自噬调控肾脏衰老的分子机制及中药的干预作用
2017-02-16涂玥孙伟陈涤平万毅刚吴薇姚建
涂玥+孙伟+陈涤平+万毅刚+吴薇+姚建
[摘要]衰老是生物体在遗传和环境等多种因素共同作用下而逐渐发生的组织、器官功能性衰退。自噬是真核细胞由溶酶体介导而降解细胞质成分的过程。肾脏是典型的衰老靶器官。自噬可以调控肾脏衰老,自噬水平降低就会加速肾脏衰老,反之,自噬水平升高就能延缓肾脏衰老。在这一肾脏衰老的调控过程中,哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)及其相关信号途径,包括腺苷酸活化蛋白激酶(adenosine monophosphate activated protein kinase,AMPK)/mTOR、磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/丝氨酸-苏氨酸激酶(serine-threonine kinase,Akt)/mTOR、AMPK/沉默信息调节因子1(silent information regulation 1,Sirt1)和转化生长因子β(transforming growth factorβ,TGF-β)等通路發挥了重要作用。在体内调控这些信号途径的关键信号分子就可以干预肾脏衰老。一些经典的补肾、活血类中药及其提取物,如冬虫夏草(Cordyceps sinensis)、姜黄素(curcumin)、白藜芦醇(resveratrol)等对肾脏衰老和/或肾脏自噬具备有益的影响。因此,基于自噬调控的分子机制揭示中药抗肾脏衰老的药理作用是今后的发展方向之一。
[关键词]肾脏衰老; 中药; 自噬; 分子机制; mTOR信号通路
[Abstract]Aging is the gradual functional recession of the living tissues or organs caused by a variety of genetic and environmental factors together. Autophagy is a process of degrading cytoplasmic components mediated by lysosomes in eukaryotic cells. Kidney is a typical target organ of aging. Autophagy regulates renal aging. Decrease in autophagy can accelerate renal aging,whereas,increase in autophagy can delay renal aging. During the process of regulating renal aging,the mammalian target of rapamycin (mTOR) and its related signaling pathways including the adenosine monophosphate activated protein kinase (AMPK)/mTOR,the phosphatidylinositol 3-kinase (PI3K)/ serine-threonine kinase(Akt)/mTOR,the AMPK/silent information regulation 1 (Sirt1) and transforming growth factorβ (TGF-β) play the important roles in renal aging. Regulating the key signaling molecules in these pathwaysin vivo can control renal aging. Some Chinese herbal medicine (CHM) and their extracts with the effects of nourishing kidney or activating stasis, such asCordyceps sinensis, curcumin and resveratrol have the beneficial effects on renal aging and/or autophagy. Therefore,revealing the pharmacological effects of CHM in anti-renal aging based on the molecular mechanisms of autophagy will become one of the development trends in the future study.
[Key words]renal aging; Chinese herbal medicine; autophagy; molecular mechanisms; mTOR signaling pathway
doi:10.4268/cjcmm20162105
衰老(aging)是生物体在遗传和环境等多种因素共同作用下而逐渐发生的组织、器官功能性衰退[1]。自噬(autophagy)是真核细胞由溶酶体介导而降解细胞质成分的过程[2-3]。最近的研究表明,人体衰老往往伴有细胞自噬的异常[4],尤其在肾脏衰老的过程中,其调控机制与肾脏固有细胞自噬密切相关[5]。
1 自噬的分类和发生过程
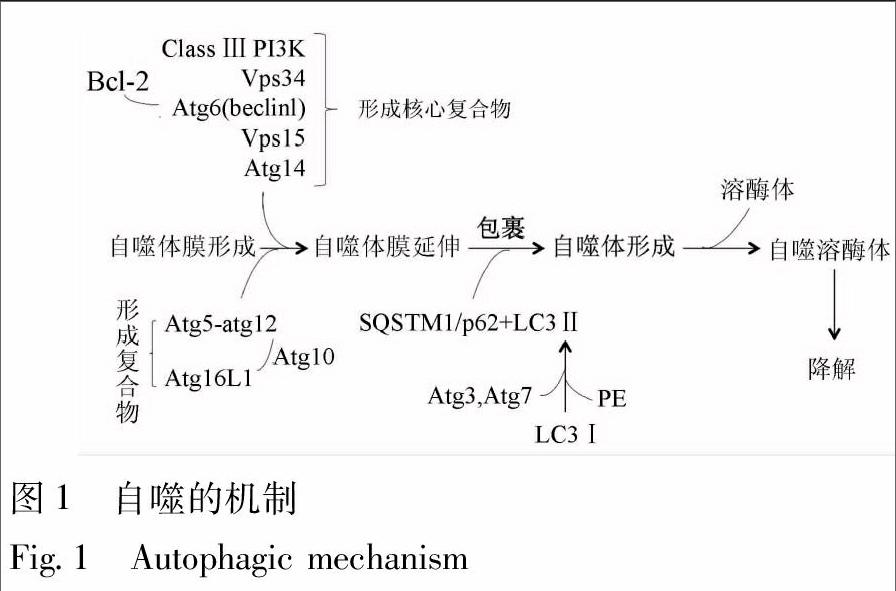
基于细胞内底物进入溶酶体的不同方式,自噬分为巨自噬、微自噬和分子伴侣介导的自噬等3类,目前,对巨自噬的研究最为深入,一般说的“自噬”主要是指巨自噬[3]。自噬的发生过程可人为地分成4个步骤。①自噬体膜(phagophore)的形成:在多种自噬刺激因素(氨基酸、生长因子缺乏、低氧、感染及细胞器受损等)的诱导下,双层结构的自噬体膜在待降解的细胞器或蛋白周围形成;②自噬体(autophagosome)的形成:隔离膜缓慢伸展,完全包裹待降解的细胞器或蛋白,形成自噬体;③自噬体的运输、融合:待降解物被自噬体运输至溶酶体,并与其融合,形成自噬溶酶体(autophagolysosome);④自噬体的裂解:溶酶体中的各种水解酶和蛋白酶溶解自噬体内膜和包裹在其中的待降解物[6]。
2 自噬的机制
近年来借助有关酵母的研究,一系列自噬相关基因(autophagy-related genes,Atgs)被相继确定,它们在自噬的诱导、产生、成熟和再循环等过程中是必不可少的。在起始阶段,最关键的调节分子是Ⅲ类磷脂酰肌醇3-激酶(class Ⅲ phosphatidylinositol 3-kinase,Class Ⅲ PI3K)和空泡分选蛋白34(vacuolar protein sorting associated protein 34,Vps34)。Vps34与Atg6(又称为“Beclin1”)相互作用,募集B-细胞淋巴瘤2(B-cell lymphoma 2,BCL-2)家族蛋白、Vps15和Atg14等自噬相关蛋白而形成“核心复合物”[7]。自噬小体的形成需要2种重要的泛素-蛋白酶系统支持,即“Atg5-Atg12系统”和“微管相关蛋白轻链3(microtubule associated protein light chain 3,LC3)/Atg8系統”。对于前者,Atg12能通过Atg7发挥类似E1泛素活化酶的作用,使其连接到Atg5,进而,Atg10发挥类似E2泛素结合酶的作用, 使得Atg5-Atg12结合物通过非共价的形式与Atg16L1形成复合物;对于后者,LC3通过Atg7(E1样蛋白)与Atg3(E2样蛋白)、磷脂酰乙醇胺(phosphatidyl ethanolamine,PE)结合,形成Ⅱ型LC3。这里,LC3作为“自噬核心蛋白”,它是自噬小体形成的关键步骤,与泛素样结合蛋白SQSTM1/p62相互作用,加速自噬小体至溶酶体的运输过程,并实现降解[8] (图1)。
3 自噬与肾脏衰老的关系
细胞衰老的普遍特征就是清除代谢废物的功能减退,导致受损蛋白质、细胞器过度积累而降低细胞的生存能力;细胞自噬的重要功能就是分解和循环利用受损蛋白质、细胞器而提高细胞的生存能力,延缓其衰老[9-10]。因此,自噬缺乏就会加速细胞衰老。
肾脏是典型的衰老靶器官。自噬在维持足细胞和近端肾小管上皮细胞的功能和稳态方面发挥着重要作用[11-12]。研究表明,老龄大鼠和小鼠的肾脏内会出现线粒体形态改变和衰老相关蛋白质累积,同时,伴有自噬活性的降低[13-14]。敲除小鼠足细胞或近端肾小管上皮细胞特异性Atg5基因都会导致细胞内损伤线粒体和泛素化蛋白(衰老相关蛋白质)的累积,进而,引起肾脏细胞衰老[15-16]。这些研究结果提示,对于足细胞和肾小管上皮细胞而言,自噬水平降低会加速肾脏衰老。
4 自噬调控肾脏衰老的分子机制
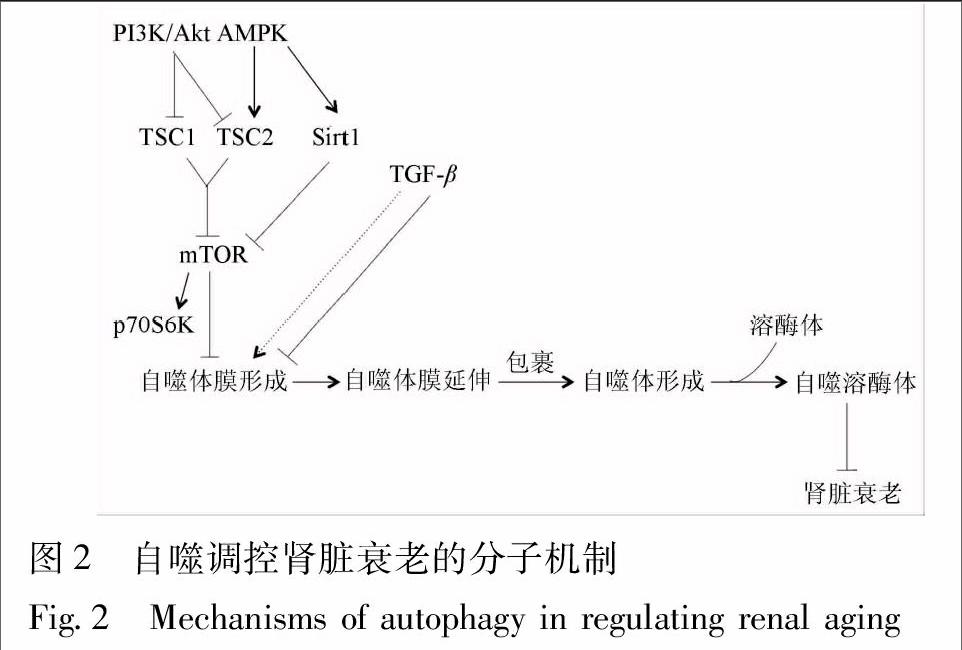
4.1 mTOR信号途径 哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)是调控细胞自噬的关键信号分子,磷酸化70kD核糖体S6激酶(phosphoprotein 70 S6Kinase,p70S6K)是其下游的特异性信号分子。当mTOR/p70S6K信号通路激活,使得核糖体与内质网黏附增强,影响内质网膜脱落,抑制自噬体膜形成,阻断细胞自噬[17]。因此,抑制mTOR信号通路活性可以促进细胞自噬[18-19]。研究显示,衰老大鼠的肾脏组织中磷酸化mTOR(phosphorylated mTOR,p-mTOR)蛋白表达增加,经mTOR抑制剂——雷帕霉素(rapamycin)干预后,L-亮氨酸所诱导的细胞衰老表型得到改善[20]。Ning等发现[21],能量限制可降低老年雄性SD大鼠肾组织中mTOR蛋白表达,增加LC3自噬蛋白表达,减轻大鼠肾脏衰老。这些研究结果提示,抑制肾脏mTOR表达可以促进细胞自噬,减轻肾脏衰老(图2)。
4.2 AMPK信号途径 腺苷酸活化蛋白激酶(adenosine monophosphate activated protein kinase,AMPK)被称为代谢的主开关,它的活性由细胞的能量状态,也就是一磷酸腺苷(adenosine monophosphate,AMP)/三磷酸腺苷(adenosine triphosphate,ATP)比值所调节的[22]。在饥饿、氧化应激、缺血、缺氧、代谢产物堆积等因素作用下,AMP/ATP比值升高,激活AMPK,介导结节性硬化复合物(tuberous sclerosis complex,TSC)2磷酸化,抑制mTOR,进而,诱导细胞自噬[23]。据报道[21],能量限制能增加老年雄性SD大鼠肾组织中的AMPK蛋白表达,减少mTOR蛋白表达,增加细胞自噬蛋白表达。这些研究结果提示,调控肾脏AMPK信号通路活性可以促进细胞自噬,减轻肾脏衰老(图2)。
4.3 PI3K/Akt信号途径 磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/丝氨酸-苏氨酸激酶(serine-threonine kinase,Akt)信号通路也是mTOR上游的信号途径。胰岛素、生长因子与胰岛素样受体结合后,激活I型PI3K,进而,活化Akt/蛋白激酶(protein kinase,PK)B信号途径。其中,活化的Akt可以抑制TSC1/2复合物,激活mTOR/p70S6K通路,从而抑制细胞自噬[24]。Li等发现,在机械应力(mechanical stress)作用下,足细胞p85-PI3K,p-Akt和p-mTOR蛋白表达增加, PI3K/Akt/mTOR信号通路活化,减少LC3,Atg5以及整合素1(integrinβ1)蛋白表达,降低自噬水平,促进足细胞衰老;而螺内酯(spironolactone)能抑制PI3K/Akt/mTOR信号通路活性,提高自噬水平,减轻机械应力引起的足细胞衰老和黏附功能损伤[25]。Kawai等发现,无机磷酸盐能激活Akt/mTOR信号通路,同时缩短小鼠的寿命;但是,在敲除抗衰老基因αKlotho(Kl-/-)的小鼠中,雷帕霉素不但能阻断mTOR通路,而且,能减轻高浓度无机磷酸盐诱导的小鼠衰老,延长小鼠寿命[26]。这些研究结果提示,抑制肾脏PI3K/Akt信号通路活性可以促进细胞自噬,减轻肾脏衰老(图2)。
4.4 Sirt1信号途径 沉默信息调节因子1(silent information regulation 1,Sirt1)是Sirtuins家族成员之一,它是一种依赖于烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD+)的去乙酰化酶。在机体内,Sirt1的激活受到AMPK调控,AMPK能够增加烟酰胺磷酸核糖转移酶(nicotinamide phosphoribosyltransferase,NAMPT)活性,将烟酰胺转化成烟酰胺单核苷酸,增加NAD+表达水平,促进Sirt1转录[27-28],随着细胞内NAD+/还原型烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide hydrogen,NADH)比率的增加,AMPK介导Sirt1去乙酰化[29]。据报道[30],Sirt1的活化与能量限制介导的寿命延长有着密切的联系。一方面,Sirt1可以活化自噬信号途径中的关键信号分子——Atg7,Atg5 和LC3,促进自噬小体形成,从而,提高肾脏细胞自噬水平;另一方面,在叉头转录因子(fork head transcription foctor,FOXO)3A参与下,Sirt1还可以调控近端肾小管上皮细胞自噬,延缓肾脏衰老。研究表明,能量限制不仅能增加老年雄性Sprague Dawley大鼠肾组织中的AMPK蛋白表达,减少mTOR蛋白表达,还能增加Sirt1蛋白表达,促进细胞自噬,减轻肾脏衰老[21]。此外,能量限制能提高雄性糖尿病肥胖大鼠(fa/fa)近端肾小管上皮细胞内 Sirt1表达水平,继而促进细胞自噬,延缓肾脏衰老[31]。对于体外培养的衰老小鼠的近端肾小管上皮细胞,经能量限制干预后,Sirt1蛋白表达增加,FOXO3A去乙酰化加剧,其下游的Bcl-2/腺病毒E1V19-kDa相互作用蛋白质3(Bcl-2/adenovirus E1V 19-kDa interacting protein 3,Bnip3)表达上调,进而,促进 Bnip3介导的自噬。这些研究结果提示,增加肾脏Sirt1表达可以促进细胞自噬,减轻肾脏衰老(图2)。
4.5 TGF-β信号途径 转化生长因子β(transforming growth factorβ,TGF-β)不仅是公认的致肾脏纤维化因子,也是影响肾脏衰老的重要因素之一[32]。一方面,TGF-β1可以诱导人近端肾小管上皮细胞中自噬相关基因Atg5,Atg7和Beclin1表达上调,增加自噬小体累积,激活自噬[33-34];另一方面,在某些条件下,TGF-β1还可以抑制自噬[35-37]。TGF-β的双重功能取决于特定的细胞类型和病理条件,尽管如此,国内外的学者认为,TGF-β诱导肾脏纤维化和加速肾脏衰老的负面作用是肯定的(图2)。
5 中药对肾脏衰老和/或肾脏自噬的干预作用
近来的研究显示,一些中药及其提取物对肾脏衰老和/或肾脏自噬具备有益的影响。Zhang等发现,高糖(30 mmol·L-1)干预系膜细胞96 h后能明显抑制Sirt1活性,诱导系膜细胞衰老,而白藜芦醇(resveratrol)1 mg·L-1聯合干预后,Sirt1活性有所增强,系膜细胞衰老得到改善[38-39]。李春花等报道[40],在链脲佐菌素(streptozotocin,STZ)腹腔注射诱导的糖尿病肾病(diabetic nephropathy,DN)大鼠模型中,每天白藜芦醇(20 mg·kg-1)灌胃,连续12周,可以明显增加模型鼠肾组织中自噬标志性蛋白LC3-Ⅱ和beclin1表达水平,减轻DN肾脏损伤。Gu等借助阿霉素肾病模型而发现阿霉素能抑制AMPK活性,并促进肾脏细胞凋亡,而白藜芦醇能通过激活AMPK/mTOR信号通路,诱导细胞自噬,减轻肾脏细胞凋亡[41]。Shen等发现,在果蝇食物中添加姜黄素(curcumin),干预3周,可以增加果蝇的寿命[42-43];基于此,Xu等进一步发现,姜黄素能明显增加H2O2干预的肾小球系膜细胞LC3Ⅱ蛋白表达水平和LC3Ⅱ/I比值,提高细胞自噬水平,减轻H2O2诱导的肾脏损伤[44]。黄可等发现,对于STZ腹腔注射而诱导的DN大鼠模型,肾组织Klotho核酸和蛋白(抗衰老标记物)表达水平明显降低;每天经冬虫夏草(Cordyceps sinensis)5 g·kg-1灌胃,干预24周后,肾组织Klotho核酸和蛋白表达水平明显上调,肾脏衰老得到延缓[45-46]。Pan等发现,在5/6肾切除大鼠残余肾组织中TGF-β1及其受体的核酸和蛋白表达水平增加,自噬水平降低;经冬虫夏草(2 g·kg-1)灌胃,干预12周后,TGF-β1及其受体的核酸和蛋白水平下降,自噬水平升高[47]。
6 结论与展望
自噬可以调控肾脏衰老,自噬水平降低就会加速肾脏衰老,反之,自噬水平升高就能延缓肾脏衰老。在这一肾脏衰老的调控过程中,mTOR及其相关信号途径,包括AMPK/mTOR,PI3K/Akt/mTOR,AMPK/Sirt1和TGF-β等通路发挥了重要作用。在体内调控这些信号途径的关键信号分子就可以干预肾脏衰老。国内初步的体内研究结果提示,一些经典的补肾、活血类中药及其提取物不仅能延缓肾脏衰老,而且,其药理作用很可能与自噬调控的分子机制有关。然而,令人遗憾的是,“肾脏衰老”与“自噬调控”在中药抗衰老的分子药理学研究领域尚未得到有机结合。笔者所属团队的前期研究表明,对于D-半乳糖诱导的衰老模型鼠,冬虫夏草提取物——虫草素(cordycepin)不但可以改善模型鼠肾功能,还可以下调其自噬蛋白LC3和抗衰老蛋白Klotho的表达;大黄的活性成分——大黄酸(rhein)能够改善Hank′s平衡盐溶液诱导的大鼠肾小管上皮(NRK-52E)细胞自噬,其机制可能与调控mTOR/p70S6K信号通路中的关键蛋白p-mTORSer2448和p-p70S6K表达有关[48]。因此,笔者认为,基于自噬调控的分子机制而揭示中药(尤其是补肾类中药)抗肾脏衰老的药理作用是今后的发展方向之一。
[參考文献]
[1]van Deursen J M. The role of senescent cells in ageing[J]. Nature,2014,509(7501):439.
[2]Levine B,Mizushima N,Virgin H W. Autophagy in immunity and inflammation[J]. Nature,2011,469(7330):323.
[3]Galluzzi L, Pietrocola F, Levine B,et al. Metabolic control of autophagy[J]. Cell,2014,159(6):1263.
[4]Rubinsztein D C,Marino G,Kroemer G. Autophagy and aging[J]. Cell,2011,146(5):682.
[5]Wang Z,Choi M E. Autophagy in kidney health and disease[J]. Antioxid Redox Signal,2014,20(3):519.
[6]Rabinowitz J D,White E. Autophagy and metabolism[J]. Science,2010,330(6009):1344.
[7]He C,Levine B. The beclin1 interactome[J]. Curr Opin Cell Biol,2010,22(2):140.
[8]Ravikumar B,Sarkar S,Davies J E,et al. Regulation of mammalian autophagy in physiology and pathophysiology[J]. Physiol Rev,2010,90(4):1383.
[9]Martinez-Lopez N, Athonvarangkul D, Singh R. Autophagy and aging[J]. Adv Exp Med Biol,2015,847:73.
[10]Cuervo A M,Bergamini E,Brunk U T,et al. Autophagy and aging:the importance of maintaining "clean" cells[J]. Autophagy,2005,1(3):131.
[11]Hartleben B, Wanner N, Huber T B. Autophagy in glomerular health and disease[J]. Semin Nephrol, 2014,34(1):42.
[12]Fougeray S, Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys[J]. Nat Rev Nephrol, 2015,11(1):34.
[13]Yamamoto T, Takabatake Y, Kimura T, et al. Time-dependent dysregulation of autophagy: implications in aging and mitochondrial homeostasis in the kidney proximal tubule[J]. Autophagy,2016,12(5):801.
[14]Cui J,Bai X Y,Shi S,et al. Age-related changes in the function of autophagy in rat kidneys[J]. Age(Dordr),2012,34(2):329.
[15]Hartleben B,Gdel M,Meyer-Schwesinger C,et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice[J]. J Clin Invest,2010,120(4):1084.
[16]Kimura T,Takabatake Y,Takahashi A,et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury[J]. J Am Soc Nephrol,2011,22(5):902.
[17]Wullschleger S,Loewith R,Hall M N. TOR signaling in growth and metabolism[J]. Cell,2006,124(3):471.
[18]Jung C H,Ro S H,Cao J,et al. mTOR regulation of autophagy[J]. FEBS Lett,2010,584(7):1287.
[19]Klionsky D J,Meijer A J,Codogno P. Autophagy and p70S6 kinase[J]. Autophagy,2005,1(1):59.
[20]Zhuo L, Cai G, Liu F,et al. Expression and mechanism of mammalian target of rapamycin in age-related renal cell senescence and organ aging[J]. Mech Ageing Dev,2009,130(10):700.
[21]Ning Y C,Cai G Y,Zhuo L,et al. Short-term calorie restriction protects against renal senescence of aged rats by increasing autophagic activity and reducing oxidative damage[J]. Mech Ageing Dev,2013,134(11/12):570.
[22]Chen S,Rehman S K,Zhang W,et al. Autophagy is a therapeutic target in anticancer drug resistance[J]. Biochim Biophys Acta,2010,1806(2):220.
[23]Shrivastava S,Bhanja Chowdhury J,Steele R,et al. Hepatitis C virus upregulates beclin1 for induction of autophagy and activates mTOR signaling[J]. J Virol,2012,86(16):8705.
[24]Ma X,Hu Y. Targeting PI3K/Akt/mTOR cascade:the medicinal potential, updated research highlights and challenges ahead[J]. Curr Med Chem,2013,20(24): 2991.
[25]Li D,Yan T,Xu Z,et al. Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR pathway and reduce adhesive damage in podocytes under mechanical stress[J]. Biosci Rep,2016,36(4):e00355.
[26]Kawai M,Kinoshita S,Ozono K,et al. Inorganic phosphate activates the AKT/mTORC1 pathway and shortens the life span of anαKlotho-Deficient model[J]. J Am Soc Nephrol,2016,27(9):2810.
[27]Maiese K. mTOR:driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus[J]. World J Diabetes,2015,6(2):217.
[28]Maiese K. New insights for oxidative stress and diabetes mellitus[J]. Oxid Med Cell Longev,2015,2015:875961.
[29]Hardie D G,Ross F A,Hawley S A. AMP-activated protein kinase:a target for drugs both ancient and modern[J]. Chem Biol,2012,19(10):1222.
[30]Kume S,Uzu T,Horiike K,et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney[J]. J Clin Invest,2010,120(4):1043.
[31]Kitada M,Takeda A,Nagai T,et al. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty(fa/fa)rats:a model of type 2 diabetes[J]. Exp Diabetes Res,2011,2011:908185.
[32]Bttinger E P,Bitzer M. TGF-beta signaling in renal disease[J]. J Am Soc Nephrol,2002,13(10):2600.
[33]Kiyono K,Suzuki H I,Matsuyama H,et al. Autophagy is activated by TGF-beta and potentiates TGF-beta-mediated growth inhibition in human hepatocellular carcinoma cells[J]. Cancer Res,2009,69(23):8844.
[34]Xu Y,Yang S,Huang J,et al. TGF-β1 induces autophagy and promotes apoptosis in renal tubular epithelial cells[J]. Int J Mol Med,2012,29(5):781.
[35]Li C,Wang Q,Wang J F. Transforming growth factor-β(TGF-β)induces the expression of chondrogenesis-related genes through TGF-β receptor Ⅱ(TGFRⅡ)-AKT-mTOR signaling in primary cultured mouse precartilaginous stem cells[J]. Biochem Biophys Res Commun,2014,450(1):646.
[36]Carracedo A,Ma L,Teruya-Feldstein J,et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer[J]. J Clin Invest,2008,118(9):3065.
[37]Patschan D,Krupincza K,Patschan S,et al. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia:modulation by ischemic preconditioning[J]. Am J Physiol Renal Physiol,2006,291(1):F176.
[38]Yang T,Wang L,Zhu M,et al. Properties and molecular mechanisms of resveratrol:a review[J]. Pharmazie,2015,70(8):501.
[39]Zhang S,Cai G,Fu B,et al. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence[J]. Mech Ageing Dev,2012,133(6):387.
[40]李春花,张英,李可心,等. 白藜芦醇通过诱导自噬保护糖尿病肾病大鼠肾功能的研究[J]. 中国实验诊断学,2014,18(12):1920.
[41]Gu J,Hu W,Song Z P,et al. Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity[J]. Int Immunopharmacol,2016,32:1.
[42]Prasad S,Gupta S C,Tyagi A K,et al. Curcumin,a component of golden spice:from bedside to bench and back[J]. Biotechnol Adv,2014,32(6):1053.
[43]Shen L R,Xiao F,Yuan P,et al. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila[J]. Age(Dordr),2013,35(4):1133.
[44]Xu J,Meng K,Zhang R,et al. The use of functional chemical-protein associations to identify multi-pathway renoprotectants[J]. PLoS ONE,2014,9(5):e97906.
[45]Paterson R R. Cordyceps:a traditional Chinese medicine and another fungal therapeutic biofactory [J]. Phytochemistry,2008,69(7):1469.
[46]黃可,谢淑华,安宁,等. 冬虫夏草通过抗氧化及抗衰老减轻糖尿病肾病大鼠肾小管损伤的研究[J]. 中国医学创新,2014,11(22):15.
[47]Pan M M,Zhang M H,Ni H F,et al. Inhibition of TGF-β1/Smad signal pathway is involved in the effect ofCordyceps sinensis against renal fibrosis in 5/6 nephrectomy rats[J]. Food Chem Toxicol,2013,58:487.
[48]涂玥,孙伟,顾刘宝,等. 大黄酸调控mTOR信号通路活性抑制肾小管上皮细胞自噬蛋白表达的分子机制[J]. 中国中药杂志,2014,39(21):4090.
[责任编辑 马超一]
