH.Data were expressed as mean±SD (n=3).Significance *P<0.05 compared to the normal group,#P<0.05 compared to the model group.Fig 3 Effects of ICA on the protein expression of Aβ1-42 (A),APP (B),BACE1 (C),PS1 (D) in cortex
3 Discussion
The results of this study showed that Aβ1-42levels are higher in the cortex of 8-month-old SAMP8 mice than those of age-matched SAMR1 mice.Administration of ICA significantly reduced the synthesis of both β-Secretase and γ-Secretase,the reduction of BACE1 and PS1 expression resulted in decrease of Aβ1-42peptide and APP levels.Which could contribute to decreased Aβ1-42levels in SAMP8 mice treat with ICA.Early research showed that ICA improved learning and memory,regulated autophagy and antioxidant damage[16].In this study,we can see that ICA improved neuronal cells damage and increased the number of Nissl bodies,these results indicated ICA could alleviate neuronal damage and decrease Aβ deposition.
A lot of evidence has proved that amyloid deposition plays an important role in the development of AD[17].At present,AD research commonly used transgenic mice as the model,such as APP/PS1 mice[18].The most of AD case are late-onset patients,which is aging-related and may be associated with environmental and genetic factors[19].AD has learning and memory deficits,the senescence accelerate mouse P8 (SAMP8) is an excellent model of early learning and memory impairment.The SAMP8 overproduce amyloid precursor protein and amyloid-beta protein in the brain,and have increased phosphorylation of tau[20].This suggested that SAMP8 may be used as the AD model.
Mounting evidence has indicated Aβ is the pathogenesis of AD.Aβ deposition in the brain may lead to oxidative stress,neuronal damage,inflammatory response,neurofibrillary tangles (NFT),synaptic loss,and so on.At the same time of neuronal damage,a large number of synapses loss could affect neurological dysfunction,cause learning and memory cognitive behavioral changes,with increased Aβ production throughout thedevelopment of AD.
APP is the precursor protein of Aβ,and has two major metabolic pathways[21].A pathway does not produce Aβ,the other way could directly produce Aβ[22].The Aβ metabolic pathway produces APP by β-Secretase and γ-Secretase hydrolysis[23],β-Secretase is a rate-limiting enzyme which is directly involved in the secretion of Aβ.The final stage of γ-Secretase catalyzes Aβ completion plays a key role in the regulation of Aβ40/Aβ42.
In the APP metabolic pathway,β-Secretase cleaves APP at the N-terminal position of Aβ[24].BACE1 is called β-site APP cleaving enzyme and is directly involved in the formation of Aβ[25],which is the rate-limiting enzyme that APP hydrolyzes to Aβ[26].Excessive expression of BACE1 does not affect the production of APP,but it can increase its hydrolysate,eventually leading to increased Aβ[27].It was reported that ICA could reduce the amyloid plaque and Aβ burden deposition in the hippocampus of APP transgenic mice by decreasing the APP and BACE1 levels[28].
γ-Secretase plays a physiological role in the production of Aβ fragments.Presenilin (PS1) is one of the complex membrane proteins.It can affect the biological behavior of nerve cells by regulating APP metabolism,calcium signal regulation,apoptosis and other means[29].Studies have showed that PS1 mutations are a major factor that causes Aβ42overgrowth and inhibits γ-Secretase activity[30].Mutation of PS1 gene in AD patients may alter γ-Secretase activity,increase Aβ42content and promote senile plaque formation[31].
In conclusion,this study demonstrated that ICA could reduce the damage of neurons,and through decreasing the expression of APP,BACE1 and PS1,ICA reduced the levels of Aβ1-42in the cortex of SAMP8 mice.Consequently,the Aβ deposition in SAMP8 mice was improved upon ICA treatment.
[1] Orejana L,Barrosmiћones L,Jordan J,et al.Sildenafil decreases BACE1 and cathepsin B levels and reduces APP amyloidogenic processing in the SAMP8 mouse[J].Journals of Gerontology,2014,70(6):2-12.
[2] Dai R,Zhang S,Duan W,et al.Enhanced autophagy contributes to protective effects of GM1 ganglioside against Abeta1-42-Induced neurotoxicity and cognitive deficits[J].Neurochem Research,2017:1-10.
[3] Bodendorf U,Danner S,Fischer F,et al.Expression of human beta-secretase in the mouse brain increases the steady-state level of beta-amyloid[J].Journal of Neurochemistry,2002,80(5):799-806.
[4] Willander H,JohanssonJ.High-resolution structure of a BRICHOS domain and its implications for anti-amyloid chaperone activity on lung surfactant protein C[J].Proceedings of the National Academy of Sciences of the United States of America,2012,109(7):2325-2329.
[5] Leveugle B,Ding W,Durkin J T,et al.Heparin promotes beta-secretase cleavage of the Alzheimer's amyloid precursor protein[J].Neurochemistry International,1997,30(6):543-548.
[6] De S B,Saftig P,Craessaerts K,et al.Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein[J].Nature,1998,391(6665):387.
[7] Robert V.BACE1,the Alzheimer's beta-secretase enzyme,in health and disease[J].Molecular Neurodegeneration,2012,7(1):1.
[8] 黄金兰.三七总皂苷对快速老化痴呆模型小鼠SAMP8大脑β淀粉样蛋白代谢通路影响的研究[D].南宁:广西医科大学,2013.
[9] Butterfield D A,Poon H F.The senescence-accelerated prone mouse (SAMP8):a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer's disease[J].Exp Gerontology,2005,40(10):774-783.
[10]周伟勤,毕明刚,杜冠华.快速老化小鼠SAMP8研究进展[J].中国药理学通报,2009,25(5):565-568.
[11]Akiguchi I,Pallas M,Budka H,et al.SAMP8 mice as a neuropathological model of accelerated brain aging and dementia:Toshio Takeda's legacy and future directions[J].Neuropathology,2017(Suppl4)(37):293-305.
[12]贾亮亮,袁丁,王洪武,等.淫羊藿苷药理作用的研究进展[J].现代生物医学进展,2010,10(20):3976-3979.
[13]Chen Y,Han S,Huang X,et al.The Protective effect of icariin on mitochondrial transport and distribution in primary hippocampal neurons from 3x Tg-AD mice[J].International Journal of Molecular Sciences,2016,17(2):163.
[14]Li F,Dong H X,Gong Q H,et al.Icariin decreases both APP and Abeta levels and increases neurogenesis in the brain of Tg2576 mice[J].Neuroscience,2015,304:29-35.
[15]Chen Y,Zheng H,Huang X,et al.Neuroprotective effects of icariin on brain metabolism,mitochondrial functions,and cognition in triple-transgenic Alzheimer's Disease mice[J].CNS Neuroscience & Therapeutics,2016,22(1):63-73.
[16]陈发菊.淫羊藿苷抗SAMP8小鼠衰老作用及机制研究[D].遵义:遵义医学院,2016.
[17]Rosenberg R N.The molecular and genetic basis of AD:the end of the beginning:the 2000 Wartenberg lecture[J].Neurology,2000,54(11):2045-2054.
[18]Jin F,Gong Q H,Xu Y S,et al.Icariin,a phoshphodiesterase-5 inhibitor,improves learning and memory in APP/PS1 transgenic mice by stimulation of NO/cGMP signalling[J].International Journal of Neuropsychopharmacology,2014,17(6):871-881.
[19]Jonsson T,Stefansson H,Steinberg S,et al.Variant of TREM2 associated with the risk of Alzheimer's disease-NEJM[J].New England Journal of Medicine,2013,368(2):107-116.
[20]Morley J E,Farr S A,Kumar V B,et al.The SAMP8 mouse:a model to develop therapeutic interventions for Alzheimer's disease[J].Current Pharmaceutical Design,2012,18(8):1123.
[21]Roychaudhuri R,Yang M,Hoshi M M,et al.Amyloid beta-protein assembly and Alzheimer disease[J].Journal of Biological Chemistry,2008,284(8):4749-4753.
[22]Reddy P H.Amyloid precursor protein-mediated free radicals and oxidative damage:implicationsfor the development and progression of Alzheimer's disease[J].Journal of Neurochemistry,2006,96(1):1-13.
[23]Jin P,Jin-A K,Dong-Young C,et al.Anti-inflammatory and anti-amyloidogenic effects of a small molecule,2,4-bis(p-hydroxyphenyl)-2-butenal in Tg 2576 Alzheimer’s disease mice model[J].Journal of Neuroinflammation,2013,10(1):2.
[24]Sastre M,Walter J,Gentleman S M.Interactions between APP secretases and inflammatory mediators[J].Journal of Neuroinflammation,2008,5(1):1-11.
[25]Vassar R,Bennett B D,Babukhan S,et al.Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE[J].Science,1999,286(5440):735.
[26]Citron M.Beta-secretase as a target for the treatment of Alzheimer's disease[J].Journal of Neuroscience Research,2002,70(3):373-379.
[27]Fukumoto H,Cheung B S,Hyman B T,et al.Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease[J].Archives of Neurology,2002,59(9):1381-1389.
[28]Lan Z,Cong S,Jin C,et al.Icariin decreases the expression of APP and BACE-1 and reduces the beta-amyloid burden in an APP transgenic mouse model of Alzheimer's disease[J].International Journal of Biological Sciences,2014,10(2):181-191.
[29]Zhang S,Zhang M,Cai F,et al.Biological function of Presenilin and its role in AD pathogenesis[J].Translational Neurodegeneration,2013,2(1):1-13.
[30]Bergmans B A,De S B.Gamma-secretases:from cell biology to therapeutic strategies[J].Lancet Neurology,2010,9(2):215-226.
[31]Wolfe M S.When loss is gain:reduced presenilin proteolytic function leads to increased Aβ42/Aβ40[J].Embo Reports,2007,8(2):136-140.
[收稿2017-09-11;修回2017-11-12]
(编辑:谭秀荣)
EffectsofIcariinonproteinexpressionsrelatedtoAβmetabolicpathwayinSAMP8mousecortex
LyuLingli,HuangJuan,LiuJing,ChenHong,XuYunyan,WuQin,ShiJingshan
(Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education,Zunyi Guizhou 563099,China)
ObjectiveThe aim of this study is to investigate the effects of Icariin (ICA) on the expression of Aβ1-42,APP ,BACE1 and PS1 in the Aβ metabolic pathway of SAMP8 mouse cortex.Methods8-month-old male SAMP8 mice were administered with different doses of ICA (20,40,80 mg/kg) for 3 months,while SAMR1 mice of the same age were used as the normal group,12 mice in each group.Model of SAMP8 and normal of SAMR1 mice were given equal volume of distilled water.The morphological change of cortex neurons were observed by H&E and Nissl staining,Western blot was used to detect the protein expression of Aβ1-42,APP,PS1 and BACE1 in mouse brain.ResultsCompared with SAMR1 mice,the numbers of neurons in the 8-month-old SAMP8 mice were decreased.The abnormal neuron cells were reduced after administration of ICA.The protein expression of Aβ1-42,PS1 in 8-month-old SAMP8 mice cortex were increased(P<0.05),but the expression of APP and BACE1 showed no significant difference ,while the expression were decreased after administration with ICA.ConclusionThis study demonstrated that ICA could reduce the damage of neurons and down-regulates the protein levels of Aβ1-42,APP,BACE1 and PS1.
Icariin;SAMP8; amyloid beta 1-42; amyloid precursor protein
国家自然科学基金资助项目(NO:81473201)。
石京山,男,教授,博士生导师,研究方向:神经药理学,E-mail:zmcshijs@163.com。
R927.2
A
1000-2715(2017)06-0587-07
