人参中三萜类化学成分的研究△
2016-09-25杨秀伟
杨秀伟
(北京大学 天然药物及仿生药物国家重点实验室,北京大学 药学院 天然药物学系,北京 100191)
人参中三萜类化学成分的研究△
杨秀伟*
(北京大学 天然药物及仿生药物国家重点实验室,北京大学 药学院 天然药物学系,北京100191)
人参皂苷系人参根和根茎的活性成分,参与调节多种生理活性。在我们前文总结的基础上,本文概述人参根和根茎、茎叶、果实、种子和红参中三萜类化学成分的研究,为源于人参的现代中药的研究与开发提供科学依据。迄今,已从人参分离鉴定了201个三萜类化合物,其中189个归属为达玛烷型三萜及其衍生物,10个归属为齐墩果酸型三萜,2个归属为羽扇豆烷型三萜;不但在含量上,而且在化学结构多样性上,达玛烷型三萜及其衍生物占绝对优势地位。
人参;茎叶;花蕾;果实;种子;红参;人参皂苷;三萜
五加科(Araliaceae)人参属(PanaxL.)植物人参PanaxginsengC.A.Meyer系驰名中外的植物药,传统药用部位为干燥根和根茎(Ginseng Radix et Rhizoma),始载于《神农本草经》。《本草纲目拾遗》亦记载人参叶代人参根之药用,“味苦微甘”,“清肺、生津、止渴”;近代,《中华人民共和国药典》一部不但收载了人参根和根茎为法定药用部位,《中华人民共和国药典》2005版亦收载了人参叶、《中华人民共和国药典》2010版和2015版收载了人参总皂苷和人参茎叶总皂苷。现代药理学和生物学活性研究表明人参中的三萜或三萜皂苷类化合物几乎反映了人参的全部药物学作用。人参根和根茎中的人参皂苷(ginsenosides,G)含量约为3~5%,以达玛烷型(dammarane-type)四环三萜及其皂苷为特征性成分,根据苷元结构不同,可分为原人参二醇型(protopanaxadiol-type,I型),如G-Rb1、Rb2、Rc和Rd;原人参三醇型(protopanaxatriol-type,II型),如G-Re、Rf、Rg1;为主要人参皂苷,含量之和约占总皂苷的90%以上,较高,称之为常见人参皂苷。而一些低极性的人参皂苷如G-Rg2、Rg3、Rg5、Rg6、Rh1、Rh2、Rk1、Rk3、F4等,在人参根和根茎中含量很低或不含(但在红参或地上部分中含有),称之为稀有人参皂苷(rare ginsenosides)。稀有人参皂苷多为常见人参皂苷的脱糖基化产物,疏水性和穿越细胞性增强,如G-Rg3、Rh1、Rh2、Rh3有更强的抗肿瘤、抗癌细胞转移、保肝、保护神经、免疫刺激和血管扩张活性。人参化学研究表明,人参地上部分含有化学结构多样性的稀有人参皂苷。此外,人参中还含有齐墩果酸型(oleanolic acid-type,III型)苷元结构的皂苷,以及少量奥克梯隆型(ocotillone-type,IV型)苷元结构的皂苷(见图1),严格地讲,奥克梯隆型是达玛烷型的衍生物。为全面揭示人参药物学作用的物质基础、阐明人参三萜类化合物结构多样性与生物学活性多样性的关系、寻找优秀的药物分子以研究开发源于人参的现代中药奠定基础,前文总结了人参根和根茎及其工业产品红参[1]、人参茎叶[2]化学成分的研究,本文进行补遗,并总结人参花蕾、果实等中的三萜类化学成分的研究。
1 人参根、根茎及其工业产品红参
前文[1]总结了人参根、根茎及其工业产品红参中的70个三萜皂苷。2012年,文献报道[3]以正交色谱-液相色谱质谱联用为导向,从人参根分离鉴定了2个新的化合物,人参皂苷(ginsenoside)IV(1)和V(2),以及已知结构的三七皂苷(notoginsenoside;NG)A(3)、K(4)。2015年又报道从人参根分离鉴定出达玛烷型的绞股蓝皂苷V(gypenosideV)、6-O-[α-L-吡喃鼠李糖基-(1→2)-β-D-吡喃葡萄糖基]-20-O-β-D-吡喃葡萄糖基-3β,12β,20(S)-二羟基-达玛-25-烯-24-酮{6-O-[α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosyl]-20-O-β-D-glucopyranosyl-3β,12β,20(S)-dihydroxy-dammar-25-en-24-one}、珠子参皂苷F6(majoroside F6)、拟人参皂苷Rt3(pseudogin-senoside-Rt3)、越南人参皂苷R15(vinaginsenoside-R15)和齐墩果烷型的金盏花皂苷B(齐墩果酸-3-O-β-D-吡喃葡萄糖醛酸苷;calenduloside B)[4]。从红参分离鉴定了人参皂苷Re2(ginsenoside Re2)、20(R)-人参皂苷Rs3[20(R)-ginsenoside Rs3,5][5]、20(S)-人参皂苷-Rf-1a[20(S)-ginsenoside-Rf-1a,6][6]、20(R)-人参皂苷Rf[20(R)-ginsenoside Rf,7]、20(R)-三七皂苷R2[20(R)-notoginsenoside R2,8]、20(22)Z-人参皂苷Rs4[20(22)Z-ginsenoside Rs4=20(Z)-ginsenoside Rs4,9][6]、人参皂苷Rz1(ginsenoside Rz1,10)[5]、20(22)E-人参皂苷Rg9[20(22)E-ginsenoside Rg9=20E-ginsenoside Rg9,11][5,7]、3β,12β-二羟基达玛-20(22)E,24-二烯-6-O-β-D-吡喃木糖基-(1→2)-O-β-D-吡喃葡萄糖苷(3β,12β-dihydroxydammar-20(22)E,24-diene-6-O-β-D-xylopyranosyl-(1→2)-O-β-D-glucopyranoside,12)[5]、12-O-葡萄糖基人参皂苷Rh4(12-O-glucoginsenoside Rh4,13)[8]、20(22)Z-人参皂苷Rg9[20(22)Z-ginsenoside Rg9=20Z-ginsenoside Rg9,14][7]、20(22)Z-人参皂苷Rh4[20(22)Z-ginsenoside Rh4,15]、20(22)Z-人参皂苷F4[20(22)Z-ginsenoside F4=ginsenoside F4=ginsenoside Rg4,16][5]、人参皂苷Rg10[ginsenoside Rg10,17][7]、12β,25-二羟基达玛-20(22)E-烯-3-O-β-D-吡喃葡萄糖基-(1→2)-O-β-D-吡喃葡萄糖苷[12β,25-dihydroxydammar-20(22)E-ene-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranoside,18][5]、人参皂苷Rh10(ginsenoside Rh10,19)、人参皂苷Rg11(ginsenoside Rg11,20)[8]、23-O-甲基人参皂苷Rg11(23-O-methylginsenoside Rg11,21)[6],上述化合物均为达玛烷型三萜皂苷。同时,从红参还分离鉴定了齐墩果酸型皂苷:人参皂苷Ro-甲酯(ginsenoside Ro methyl ester)、聚乙炔基人参皂苷Ro(polyacetyleneginsenoside Ro)[5]、人参皂苷Ro-6′-丁酯(ginsenoside-Ro-6′-butyl ester,22)[6]、竹节参苷IVa甲酯(chikusetsusaponin IVa methyl ester,23)、竹节参苷IVa丁酯(chikusetsu-saponin IVa butyl ester,24)、姜状三七苷R1-6′-丁酯(zingibroside R1-6′-butyl ester],25)和姜状三七苷R1-6′-甲酯(zingibroside R1-6′-methyl ester,26)[5]。
2 人参茎叶
由于人参茎叶中含有结构多样性的稀有达玛烷型三萜及其皂苷,近年来得到重视。前文[2]总结了从人参茎叶中分离鉴定的58个三萜类化合物。近年来又从人参茎叶中分离鉴定出人参皂苷(ginsenoside)Rh14(27)、Rh15(28)、Rh16(29)、Rh17(30)[9]、Rh18(31)、Rh19(32)、Rh20(33)[10],达玛-20(22)E,25-二烯-3β,6α,12β,24S-四醇[dammara-(20)22E,25-diene-3β,6α,12β,24S-tetrol,34],12β,23R-环氧达玛-24-烯-3β,6α,20S-三醇(12β,23R-epoxydammara-24-ene-3β,6α,20S-triol,35)[10],三七皂苷B1(sanchinoside B1,36),3β,6α,12β,25-四羟基达玛-20(22)E-烯-6-O-α-L-吡喃鼠李糖基-(1→2)-β-D-吡喃葡萄糖苷[3β,6α,12β,25-tetrahydroxydammar-20(22)E-ene-6-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside,37],达玛-20(22)E-烯-3β,6α,12β,25-四醇[dammar-20(22)E-ene-3β,6α,12β,25-tetraol,38],人参皂苷(ginsenoside)Rg5、Rh4、Rk1、Rk3,达玛-20(21),24-二烯-3β,6α,12β-三醇[dammar-20(21),24-diene-3β,6α,12β-triol,39],三七皂苷T2(notoginsenoside T2,40)[11],20(S)-原人参二醇[20(S)-protopanaxadiol,41],20(S)-原人参三醇[20(S)-protopanaxatriol,42],20(S)-人参皂苷Rf2[20(S)-Ginsenoside-Rf2,43][12]。从人参叶分离鉴定出对肝癌HepG2细胞有细胞毒活性的20(R),22(ζ),24(S)-达玛-25(26)-烯-3β,6α,12β,20,22,24-六醇[20(R),22(ζ),24(S)-dammar-25(26)-ene-3β,6α,12β,20,22,24-hexanol,44][13];对肝癌Hep3B和肺癌A549细胞有细胞毒活性的3-O-β-D-吡喃葡萄糖基-20(S)-原人参三醇[3-O-β-D-glucopyranosyl-20(S)-proto-panaxtriol,45]、3-甲酰氧基-20-O-β-D-吡喃葡萄糖基-20(S)-原人参三醇[3-formyloxy-20-O-β-D-glucopyranosyl-20(S)-protopanaxatriol,46]和26-羟基-24(E)-20(S)-原人参三醇[26-hydroxyl-24(E)-20(S)-protopanaxatriol,47][14];对脂多糖刺激的鼠性巨噬细胞白细胞介素-12表达有促进作用的3β,20(S)-二羟基达玛-24-烯-12β,23β-环氧-20-O-β-D-吡喃葡萄糖苷[3β,20(S)-dihydroxydammar-24-en-12β,23β-epoxy-20-O-β-D-glucopyranoside,48]和27-去甲基-(E,E)-20(22),23-二烯-3β,6α,12β-三羟基达玛-25-酮[27-demethyl-(E,E)-20(22),23-dien-3β,6α,12β-trihydroxydammar-25-one,49][15];对沉默信息调节因子2基因1(silent information regulator two homologue 1,SIRT1)有激活作用的达玛-20(22)E,24-二烯-3β,6α,12β-三醇[dammar-20(22)E,24-diene-3β,6α,12β-triol,50]、6α,20(S)-二羟基达玛-3,12-二酮-24-烯[6α,20(S)-dihydroxydammar-3,12-dione-24-ene,51]和6α,20(S),25-三羟基达玛-3,12-二酮-23-烯[6α,20(S),25-trihydroxydammar-3,12-dione-23-ene,52],以及无活性的6α,20(S),24(S)-三羟基达玛-3,12-二酮-25-烯[6α,20(S),24(S)-trihyd-roxydammar-3,12-dione-25-ene,53][16]。Qiu等[17]声称采用2D LC/LTQ-Orbitrap-MS/NMR技术从人参茎叶中检出646个人参皂苷,不过许多的结构需要进一步获得纯单体化合物后确认。
3 人参花蕾
人参花蕾化学成分的研究始于20世纪80年代末,分离鉴定出人参皂苷(ginsenoside)Ro、Rb1、Rb2、Rb3、Rc、Rd、Re、Rf、Rg1、Rg2[18-19]、I(54)、II(55)[20]、III(56)[21]、F1[22-23]、F3[22]、F5[22-23]、M7cd[24],20-葡萄糖基人参皂苷Rf[18],20(R)-人参皂苷Rh1[19,25],20(S)-人参皂苷Rh1[25],20(S)-人参皂苷Rg2,20(R)-人参皂苷Rg2,20(R)-原人参三醇[19],20(S)-原人参三醇[25],人参花皂苷(floralginsenoside)A(57)、B(58)、C(59)、D(60)、E(61)、F(62)[24]、G(63)、H(64)、I(65)、J(66)、K(67)[26]、Ka(68)、Kb(69)、Kc(70)[24]、La(71)、Lb(72)[24,26]、M、N[24,27]、O(73)、P(74)[27]、Ta、Tb(75)、Tc(76)、Td(77)[23],绞股蓝皂苷XVII(gypenoside X)[19,22],珠子参皂苷(majoroside)F1(78)[24]、F6(79)[26],三七皂苷E(notoginsenoside E,80)[19,26],拟人参皂苷(pseudoginsenoside)RC1[22]、RS1(81)[26],越南人参皂苷(vina-ginsenoside)R4[24,26]、R9(82)、R15(83)[24],达玛-20(21),24-二烯-3β,6α,12β-三醇,达玛-20(22)E,24-二烯-3β,6α,12β-三醇[25]。
4 人参果实(浆果)
人参果实化学成分的研究始于20世纪80年代末,分离鉴定出人参皂苷(ginsenoside)Rb1[28-30]、Rb2、Rc[28-29]、Rd、Re、Rg1[28-30]、Rg2、Rg3[30]、Rh1[28]、Rh2[28,30]、Rh4、F1[31],20(R)-人参皂苷Rg2[32-33],20(R)-人参皂苷Rg3,20(R)-人参皂苷Rf2[20(R)-ginsenoside Rf2=25-hydroxy-20(R)-ginsenoside-Rg2][33],20(R)-人参皂苷Rh2[34],人参皂苷化合物K(ginsenoside compound K;20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol,84)[29],异人参皂苷Rh3(isoginsenoside Rh3,85)[28-29],20(S)-原人参二醇[30-31],20(S)-原人参三醇,20(R)-原人参三醇[31,33],20(R)-达玛烷-3β,12β,20,25-四醇[20(R)-dammarane-3β,12β,20,25-tetrol,86],20(R)-达玛烷-3β,6α,12β,20,25-五醇[20(R)-dammarane-3β,6α,12β,20,25-pentol][30]。
5 人参种子
与人参其他部位的三萜类化合物研究相比,人参种子的研究相对滞后。2009年报道从人参种子分离鉴定出达玛烷型的三萜类化合物20(S)-原人参三醇,3-酮基-20(S)-原人参三醇[3-keto-20(S)-protopanaxatriol,87],人参皂苷Rd、Re、Rg2[35];奥克梯隆(ocotillone)型的三萜类化合物人参三萜二酮[panaxadione;(6α,24R)-20,24-epoxy-6,25-dihydroxy-dammarane-3,12-dione,88][35];以及羽扇豆烷(lupane)型三萜类化合物3β-反式阿魏酰氧基-16β-羟基羽扇豆-20(29)-烯[3β-trans-feruloyloxy-16β-hydroxylup-20(29)-ene,89][35-36],3β-顺式阿魏酰氧基-16β-羟基羽扇豆-20(29)-烯[3β-cis-feruloyloxy-16β-hydroxylup-20(29)-ene,90][36]。
化合物1~90的化学结构见图2。
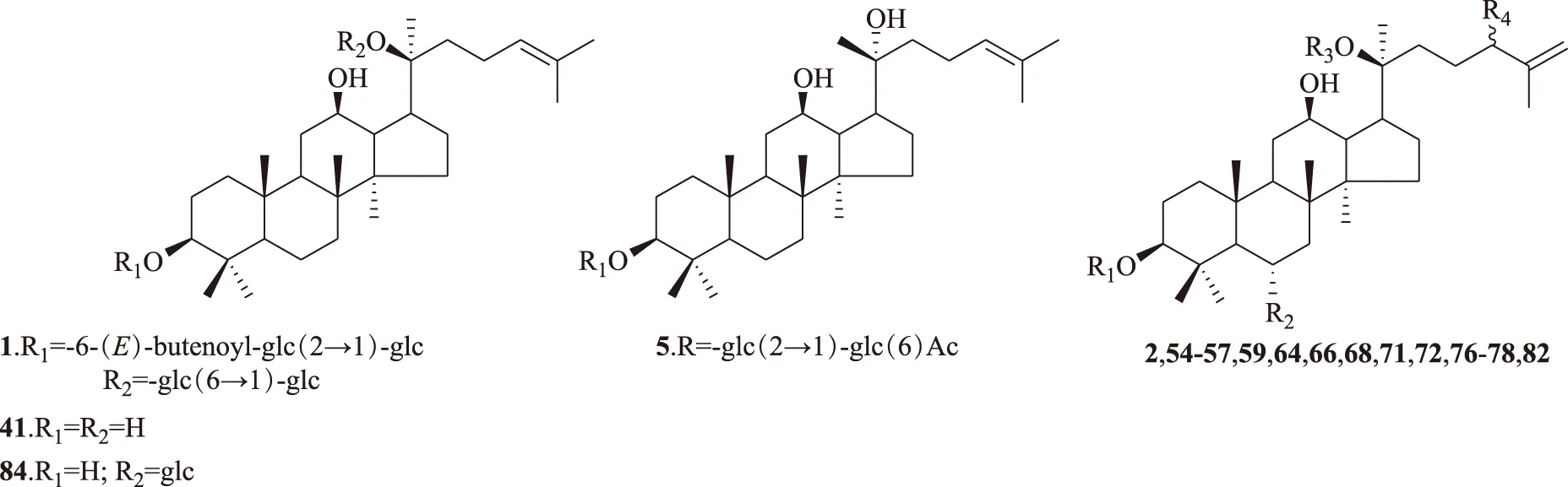

R1R2R3R42.glc(2→1)-glcH-glc(6→1)-glcR-OH54.-glc(2→1)-glcHglcS-OOHorR-OOH55.-glc(2→1)-glcHglcR-OOHorS-OOH56.-glc(2→1)-glcHglc=O57.H-O-glcglc-OOH59.HOH-glc(6→1)-ara(p)-OOH64.-glc(2→1)-glc(6)AcHglc-OOH66.H-O-glc(2→1)-rhaglc-OOH68.HOHglc-OOH71.H-O-glc(2→1)-rhaglcS-OH72.H-O-glc(2→1)-rhaglcR-OH76.-glc(2→1)-glcH-glc(6→1)-ara(p)R-OOHorS-OOH77.-glc(2→1)-glcH-glc(6→1)-ara(p)S-OOHorR-OOH78.-glc(2→1)-glcHglcR-OH82.-glc(2→1)-glcHglcS-OH
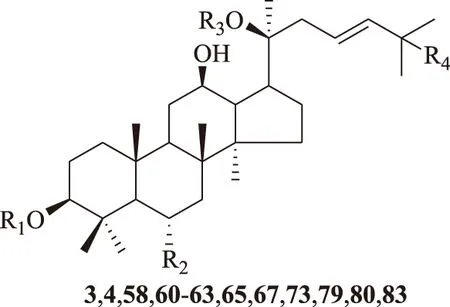

R1R2R3R43.-glc(2→1)-glcH-glc(6→1)-glcOH4.-glc(2→1)-glcH-glc(6→1)-glcOOH58.H-O-glcglcOOH60.HOH-glc(6→1)-ara(f)OOH61.-glc(2→1)-glcHHOOH62.glcHglcOOH63.-glc(2→1)-glc(6)AcHglcOOH65.H-O-glc(2→1)-rhaglcOOH67.-glc(2→1)-glcOHglcOOH73.-glc(2→1)-glcH-glc(6→1)-ara(f)OOH79.H-O-glc(2→1)-L-rhaglcOH80.-glc(2→1)-glcHglcOOH83.H-O-glcglcOH


R1R2R36.OH-glc(4→1)-α-D-glcH7.OH-glc(2→1)-glcH8.OH-glc(2→1)-xylH30.=O-glc(2→1)-rhaH32.OglcHH42.OHHH45.OglcHH46.-OOCHHglc74.-O-glc(2→1)-glcH-glc(6→1)-ara(p)81.OH-glc(6)Ac(2→1)-rhaglc


R1R2R311.H-O-glc(2→1)-glcH12.H-O-glc(2→1)-xylH13.H-O-glcglc14.H-O-glc(2→1)-glcH15.H-O-glcH16.H-O-glc(2→1)-rhaH29.glcOHH50.HOHH85.glcHH


R1R218.-glc(2→1)-glcH19.glcH36.H-O-glc37.H-O-glc(2→1)-rha38.HOH

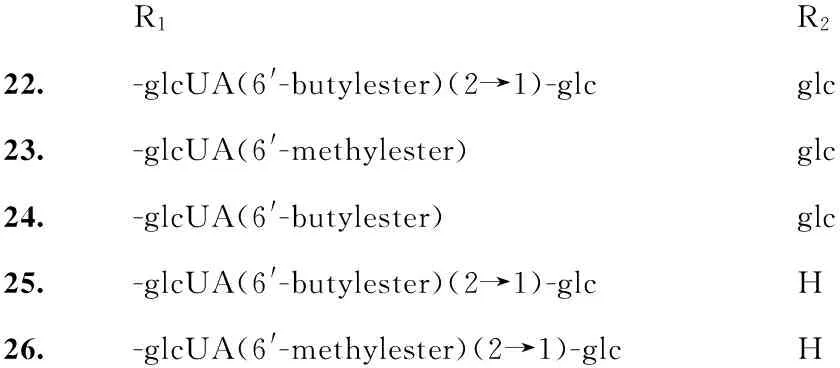
R1R222.-glcUA(6'-butylester)(2→1)-glcglc23.-glcUA(6'-methylester)glc24.-glcUA(6'-butylester)glc25.-glcUA(6'-butylester)(2→1)-glcH26.-glcUA(6'-methylester)(2→1)-glcH



注:1.本文化合物取代基部分,glc:β-D-glucopyranosyl;rha:α-L-rhamnopyranosyl;ara(p):α-L-arabinopyranosyl;ara(f):α-L-arabinofuranosyl;xyl:β-D-xylopyranosyl;Ac:acetyl;glcUA:β-D-glucopyranosiduronic acid;2.达玛烷型的C-20和C-22双键有Z型和E型之分,其写法本文规范化为20(22)Z-或20(22)E-。3.已在前文[1-2]出现过的化合物结构,本文省略。图2 化合物1~90的化学结构
6 结语
人参是驰名古今中外的药用植物,至少有3000多年的药用历史[37]。早年的研究多集中在它的功能描述和评价,但直到1963年从人参中分离鉴定出人参皂苷[38-39],人参的研究才步入分子水平。1975年以后,人参的研究报道呈指数增加。现在,人参的功效和药理作用得到国际公认[40],表明一定有药效物质基础存在。已有研究结果表明,人参皂苷的药理作用和生物学活性,几乎反映了人参的全部功能。本文综合前文结果[1-2],迄今已从人参根和根茎、茎叶、花蕾、浆果、种子和红参中共分离鉴定了201个单体化合物,具有翔实的谱学数据支持结构鉴定,其中189个归属为达玛烷型三萜及其衍生物,10个归属为齐墩果酸型三萜,2个归属为羽扇豆烷型三萜。皂苷的糖基部分主要为β-D-吡喃葡萄糖基(β-D-glucopyranosyl group),其次为α-L-吡喃鼠李糖基(α-L-rhamnopyranosyl group),少数结合α-L-吡喃/呋喃阿拉伯糖基(α-L-arabinopyranosyl/α-L-arabinofuranosyl group)和β-D-吡喃木糖基(β-D-xylopyranosyl group),而β-D-吡喃葡萄糖醛酸基(β-D-glucopyranosiduronyl group)仅呈现在以齐墩果酸为苷元的皂苷中。在达玛烷型三萜皂苷中,-β-D-吡喃葡萄糖基(2→1)-β-D-吡喃葡萄糖基寡糖链发生率较高,多结合在苷元的C-3位,成氧糖苷;-β-D-吡喃葡萄糖基(2→1)-α-L-吡喃鼠李糖基寡糖链多结合在苷元的C-6位,成氧糖苷;无论是原人参二醇和/或三醇型苷元,四环母核比较稳定,取代基发生在C-3、C-6和C-12,C-17侧链常常发生氧化、还原、环合、差向异构化等,衍生出多样性的化学结构。生晒参(sun-dried ginseng)、水参(fresh ginseng)中的三萜结构类型相对比较简单,但水参加工为红参,热动力学过程使C-17侧链发生氧化、还原、环合、差向异构化、脱糖基化等,加热温度和时间不同,转化程度不同[1]。如果C-20和C-22呈双键键合,以E型为优势构型。人参地上部分富含氧化态的达玛烷型三萜及其皂苷,这与地上部分暴露在空气中有关。因此,人参地上部分比根和根茎含有更丰富的、化学结构更多样性的达玛烷型三萜及其皂苷,是寻找新药优秀先导化合物的宝贵天然资源。由于人参为种子类繁育生物,花蕾、果实、种子开发利用潜力受资源限制。茎叶每年可以再生,且茎叶中20(S/R)-人参皂苷Rh1、Rh2、Rg2、Rg3等稀有人参皂苷含量更高,受红参中稀有人参皂苷生物活性研究结果的启迪,人参茎叶更有广阔的开发利用前景。
随着高效液相色谱与各种类型质谱联用技术的飞跃发展,生晒参[3,41]、红参[41]、人参叶[42]等中的化学成分数目不断被刷新,每个部位就含有近百个或以上,在这些技术的导向下,人们将从人参分离鉴定出更多的单体化合物,应用各种谱学技术确定其精细结构,丰富人参化学结构的多样性,并进而阐明人参的药效物质基础和开发利用价值。
[1] 杨鑫宝,杨秀伟,刘建勋.人参中皂苷类化学成分的研究[J].中国现代中药,2013,13(5):349-358.
[2] 李珂珂,杨秀伟.人参茎叶化学成分的研究进展[J].中国现代中药,2012,14(1):47-50.
[3] Yang W Z,Ye M,Qiao X,et al.A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis:Its application inPanaxginseng,PanaxquinquefoliumandPanaxnotoginsengto characterize437potential new ginsenosides[J].Anal Chim Acta,2012,739:56-66.
[4] Lee D G,Lee J M,Yang S H,Identification of dammarane-type triterpenoid saponins from the root ofPanaxginseng[J].Nat Prod Sci,2015,21(2):111-121.
[5] 周琪乐,徐嵬,杨秀伟.中国红参化学成分研究[J].中国中药杂志,2016,41(2).DOI:10.4268cjcmm2016024.
[6] Zhou Q L,Yang X W.Four new ginsenosides from red ginseng with inhibitory activity on melanogenesis in melanoma cells[J].Bioorg Med Chem Lett,2015,25(16):3112-3116.
[7] Lee S M,Seo H K,Oh J S,et al.Updating chemical profiling of red ginseng via the elucidation of two geometric isomers of ginsenosides Rg9and Rg10[J].Food Chem,2013,141(4):3920-3924.
[8] Cho J G,Lee D Y,Shrestha S,et al.Three new ginsenosides from the heat-processed roots ofPanaxginseng[J].Chem Nat Compd,2013,49(5):882-887.
[9] Li K K,Yao C M,Yang X W.Four new dammarane-type triterpene saponins from the stems and leaves ofPanaxginsengand their cytotoxicity on HL-60cells[J].Planta Med,2012,78(2):189-192.
[10] Li K K,Yang X W.Minor triterpene compounds from the stems and leaves ofPanaxginseng[J].Fitoterapia,2012,83(6):1030-1035.
[11] 李珂珂,杨秀伟.人参茎叶中1个新三萜类天然产物[J].中草药,2015,46(2):169-173.
[12] 杨秀伟,李珂珂,周琪乐.20(S)-人参皂苷Rf2,人参茎叶中1个新皂苷[J].中草药,2015,46(21):3137-3145.
[13] Huang J,Tang X H,Ikejima T,et al.A new triterpenoid fromPanaxginsengexhibits cytotoxicity through p53and the caspase signaling pathway in the HepG2cell line[J].Arch Pharm Res,2008,31(3):323-329.
[14] Ma H Y,Gao H Y,Huang J,et al.Three new triterpenoids fromPanaxginsengexhibit cytotoxicity against human A549and Hep-3B cell lines[J].J Nat Med,2012,66(3):576-582.
[15] Tran T L,Kim Y R,Yang J L,et al.Dammarane triterpenes from the leaves ofPanaxginsengenhance cellular immunity[J].Bioorg Med Chem,2014,22(1):499-504.
[16] Yang J L,Ha T K,Dhodary B,et al.Dammarane triterpenes as potential SIRT1activators from the leaves ofPanaxginseng[J].J Nat Prod,2014,77(7):1615-1623.
[17] Qiu S,Yang W Z,Shi X J,et al.A green protocol for efficient discovery of novel natural compounds:Characterization of new ginsenosides from the stems and leaves ofPanaxginsengas a case study[J].Anal Chim Acta,2015.893:65-76.
[18] Shao C J,Xu J D,Kasai R,et al.Saponins from flower-buds ofPanaxginsengcultivated at Jilin,China[J].Chem Pharm Bull,1989,37(7):1934-1935.
[19] 邱峰,马忠泽,裴玉萍,等.人参花蕾化学成分的研究[J].中国药物化学杂志,1998,8(3):205-207.
[20] Qiu F,Ma Z Z,Xu S X,et al.A pair of24-hydroperoxyl epimeric dammarane saponins from flower-buds ofPanaxGinseng[J].J Asian Nat Prod Res,2001,3(3):235-240.
[21] Qiu F,Ma Z Z,Xu S X,et al.Studies on dammarane-type saponins in the flower-buds ofPanaxginsengC.A.Meyer[J].J Asian Nat Prod Res,1998,1(2):119-123.
[22] Yoshikawa M,Sugimoto S,Nakamura S,et al.Medicinal flowers.XI.Structures of new dammarane-type triterpene diglycosides with hydroperoxide group from flower buds ofPanaxginseng[J].Chem Pharm Bull,2007,55(4):571-576.
[23] Tung N H,Song G Y,Kim J A,et al.Dammarane-type saponins from the flower buds ofPanaxginsengand their effects on human leukemia cells[J].Bioorg Med Chem Lett,2010,20(1):309-314.
[24] Tung N H,Song G Y,Nhiem N X,et al.Dammarane-type saponins from the flower buds ofPanaxginsengand their intracellular radical scavenging capacity[J].J Agric Food Chem,2010,58(2):868-874.
[25] 徐斐,李珂珂,陈丽荣,等.人参花醇提物中的皂苷类化学成分[J].中国现代中药,2016,18(1):56-62.
[26] Nakamura S,Sugimoto S,Matsuda H,et al.Structures of dammarane-type triterpene triglycosides from the flower buds ofPanaxginseng[J].Heterocycles,2007,71(3):577-588.
[27] Yoshikawa M,Sugimoto S,Nakamura S,et al.Medicinal flowers.XVI.New dammarane-type triterpene tetraglycosides and gastroprotective principles from flower buds ofPanaxginseng[J].Chem Pharm Bull,2007,55(7):1034-1038.
[28] Wang J Y,Li X G,Zheng Y N,et al.Isoginsenoside-Rh3,a new triterpenoid saponin from the fruits ofPanaxginsengC.A.Mey.[J].J Asian Nat Prod Res,2004,6(4):289-293.
[29] 王继彦,李向高,杨秀伟.人参果中一个新的天然化合物的分离[J].中草药,2006,37(12):1761-1764.
[30] Wang W,Zhao Y Q,Rayburn E R,et al.Invitroanti-cancer activity and structure-activity relationships of natural products isolated from fruits ofPanaxginseng[J].Cancer Chemother Pharmacol,2007,59(5):589-601.
[31] 徐敏,占扎君,章小永.人参果的化学成分研究[J].中草药,2007,38(5):667-669.
[32] Bai X G,Xu J D,Jiang X K,et al.Study on dammarane-type saponins of ginseng fruit-isolation and identification of two configurational isomers[J].Sci Bull,1987,32(8):536-539.
[33] 于明,赵余庆.人参果中三萜类成分的化学研究[J].中草药,2004,35(11):1221-1223.
[34] 赵余庆,袁昌鲁,吕浩然.人参果中抗癌活性成分20(R)-人参皂甙-Rh2的分离与鉴定[J].中国中药杂志,1991,16(11):678-679,704.
[35] Sugimoto S,Nakamura S,Matsuda H,et al.Chemical constituents from seeds ofPanaxginseng:structure of new dammarane-type triterpene ketone,Panaxadione,and HPLC comparisons of seeds and flesh[J].Chem Pharm Bull,2009,57(3):283-287.
[36] Kim J A,Son J H,Yang S Y,et al.A new lupane-type triterpene from the seeds ofPanaxginsengwith its inhibition of NF-κB[J].Arch Pharm Res,2012,35(4):647-651.
[37] Zheng B C.Shennong’s herbal-One of the world’s earliest pharmacopoeia[J].J Tradit Chin Med,1985,5:236.
[38] Shibata S,Fujita M,Itokawa H,et al.Studies on the constituents of Japanese and Chinese crude drugs.XI.Panaxadiol,a sapogenin of ginseng roots.(1).Chem Pharm Bull,1963,11(6):759-761.
[39] Shibata S,Tanaka O,Soma K,et al.Studies on saponins and sapogenins of ginseng.The structure of panaxatriol[J].Tetrahedron Lett,1965,42:207-213.
[40] Gillis C N.Panaxginsengpharmacology:A nitric oxide link?[J].Biochem Pharmacol,1997,54(1):1-8.
[41] Wu W,Sun L,Zhang Z,et al.Profiling and multivariate statistical analysis ofPanaxginsengbased on ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry[J].J Pharm Biomed Anal,2015,107:141-150.
[42] Mao Q,Bai M,Xu J D,et al.Discrimination of leaves ofPanaxginsengandP.quinquefoliusby ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach[J].J Pharm Biomed Anal,2014,97:129-140.
TriterpenoidsinPanaxginseng
YANG Xiuwei*
(StateKeyLaboratoryofNaturalandBiomimeticDrugs,DepartmentofNaturalMedicines,SchoolofPharmaceuticalSciences,PekingUniversity,Beijing100191,China)
Ginseng is members of the genusPanaxin the Araliaceae family,which is well-known as the king of herbs,and has been an important medicinal resource all over the world.Many studies have been conducted to elucidate magical healing activities of ginsengs.Since most studies have been focused on the roots and rhizomes of ginseng as traditional medicinal parts,scientists are less attracted by ginseng stems-leaves.The prices of the roots and rhizomes of ginseng are expensive because it takes4-6years for growing from the seed,whereas ginseng stems-leaves which the prices are low and can be harvested every year.Therefore,if the ingredients from the stems-leaves have a similar or new biological activity as those from the roots and rhizomes,their utility values will be improved.Ginsenosides,the active components of the roots and rhizomes of ginseng,are shown to be involved in modulating multiple physiological activities.As a continuation of our summary on ginseng,this article will review the triterpenoids isolated from the roots and rhizomes,stems-leaves,fruits and seeds of ginseng as well as red ginseng to provide the scientific basis for research and development of modern Chinese medicine derived from ginseng.Until now,201triterpenoid compounds have been isolated and identified from ginseng.Among them,189compounds are classified into dammarane-type and their derivatives,10ones are classified into oleanolic acid-type and2ones are classified into lupane-type,which dammarane-type triterpenoids and their derivatives are predominantly dominant type not only in amount but also in structural varieties.
PanaxginsengC.A.Meyer;stems-leaves;flower buds;fruits;seeds;red ginseng;ginsenosides;triterpenoids
10.13313/j.issn.1673-4890.2016.1.003
2015-10-07)
“十二五”国家科技支撑专项(2011BAI03B01,2011BAI07B08)
*
杨秀伟,教授,博士生导师,研究方向:天然产物化学与药物代谢;E-mail:xwyang@bjmu.edu.cn