结肠癌患者血浆外泌体表面膜结合型转化生长因子β1表达及其生物学作用
2017-01-06居颂文
居颂文
(南京医科大学附属苏州医院北区,江苏苏州215008)
·论著·
结肠癌患者血浆外泌体表面膜结合型转化生长因子β1表达及其生物学作用
居颂文
(南京医科大学附属苏州医院北区,江苏苏州215008)
目的 观察结肠癌患者血浆外泌体(exosomes)表面膜结合型转化生长因子β1(mTGF-β1)表达,探讨其生物学作用。方法 选取结肠癌患者20例(观察组),同期体检健康者10例(对照组),提取两组血浆exosomes,采用流式细胞仪分析exosomes表面mTGF-β1表达。从结肠癌患者肝素抗凝全血中分离外周血单个核细胞(PBMCs),分选获得纯化的CD4+T细胞,采用CD3、CD28诱导细胞增殖,将细胞随机分为三组,A组加入TGF-β1阻断性单抗处理的结肠癌患者血浆exosomes(1 μg/mL),B组加入IgG1同型对照抗体处理的结肠癌患者血浆exosomes(1 μg/mL),C组加入健康人血浆exosomes(1 μg/mL),培养3天时进行台盼蓝染色计数活细胞数;采用ELISA法检测细胞培养上清液中TNF-α和IFN-γ水平。结果 观察组血浆exosomes表面mTGF-β1表达阳性率为36.52%±4.26%,高于对照组的9.23%±3.77%(P<0.01)。与C组比较,B组CD4+T细胞数量及其分泌的TNF-α、IFN-γ水平均降低(P均<0.05);与B组比较,A组CD4+T细胞数量及其分泌的TNF-α、IFN-γ水平均增加(P均<0.05)。结论 结肠癌患者血浆exosomes表面高表达mTGF-β1,mTGF-β1表达增加可能是构成结肠癌免疫抑制环境的重要因素,可促进结肠癌的发生、发展。
结肠癌;膜结合型转化生长因子β1;外泌体
外泌体(exosomes)是由多种动物活细胞分泌的来源于多囊体的小囊泡,具有双层膜结构,是直径30~100 nm的扁平或球形小体,广泛分布于人体血浆、唾液、乳汁、尿液等体液中[1]。近年研究表明,exosomes不仅可促进细胞新陈代谢过程中代谢产物的排出,也是细胞间进行物质、信息交流的重要媒介,其中肿瘤细胞来源的exosomes参与肿瘤侵袭、转移及耐药等病理过程[2,3]。转化生长因子β1(TGF-β1)是具有免疫抑制作用的细胞因子,可抑制机体的抗肿瘤免疫反应[4],而膜结合型转化生长因子β1(mTGF-β1)是调节性T细胞(Treg)及骨髓来源的抑制性细胞(MDSCs)发挥免疫抑制作用的重要效应分子[5,6]。 本研究观察结肠癌患者血浆exosomes表面TGF-β1表达,探讨其生物学作用。
1 资料与方法
1.1 临床资料 选取2014年1月~2015年6月南京医科大学附属苏州医院北区及苏州大学附属第二医院收治的结肠癌患者20例(结肠癌组),男13例、女7例,年龄31~75岁、平均56.8岁,TMN分期Ⅱ期1例、Ⅲ期13例、Ⅳ期6例,入组前均未进行放化疗。选取同期体检健康者10例作为对照组,男5例、女5例,年龄28~60岁、平均39.3岁。两组性别、年龄具有可比性。
1.2 血浆exosomes表面mTGF-β1表达检测 观察组入院时、对照组体检时均留取外周静脉血,离心取血浆,采用ExoQuick exosome precipitation solution试剂盒(SBI公司)提取血浆中的exosomes,具体操作参照试剂盒说明书。采用微珠(Aldehyde/Sulfate Latex Beads,4% w/v,4 μm,Life technologies)包被exosomes,取30 μg exosomes与微珠混合,加入PBS调整体积至100 μL,室温反应2 h,加入100 mmol/L甘氨酸溶液终止反应。包被exosomes的微珠标记不同的荧光抗体和同型对照抗体,流式细胞仪检测exosomes表面CD9、CD63表达,结果显示结肠癌患者血浆中分离获得的exosomes表达CD9、CD63,两组表达量相近,提示血浆exosomes提取成功。采用流式细胞仪检测两组血浆exosomes表面mTGF-β1表达阳性率。
1.3 外周血单个核细胞(PBMCs)CD4+T细胞增殖情况及TNF-α、IFN-γ分泌水平检测 采用聚蔗糖-泛影葡胺密度梯度离心法从结肠癌患者肝素抗凝全血中分离PBMCs,采用流式细胞仪(BD FACSAria Ⅱ)分选获得纯化的CD4+T细胞。采用CD3单抗(1 μg/mL)包被96孔板,4 ℃过夜,弃包被液,备用。采用含10%胎牛血清的RPMI 1640培养基培养纯化的CD4+T细胞,将细胞密度调整至3×105个/mL,接种至包被CD3单抗的96孔板,100 μL/孔,加入CD28单抗(1 μg/mL)。将细胞随机分为三组,A组加入TGF-β1阻断性单抗处理的结肠癌患者血浆exosomes(1 μg/mL),B组加入IgG1同型对照抗体处理的结肠癌患者血浆exosomes(1 μg/mL),C组加入健康人血浆exosomes(1 μg/mL),培养3天时行台盼蓝染色计数活细胞数;收集细胞培养液上清,采用ELISA法检测细胞因子TNF-α和IFN-γ水平。
TGF-β1阻断性单抗处理的结肠癌患者血浆exosomes获得方法:exosomes与TGF-β1阻断性抗体(Biolegend公司)混合(质量比1∶1),4 ℃反应15 min,加入PBS,超速离心(36 000 r/min,1.5 h),弃上清,洗涤两遍,去除未结合的抗体,收集阻断TGF-β1的exosomes沉淀,加入PBS重新溶解,备用;TGF-β1同型对照处理的结肠癌患者血浆exosomes获得方法:exosomes与IgG1同型对照抗体混合(质量比1∶1),其余操作同前。
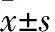
2 结果
观察组、对照组血浆exosomes表面mTGF-β1表达阳性率分别为36.52%±4.26%、9.23%±3.77%,两组比较P<0.01。与C组比较,B组CD4+T细胞数量及其分泌的TNF-α、IFN-γ水平均降低(P均<0.05);与B组比较,A组CD4+T细胞数量及其分泌的TNF-α、IFN-γ水平均增加(P均<0.05)。见表1。
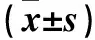
表1 三组CD4+T细胞数量及其分泌的TNF-α、IFN-γ水平比较
注:与B组比较,*P<0.05。
3 讨论
Exosomes是由多种动物活细胞分泌的小囊泡体,含有大量的蛋白质、脂质、RNA和microRNA等成分,并可将这些成分传递给附近或远处的受体细胞,进而介导一系列的生物学功能[1~3]。研究发现,树突状细胞(DCs)来源的exosomes在体内可刺激CD8+T细胞依赖的抗肿瘤效应,导致肿瘤消退[7,8];肿瘤细胞来源的exosomes在肿瘤细胞侵袭、转移、耐药及免疫调节过程中发挥重要作用,亦可通过抑制DCs、NK、CD4+和CD8+T细胞等的抗肿瘤免疫反应以及诱导调节性T细胞(Treg)和骨髓来源的抑制性细胞(MDSCs)在肿瘤免疫逃逸中发挥作用[9~14]。乳腺癌细胞通过携带高水平miR-122的exosomes在转移前微环境中抑制非肿瘤细胞的葡萄糖摄取,为即将转移的肿瘤细胞提供一个高葡萄糖环境[11]。研究发现,exosomes表面的整合素种类决定着靶器官的特异性整合素移靶向性,例如与肺转移有关的exosomes表面整合素为α6β4和α6β1,整合素αvβ5则与肝转移有关[9]。在肾上皮细胞癌中,具有耐药性的肿瘤细胞会通过exosomes传递与耐药相关的长链非编码RNA(lncRNA)药物敏感性受体结合,介导肿瘤细胞耐药[10]。Exosomes能抑制单核细胞分化为DCs和抑制DCs成熟,亦能激活髓系来源的MDSCs的抑制活性[9,14]。肿瘤exosomes含有膜蛋白HSP72,通过Hsp72/TLR2途径促进MDSCs的免疫抑制功能[15]。Abusamra等[16]研究发现,前列腺癌细胞来源的exosomes表达FasL,与T细胞表面的Fas受体结合,诱导T细胞凋亡。
TGF-β1是具有免疫抑制作用的细胞因子,可抑制T细胞增殖,抑制IFN-γ、IL-12等细胞因子产生,通过抑制机体的抗肿瘤免疫反应促进肿瘤的发生、发展[17]。研究发现,TGF-β1上调杀伤抑制性受体NKG2A表达,抑制杀伤活化性受体NKG2D和NKp30在NK细胞上的表达,从而抑制NK细胞的抗肿瘤活性[18,19]。本研究结果显示,结肠癌患者血浆中exosomes表面表达mTGF-β1显著升高;结肠癌患者血浆exosomes能抑制CD4+T细胞增殖以及IFN-γ和TNF-α的产生,而采用TGF-β1阻断性单抗可显著抑制exosomes对CD4+T细胞增殖及IFN-γ、TNF-α产生的抑制作用;上述结果提示,结肠癌患者血浆exosomes表面高表达mTGF-β1,mTGF-β1表达升高可能是构成结肠癌免疫抑制环境的重要因素,可促进结肠癌的发生、发展。
[1] Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function[J]. Nat Rev Immunol, 2002,2(8):569-579.
[2] Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review[J]. Cancer Metastasis Rev, 2013,32(3-4):623-642.
[3] Beach A, Zhang HG, Ratajczak MZ, et al. Exosomes: an overview of biogenesis, composition and role in ovarian cancer[J]. J Ovarian Res, 2014(25):7-14.
[4] Yang L, Pang YL, Moses HL, et al. TGF-β and immune cells: an important regulatory axis in the tumormicroenvironment and progression[J]. Trends Immunol, 2010,31(6):220-227.
[5] Nakamura K, Kitani A, Strober W, et al. Cell contact-dependentimmunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cellsurface-bound transforming growth factor beta[J]. J Exp Med, 2001,194(4):629-644.
[6] Li HQ, Han,YM, Guo QL, et al. Cancer-expanded myeloid-derived suppressor cells induce anergyof NK cells through membrane-bound TGF-β1[J]. J Immunol, 2009,182(1):240-249.
[7] Pitt JM, Charrier M, Viaud S,et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer[J]. J Immunol, 2014,193(3):1006-1011.
[8] 张在云,李希德,刘叶,等.肺癌细胞裂解物负载对树突状细胞分泌的exosome诱导抗肿瘤作用的影响[J].山东医药,2011,51(31):4-6.
[9] Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression[J]. Clin Cancer Res, 2011,17 (5):959-964.
[10] Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments[J]. Semin Immunopathol, 2011,33(5):441-454.
[11] Fong MY, Zhou WY, Liu L, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis[J]. Nature Cell Biology, 2015,17(2):183-194.
[12] Hoshino A, Costa-Silva B, Shen TL, et al. Tumorexosome integrins determine organotropic metastasis[J]. Nature, 2015,527(7578):329-335.
[13] Qu L, Ding J, Chen C, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA[J]. Cancer Cell, 2016,29(5):653-668.
[14] Ashiru O, Boutet P, Fernández-Messina L, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes[J]. Cancer Res, 2010,70(2):481-489.
[15] Chalmin F, Sylvain Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells[J]. J Clin Invest, 2010,120(2):457-471.
[16] Abusamra AJ, Zhong Z, Zheng X, et al.Tumor exosomes expressing Fas ligand mediate CD8+T-cell apoptosis[J]. Blood Cells Mol Dis, 2005,35(2):169-173.
[17] Oh SA, Li MO. TGF-β: guardian of T cell function[J]. J Immunol, 2013,191(8):3973-3979.
[18] Witham TF, Villa L, Yang T, et al. Expression of a soluble transforming growth factor-beta (TGFbeta) receptor reduces tumorigenicity by regulating natural killer (NK) cell activity against 9L gliosarcoma in vivo[J]. J Neurooncol, 2003,64(1-2):63-69.
[19] Zhang HG, Grizzle WE. Exosomesand cancer: a newly described pathway of immune suppression[J]. Clin Cancer Res, 2011,17(5):959-964.
Expression of mTGF-β1on the surface of exosomes in plasma from patients with colorectal cancer and its biological function
JUSongwen
(NorthDistrictofSuzhouHospitalAffiliatedtoNanjingMedicalUniversity,Suzhou215008,China)
Objective To explore the membrane-bound transforming growth factor-β1(mTGF-β1) expression on the surface of exosomes in the plasma from patients with colorectal cancer and its biological function. Methods The expression of mTGF-β1on the surface of exosomes from 20 samples of patients with colorectal cancer (observation group) and 10 healthy donors (control group) were analyzed by flow cytometry. Peripheral blood mononuclear cells (PBMCs) were isolated from heparin anticoagulated whole blood of colon cancer patients, and CD4+T cells were purified and obtained by sorting. Meanwhile, CD3 and CD28 were used to induce cell proliferation, and the cells were randomly divided into three groups: groups A, B and C, group A was added with 1 μg/ml exosomes from plasma of patients treated with anti-TGF-β1monoclonal antibody (mAb), group B was added with 1 μg/ml exosomes from plasma of patients treated with control IgG1mAb and group C was added with 1 μg/mL exosomes from plasma of healthy donors. The cell count was calculated by Trypan blue stainin, and the levels of TNF-α and IFN-γ was detected by ELISA on day 3 after culture. Results The positive rate of mTGF-β1expression in the observation group was 36.52%±4.26%, which was higher than that (9.23%±3.77%) in the control group (P<0.01). Compared with group C, the number of CD4+T cells and the levels of TNF-α and IFN-γ secreted in the group B were all lower than those in the group C (allP<0.05). Compared with group B, the number of CD4+T cells and the levels of TNF-α and IFN-γ secreted in the group A were higher (allP<0.05). Conclusion The mTGF-β1expression is highly expressed on the surface of exosomes in the plasma from patients with colorectal cancer, and the increased expression of mTGF-β1may play an important role in the colon cancer immunosuppressive environment, which promotes the occurrence and development of colon cancer.
colorectal carcinoma; membrane-bound transforming growth factor-β1; exosomes
国家自然科学基金资助项目(81373149);江苏省自然科学基金资助项目(BK20151195);苏州市科技计划项目(SYS201363)。
居颂文(1977-),男,副研究员,研究方向为肿瘤免疫学。E-mail: szjusw@sina.com
10.3969/j.issn.1002-266X.2016.44.001
R735.3
A
1002-266X(2016)44-0001-03
2016-01-12)
