活性氧簇介导血管紧张素 II对延髓神经元胞内游离Ca2+水平的调节作用*
2017-01-03刘小妮曹冬青张娜娜丁滢泂金惠铭
刘小妮, 曹冬青, 张娜娜, 陶 然, 丁滢泂, 金惠铭, 卢 宁△
(1复旦大学基础医学院生理与病理生理系,上海 200032; 2复旦大学附属华山医院神经外科,上海 200040)
活性氧簇介导血管紧张素 II对延髓神经元胞内游离Ca2+水平的调节作用*
刘小妮1, 曹冬青2, 张娜娜1, 陶 然1, 丁滢泂1, 金惠铭1, 卢 宁1△
(1复旦大学基础医学院生理与病理生理系,上海 200032;2复旦大学附属华山医院神经外科,上海 200040)
目的: 探讨活性氧簇(reactive oxygen species, ROS)介导血管紧张素II(angiotensin II, Ang II)对延髓神经元胞内游离Ca2+的调节作用及其机制。方法:原代培养延髓神经元;免疫荧光双标法鉴定培养神经元的特征;给予Ang II处理后,用二氢乙啶荧光探针法测定神经元ROS水平;同时或单独给予Ang II和NADPH氧化酶抑制剂apocynin或自由基清除剂TEMPOL后,Fura-2/AM钙瞬变法记录神经元胞内游离Ca2+的水平;CCK-8法检测神经元活性。结果:原代培养的延髓神经元多数为谷氨酸阳性的神经元;Ang II(5 μmol/L)可在10 min内显著升高神经元ROS水平(P<0.01);给予Ang II处理后延髓神经元胞内Ca2+水平显著升高(P<0.01);给予apocynin/TEMPOL预处理后,Ang II引起的延髓神经元胞内Ca2+的升高则被抑制(P<0.05)。实验浓度的Ang II对神经元无毒性作用。结论:ROS介导Ang II诱导的延髓神经元胞内Ca2+的升高作用,可能是Ang II在中枢诱导氧化应激作用的潜在细胞内信号机制。
延髓神经元; 活性氧簇; 细胞内Ca2+; 血管紧张素II
已知血管紧张素II(angiotensin II, Ang II)可通过中枢调控对体液及心血管调节发挥重要调节作用。Ang II 可通过作用于心血管中枢所在部位的AT1受体,而产生升高血压、促进饮水、促进抗利尿激素的释放和引起交感神经兴奋等[1-2]。中枢Ang II信号机制的紊乱与高血压、心力衰竭等多种心血管疾病的病理过程相关[3]。但Ang II在心血管调控中枢介导的细胞内信号机制仍不明确。

细胞内钙离子浓度(intracellular Ca2+concentration, [Ca2+]i)在神经细胞内和神经细胞之间的生理与病理过程中都起着非常关键的作用,神经元胞内[Ca2+]i的增加可产生促进神经递质的释放、加强神经元之间的联系和调节突触的可塑性、促进基因转录等效应[9-10]。神经元胞内Ca2+水平的变化可直接影响神经元的兴奋性[11]。由Ang II介导产生ROS的潜在信号机制是Ca2+的相关机制[12-13]。研究表明,Ang II可引起Ca2+电流的增加和K+电流的降低,从而通过调节动作电位的产生而增强神经元放电频率[14]。ROS介导的神经元胞内[Ca2+]i的增加可能与细胞毒性及神经系统退行性疾病相关。但是,由Ang II介导的ROS及Ca2+的相关信号机制在心血管的中枢调控过程中仍有待进一步阐明。
因此,本研究主要通过给予Ang II观察其对延髓神经元的ROS及[Ca2+]i的调节,并进一步对可能的作用机制进行探讨,为阐明Ang II介导的ROS在心血管的中枢调控过程中发挥作用的细胞内信号机制提供实验依据。
材 料 和 方 法
1 主要试剂与仪器
Ang II、apocynin、TEMPOL和二氢乙啶(dihydroethidium, DHE)购自Sigma;Fura-2/AM和 CCK-8试剂盒购自Dojindo;小鼠来源MAP-2抗体购自Abcam;兔来源谷氨酸抗体购自Sigma-Aldrich;神经元培养液、B27、胎牛血清(fetal bovine serum, FBS)和2.5%胰酶购自Gibco;DAPI、FITC标记山羊抗兔IgG (H+L)和Cy3标记山羊抗小鼠IgG (H+L)购自碧云天。CO2恒温细胞培养箱(上海力申科学仪器有限公司);无菌超净台(上海博迅实业有限公司医疗设备厂);激光共聚焦系统(ZEISS);酶标仪(TECAN);钙瞬变系统(PTI)。
2 方法
2.1 原代延髓神经元培养[5]取孕14~17 d的SD大鼠(购自上海斯莱克实验动物中心),取胎鼠断头;在解剖显微镜下分离延髓,剪碎(约1 mm3);0.125%胰蛋白酶溶液于37 ℃细胞培养箱中消化10 min左右;10% 的FBS终止消化;取上清至新的离心管;1 000 r/min离心8 min;弃上清,加入Neurobasal medium 与B27的混合培养基(比例为49∶1),重悬细胞。将细胞接种到35 mm的培养皿或96孔板(前1 d用多聚赖氨酸处理)中,使细胞在37 ℃、 5% CO2条件下贴壁生长;每2 d半量换液1次,培养7 d左右,待神经元发育成熟,进行实验。
2.2 神经元的鉴定[5]细胞经培养7 d左右,用0.01 mol/L PBS洗3次,4%多聚甲醛室温固定细胞20 min;弃固定液,0.01 mol/L PBS洗3次;5%~7%胎牛血清封闭30 min;弃封闭液,加入Ⅰ抗:小鼠抗MAP-2抗体(1∶200,1%FBS配制),37℃孵育1 h后转入4 ℃过夜;弃Ⅰ抗,0.01 mol/L PBS洗3次;加入Ⅱ抗:Cy3结合的山羊抗小鼠IgG (H+L)(1∶100,Ⅱ抗稀释液配制),避光,37 ℃孵育1 h; 0.01 mol/L PBS洗3次;加入DAPI染核5 min; 0.01 mol/L PBS洗3次;采用激光共聚焦系统拍照鉴定。
2.3 延髓谷氨酸阳性神经元的鉴定 方法同2.2。第一Ⅰ抗为小鼠抗MAP-2抗体(1∶200)和第二Ⅰ抗为兔抗谷氨酸抗体(1∶100)。Ⅱ抗为FITC标记山羊抗兔IgG (H+L); Cy3标记山羊抗小鼠IgG (H+L)。

2.5 神经元胞内游离Ca2+的测定 以钙离子敏感的荧光探针Fura-2/AM来检测细胞内[Ca2+]i。Fura-2能以1∶1的比例特异性地与Ca2+结合,并且结合Ca2+后,其最大激发波长由原来Fura-2的380 nm变为其与Ca2+结合后的复合物的340 nm,通过钙瞬变系统检测延髓神经元F340/380的比值(R),采用R/R0来评估给药前后细胞内Ca2+的变化,其中R0代表药物处理前的荧光信号,R代表药物处理后的荧光信号。实验时,将已孵育好Fura-2/AM的神经元培养皿固定在载物台上,用HBSS溶液灌流细胞,待钙瞬变系统基线稳定后,用不同药物灌流细胞。以上操作由FelixGX-4.2.2软件执行。
2.6 CCK-8实验检测神经元的活性 神经元种于96孔板;培养的延髓神经元给予不同药物处理后,弃含药物的培养基,D-Hank’s溶液洗3遍;每孔加入110 μL CCK-8与培养基的混合液(二者体积比为1∶10),置于37 ℃细胞培养箱中孵育2 h;用酶标仪测定450 nm处的吸光度。
3 统计学处理
采用SPSS 19.0统计软件进行分析。实验结果用均数±标准误(mean±SEM)表示。两组实验数据采用独立样本t检验,多组数据采用单因素方差分析(one-way ANOVA)。以P<0.05为差异有统计学意义。
结 果
1 原代延髓神经元的培养及鉴定
采用激光共聚焦结合显微镜观察的方法对培养的细胞进行鉴定,图1为培养5~7 d的延髓细胞,免疫荧光染色可见红色的特异性MAP-2标记细胞,且可见原代培养的延髓细胞90%以上为神经元。
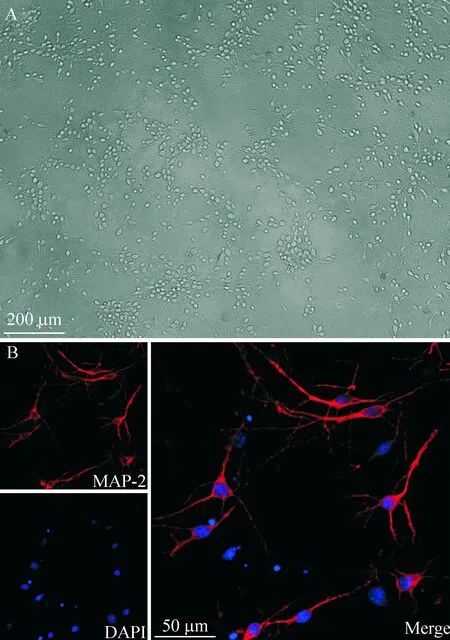
Figure 1.Identification of the primarily cultured medullary neurons. A: the white-light graph of the primarily cultured medullary neurons; B: fluorescence micrograph of the neurons stained with anti-MAP-2 antibody, fluorescence micrograph of neurons stained with DAPI, and merged graph of the former 2 photos.
图1 原代延髓神经元的培养及鉴定
2 延髓谷氨酸阳性神经元的鉴定
由于中枢调节心血管活动的神经元大多为谷氨酸阳性的神经元,因此我们用谷氨酸去标记培养的延髓神经元。图2可见红色为MAP-2标记的神经元,绿色为谷氨酸阳性的细胞,蓝色为DAPI标记的细胞核。结果显示原代培养的延髓神经元80%以上为谷氨酸阳性的神经元。

Figure 2.Identification of glutamatergic neurons. The confocal images were the fluorescence micrographs of the neurons stained with anti-MAP-2 antibody (a neuronal maker), anti-glutamate (Glu) antibody and DAPI, and the merged graph of the former 3 photos.
图2 延髓谷氨酸阳性神经元的鉴定
3 Ang II升高延髓神经元ROS的水平
前期研究显示,Ang II(5 μmol/L)可显著升高小鼠神经母细胞瘤细胞ROS水平[12]。在本研究中,给予Ang II(5 μmol/L),37 ℃分别孵育5、10 和30 min后,采用DHE荧光探针法检测延髓神经元ROS水平。结果显示Ang II可在10 min内显著升高神经元的ROS水平,见图3。
4 Ang II 引起神经元胞内游离Ca2+水平的升高
为研究Ang II对延髓神经元胞内[Ca2+]i的影响,我们首先研究单独给予Ang II对神经元包内游离Ca2+水平的影响。结果显示:Ang II可在10 min左右显著升高神经元胞内[Ca2+]i,并在其升高达到稳定并持续约2 min后,再给予HBSS冲洗后,神经元胞内[Ca2+]i开始下降,见图4。

Figure 3.The effects of Ang II on the ROS levels in the primarily cultured medullary neurons treated with or without Ang II (5 μmol/L) for 5, 10 and 30 min. ROS level was determined using the oxidant-sensitive fluorogenic probe dihydroethidium (DHE). Mean±SEM.n=12.*P<0.05,**P<0.01vs0 min group.
图3 Ang II升高延髓神经元ROS水平
5 ROS 介导Ang II对延髓神经元[Ca2+]i的升高作用
延髓神经元给予NADPH氧化酶抑制剂apocynin或自由基清除剂TEMPOL预处理后,观察Ang II 对神经元[Ca2+]i的效应,结果显示apocynin和TEMPOL均可抑制Ang II对延髓神经元[Ca2+]i的升高作用,见图5。
6 Ang II对延髓神经元无毒性作用
考虑到Ang II可能对神经元产生毒性作用,我们用CCK-8法检测了实验浓度的Ang II孵育神经元30 min后的细胞活力,结果显示实验中所用浓度的Ang II (5 μmol/L)不影响神经元的细胞活力,见图6。
讨 论
研究表明,NADPH氧化酶来源的ROS在Ang II依赖的心血管疾病的中枢调控过程中起着非常重要的调节作用[8, 15]。在高血压的发病过程中,中枢及外周Ang II 可通过穹窿下区(suofornial organ,SFO)-室旁核(paraventricularis nucleus,PVN)-延髓头端腹外侧(the rostral ventrolateral medulla, RVLM)的通路,最后通过脊髓中间外侧柱(intermediolateral column of spinal cord)的传出将兴奋传递至外周,引起交感神经兴奋,而导致高血压的发生[16]。在此过程中,RVLM区作为心血管交感活动的基本中枢,接受中枢心血管效应相关核团SFO、PVN等的纤维投射,在维持心血管中枢紧张性活动中起关键作用[16]。本课题组前期研究表明Ang II 可显著升高延髓神经元的ROS水平,且主要是通过激活NADPH氧化酶产生[5-6];RVLM区微量注射NaHS可显著降低SHR大鼠的血压的心率,其机制可能是通过抑制NADPH氧化酶p47phox亚单位的磷酸化从而降低酶的活性,使ROS生成减少而所致[17]。因此研究中枢ROS的细胞内信号机制对阐明Ang II诱导的心血管疾病中枢调控机制具有重要的应用价值。

Figure 4.The effect of Ang II on [Ca2+]iin the primarily cultured medullary neurons. A: typical elevation of [Ca2+]iinduced by Ang II at 5 μmol/L; B: typical elevation of [Ca2+]iinduced by Ang II at 5 μmol/L and washout of Ang II led [Ca2+]ito decline; C: the quantitative analysis of the peak increase in [Ca2+]iin the neurons stimulated with Ang II (5 μmol/L). Mean±SEM.n=8.**P<0.01vscontrol.
图4 Ang II引起延髓神经元胞内游离Ca2+水平的升高
在前期研究的基础上,本工作主要采用离体实验在细胞水平观察ROS是否介导Ang II对细胞内行了鉴定,结果显示培养的细胞中90%以上为神经元。由于中枢调节心血管活动的神经元大多为谷氨酸阳性的神经元,本研究重点观察了培养的延髓神经元中谷氨酸的表达变化。结果显示原代培养的延髓神经元80%以上为谷氨酸阳性神经元,为进一步研究奠定了细胞学基础。

Figure 5.The effect of Ang II on [Ca2+]iin the primarily cultured medullary neurons pretreated with apocynin (an inhibitor of NADPH oxidase) or TEMPOL (a cell membrane-permeable superoxide dismutase mimetic). A, B: typical suppression of [Ca2+]ilevel induced by Ang II (5 μmol/L) with or without apocynin (100 μmol/L) or TEMPOL (100 μmol/L); C: the quantitative analysis showed the effects of Ang II were atte-nuated by apocynin or tempol. Mean±SEM.n=6.**P<0.01vscontrol group;#P<0.05vsAng II group.
图5 ROS 介导Ang II对延髓神经元[Ca2+]i的升高作用
Ca2+水平的调节作用。并采用免疫荧光双标结合激光共聚焦显微镜的方法对原代培养的延髓神经元进已证明,Ca2+作为重要的第二信使,参与神经递质的释放、突触可塑性的调节、神经元的兴奋性及基因转录的调节[9-10];神经元[Ca2+]i的变化可直接影响神经的兴奋性[11];而[Ca2+]i在神经元之间的信号传递中起着非常重要的作用,并受细胞膜上的Ca2+通道和细胞内钙库的调控[18],提示研究神经元Ca2+的相关机制对其功能具有重要意义。为明确Ang II对延髓神经元[Ca2+]i的影响,我们采用Fura-2/AM法记录神经元胞内[Ca2+]i的变化。结果显示Ang II可在10 min左右显著升高神经元[Ca2+]i,并在其升高达到稳定后,再给予HBSS冲洗后,神经元[Ca2+]i开始下降,提示Ang II可显著升高延髓神经元[Ca2+]i。由于ROS参与了中枢Ang II所致的交感兴奋与心血管效应,且主要与NADPH氧化酶途径的激活有关[3-4, 7-8]。因此,为明确Ang II对神经元ROS的影响,我们首先给予Ang II处理后检测延髓神经元ROS水平。结果显示Ang II可在10 min内显著升高神经元ROS水平,提示Ang II可显著升高延髓神经元ROS水平。研究表明,apocynin可通过特异性地抑制NADPH氧化酶(ROS生成的关键酶)亚单位gp91ds-tat而选择性地抑制NADPH氧化酶活性,从而使ROS生成减少[8];而TEMPOL则作为一种自由基清除剂可降低细胞ROS水平[19]。在本研究中,我们给予apocynin (NADPH氧化酶抑制剂)或TEMPOL(自由基清除剂)预处理后,再观察Ang II 对神经元[Ca2+]i的效应,结果显示apocynin和TEMPOL均可抑制Ang II对延髓神经元[Ca2+]i的升高作用,提示ROS 介导Ang II对延髓神经元[Ca2+]i的升高作用。此外,考虑到Ang II可能对神经元产生毒性作用,本工作采用CCK-8法检测了实验浓度的Ang II孵育神经元30 min后的细胞活力,结果显示实验中所用浓度的Ang II不影响神经元的细胞活力,提示实验中所用浓度的Ang II对神经元无毒性作用。
本工作的实验结果提示ROS介导Ang II诱导的延髓神经元胞内Ca2+的升高作用,可能是Ang II在中枢诱导氧化应激作用的潜在细胞内信号机制。

Figure 6.The cell viability analyzed by CCK-8 assay showed no significant difference of the cell activity between Ang II group and control group. Mean±SEM.n=6.
图6 Ang II对神经元无毒性作用
[1] Giam B, Kaye DM, Rajapakse NW. Role of renal oxidative stress in the pathogenesis of the cardiorenal syndrome[J]. Heart Lung Circ, 2016, 25(8):874-880.
[2] Tamura K, Wakui H, Azushima K, et al. Angiotensin II type 1 receptor binding molecule ATRAP as a possible modulator of renal sodium handling and blood pressure in pathophysiology[J]. Curr Med Chem,2015,22(28):3210-3216.
[3] de Kloet AD, Liu M, Rodriguez V, et al. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control[J]. Am J Physiol Regul Integr Comp Physiol, 2015, 309(5):R444-R458.
[4] Lu N, Helwig BG, Fels RJ, et al. Central Tempol alters basal sympathetic nerve discharge and attenuates sympathetic excitation to central ANG II[J]. Am J Physiol Heart Circ Physiol, 2004, 287(6):H2626-H2633.
[5] 曹冬青, 刘小妮, 徐海艳, 等. 内源性H2S抑制angiotensin Ⅱ引起的神经元活性氧水平的升高[J]. 中国病理生理杂志, 2014, 30(5):837-841.
[6] 马 红, 于海云, 于 燕, 等. H2S抑制Ang Ⅱ引起的神经元活性氧水平升高的机制研究[J]. 中国病理生理杂志, 2012, 28(5):865-869.
[7] Chan SH, Chan JY. Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases[J]. Antioxid Redox Signal, 2013, 19(10):1074-1084.
[8] Sun C, Sellers KW, Sumners C, et al. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II[J]. Circ Res, 2005, 96(6):659-666.
[9] Berridge MJ. Neuronal calcium signaling[J]. Neuron,1998, 21(1):13-26.
[10]Lynch MA. Long-term potentiation and memory[J]. Phy-siol Rev,2004,84(1):87-136.
[11]Yong QC, Choo CH, Tan BH, et al. Effect of hydrogen sulfide on intracellular calcium homeostasis in neuronal cells[J]. Neurochem Int, 2010, 56(3):508-515.
[12]Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells[J]. Hypertension, 2005, 45(4):717-723.
[13]Chan SH, Chan JY. Brain stem NOS and ROS in neural mechanisms of hypertension[J]. Antioxid Redox Signal, 2014, 20(1):146-163.
[14]Sumners C, Zhu M, Gelband CH, et al. Angiotensin II type 1 receptor modulation of neuronal K+and Ca2+currents: intracellular mechanisms[J]. Am J Physiol, 1996, 271(1 Pt 1):C154-C163.
[15]Chan SH, Wu KL, Chang AY, et al. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension[J]. Hypertension, 2009, 53(2):217-227.
[16]Braga VA, Medeiros IA, Ribeiro TP, et al. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: implications in neurogenic hypertension[J]. Braz J Med Biol Res, 2011, 44(9):871-876.
[17]Yu H, Xu H, Liu X, et al. Superoxide mediates depressive effects induced by hydrogen sulfide in rostral ventrolateral medulla of spontaneously hypertensive rats[J]. Oxid Med Cell Longev, 2015, 2015:927686.
[18]Clapham DE. Calcium signaling[J]. Cell, 2007, 131(6):1047-1058.
[19]Zimmerman MC, Dunlay RP, Lazartigues E, et al. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain[J]. Circ Res, 2004, 95(5):532-539.
(责任编辑: 卢 萍, 罗 森)
ROS mediates regulation of intracellular Ca2+induced by angiotensin II in primarily cultured medullary neurons
LIU Xiao-ni1, CAO Dong-qing2, ZHANG Na-na1, TAO Ran1, DING Ying-jiong1, JIN Hui-ming1, LU Ning1
(1DepartmentofPhysiologyandPathophysiology,SchoolofBasicMedicalSciences,FudanUniversity,Shanghai200032,China;2DepartmentofNeurosurgery,HuashanHospital,FudanUniversity,Shanghai200040,China.E-mail:luning7@shmu.edu.cn)
AIM: To investigate the role of reactive oxygen species (ROS) in the regulation of intracellular Ca2 + induced by angiotensin II (Ang II) in the primarily cultured medullary neurons. METHODS: Primarily cultured medullary neurons were prepared from 14-day-old embryos of Sprague-Dawley rats in the study. The identification of medullary neurons was assessed by double-labeling immunofluorescence. To explore the role of ROS,mainly the superoxide ( O2 -· ) , the O2 -· generation was measured using the fluorogenic probe dihydroethidium (DHE) . To determine intracellular free calcium concentration ( [Ca2 +]i ) ,the neurons were loaded with the Ca2+-specific dye Fura-2 /AM. The cell viability(DHE).Todetermineintracellularfreecalciumconcentration([Ca2+]i),theneuronswereloadedwiththeCa2+-specificdyeFura-2/AM.ThecellviabilityafteraddingAngIIwasalsoexaminedusingCCK-8assay. RESULTS: Most of the cultured cells were medullary neurons, more than 80% of which were glutamate positive neurons. Ang II (5 μmol/L) increased the level of ROS within 10 min in the medullary neurons. Ang II at 5 μmol/L induced a significant [Ca2+]iincrease in the medullary neurons, and the effect of Ang II occurred rapidly and reached a peak within 20 min after administration. The level of [Ca2+]istarted to decline after washout. The Ca2+elevation induced by Ang II was significantly decreased by apocynin or TEMPOL. No significant difference in the cell viability between control group and 5 μmol/L Ang II treatment group was observed. CONCLUSION: ROS is involved in the regulation of [Ca2+]iinduced by Ang II in the primarily cultured medullary neurons, suggesting a potential intracellular signaling mechanism involved in the Ang II-mediated oxidant regulation of central neural control of blood pressure.
Medullary neurons; Reactive oxygen species; Intracellular Ca2+; Angiotensin II
1000- 4718(2016)12- 2133- 06
2016- 07- 12
2016- 09- 05
国家自然科学基金资助项目(No. 81170237);国家基础科学人才培养基金资助项目(No. J1210041)
R363.2
A
10.3969/j.issn.1000- 4718.2016.12.003
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 021-54237452; E-mail: luning7@shmu.edu.cn
