两个基于4-羧酸-2,2′∶6′,2″-三联吡啶的铜配合物的合成、晶体结构及性质
2016-07-22汪晨敏瞿志荣杭州师范大学有机硅实验室杭州311121
范 艳 汪晨敏 瞿志荣(杭州师范大学有机硅实验室,杭州 311121)
两个基于4-羧酸-2,2′∶6′,2″-三联吡啶的铜配合物的合成、晶体结构及性质
范艳汪晨敏瞿志荣*
(杭州师范大学有机硅实验室,杭州311121)
摘要:水热法合成了2个配合物{[Cu2(L)2(tp)]·2H2O}n(1)和[CuL(ClO4)]n(2)(L=4-羧酸-2,2′∶6′,2″-三联吡啶,H2tp=对苯二甲酸)。对其晶体结构进行了分析,结果表明配合物1和2都通过配体和金属离子组装成一维链结构;配合物1属于三斜晶系,P�空间群,并通过链间的氢键作用形成三维的网状结构;配合物2通过链与链间的氢键作用形成二维的层状结构。对其热稳定性和荧光性质进行了研究。
关键词:4-羧酸-2,2′∶6′,2″-三联吡啶;晶体结构;热重;荧光
0 Introduction
With the rapid development of science and technology,metal-organic frameworks(MOFs)[1]has been an active research area over the past decades. Mainly due to the configuration of different inorganic and organic building blocks,organic coordination polymers can be prepared into different dimensions structures,different metal spatial configuration and topology[2-3].Most of these compounds have a porous
*通信联系人。E-mail:quzr@hznu.edu.cnstructure,which likes a hole in the field with broad application prospects especially in materials chemistry,attracting more and more attentions[4-5].Based on this factor,the use of organic ligand with suitable binding groups is important[6-8].In order to build new 1D,2D or 3D structural complex,a new bridging trigonal bifunctional ligand 4′-carboxy-2,2′∶6′,2″-terpyridine was employed in the realm of coordination chemistry[9-13]. It has a large π-conjugated nonlinear structure with N,O donors that can offer additional hydrogen bonding and π-π interactions to consolidate the whole MOFs. Theappendedterephthalategroupshaveseveral coordination modes to act as convenient bridging units,which allow the molecules to form into different complicated structures.To the best of our knowledge,much attention has been paid on this ligand to the light-emitting properties of Ru(Ⅱ)complexes[14-16].
In this paper,the coordination configuration is relatively simple,due to the linear structural mode of the ligand.Thus,new complexes were formed with a flexible benzoic acid acted as an auxiliary ligand[17-19]. Herein,we report the syntheses and crystal structures of two new coordination compounds based on HL,namely{[Cu2(L)2(tp)]·2H2O}n(1)and[Cu(L)(ClO4)]n(2)(L=4′-carboxy-2,2′∶6′,2″-terpyridine,H2tp=terephthalic acid).Furthermore,thethermalstabilitiesand fluorescent properties of two compounds have also been investigated.
1 Experimental
1.1Chemicals and measurement
The ligand of 4′-carboxy-2,2′∶6′,2″-terpyridine was synthesized as described elsewhere[20-21].Other reagents and solvents mentioned were commercially available and used as received without further purification.IR spectra were obtained with KBr pellets from 400 to 4 000 cm-1using a Bruker VERTEX 70 spectrometer.The UV-Vis spectra were recorded on a evolution300spectrophotometer.Photoluminescent spectra were measured using a Hitachi F-2700 Fluorescence Spectrometer.Thermogravimetric(TG)analyses wereperformedonaNETZSCHTGSTA449F3 analyzer at a heating rate of 10℃·min-1under nitrogen flow with a rate of 20 mL·min-1.
1.2Preparation
1.2.1Preparation of 1
A mixture of Cu(CH3COO)2·6H2O(0.039 0 g,0.2 mmol),HL(0.054 0 g,0.2 mmol),H2tp(0.033 2 g,0.2 mmol)in solvents of CH3OH-H2O(10 mL,1∶1,V/V was placed into a Teflon-lined stainless steel vesse (25 mL).The reaction system was kept at 110℃for 5 days,followed by cooling down to room temperature Dark blue block crystals(0.055 7 g)were obtained Yield:63.2%(based on Cu).Anal.Calcd.for CuC20H1N3O5(%):C,54.73;H,2.99;N,9.57.Found(%):C 54.77;H,3.05;N,9.61.IR(KBr,cm-1):3 517(s),3 054 (s),1 678(s),1 621(s),1 473(s),1 399(s),1 324(m)1 253(m),1 084(m),1 022(m),849(m),781(s),739(s)655(s).
1.2.2Preparation of 2
A mixture of Cu(ClO4)2·6H2O(0.074 0 g,0.2 mmol),HL(0.054 0 g,0.2 mmol),H2tp(0.033 2 g,0.2 mmol)in H2O(10 mL)was placed into a Teflon-lined stainless steel vessel(25 mL).The reaction system wa kept at 150℃for 4 days,followed by cooling down t room temperature.Dark blue block crystals(0.046 3 g)were obtained.Yield:52.7%(based on Cu).Anal Calcd.for CuC16H10N3O6Cl(%):C,43.75;H,2.29;N 9.57.Found(%):C,43.79;H,2.35;N,9.61.IR(KBr cm-1):3 505(s),3 041(s),1 723(s),1 621(s),1 462(s)1 389(s),1 251(m),1 018(m),852(m),786(s),687(s).
1.3X-ray crystallography
X-ray single-crystal diffraction data of complexe 1 and 2 were collected on a Bruker SMART APEXⅡCCDdiffractometerwithgraphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at 293(2)K.The structures were solved by direct methods and refined by full-matrix least-squares on F2with SHELX-97 program[22].Allnon-hydrogen atoms were refined anisotropically.Hydrogen atoms were added theoreti cally and refined with a riding model.Detailed data collection and refinements of 1 and 2 are summarized in Table 1.Selected bond lengths and angles ar listed in Tables 2 and 3.Relevant hydrogen bondin parameters of 1 and 2 are summarized in Table 4.
CCDC:1428254,1;1438989,2.

Table 1 Crystal data and structure refinement for complexes 1 and 2

Table 2 Selected bond lengths(nm)and angles(°)for the complex 1
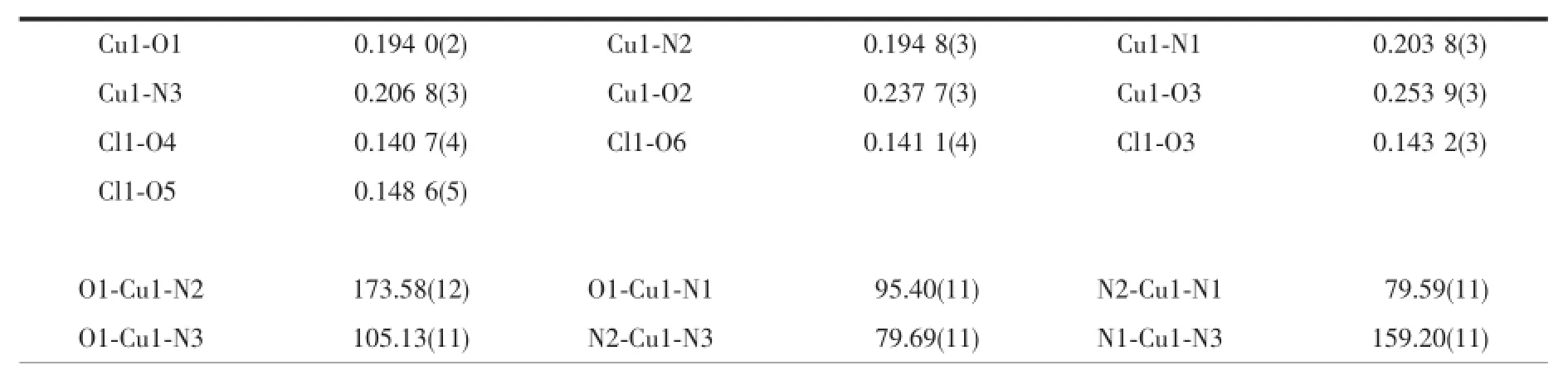
Table 3 Selected bond lengths(nm)and angles(°)for the complex 2

Continued Table 3

Table 4 Hydrogen bond parameters for the complexes 1 and 2
2 Results and discussion
2.1Structure description of 1
The crystal structure of complex 1 belongs to triclinic system,space group P1.Each Cu(Ⅱ)ion is fivecoordinate by three nitrogen atoms(Cu1-N1 0.202 4(2) nm,Cu1-N2 0.194 2(2)nm,Cu1-N3 0.202 9(2)nm from the same L ligand,one oxygen atom (Cu1-O3 0.229 15(19)nm)from another L ligand and one oxygen atom(Cu1-O1 0.190 41(19)nm)from the tp ligand in a distorted tetragonal pyramid geometry(Fig.1a).

Fig.1 (a)Crystal structure of complex 1 with 30%thermal ellipsoids;(b)Tetragonal pyramid geometry of Cu units

Fig.2 1D chain structure of complex 1
Two symmetry-related Cu(Ⅱ) ions are connected by two trans-conformational tp ligands and L ligands,resulting in a tert-nuclear ring[Cu4(L)4(tp)2](Fig.1b). Adjacent non-bonding Cu…Cu distance are 1.093 8 and 0.762 9 nm,respectively.The dihedral angle between the L and the tp ligands is 114.5°.The tertnuclear units extend repeatedly to form a 1D chain structure(Fig.2).A 2D supramolecular layer(Fig.3a)was formed by hydrogen bonds interactions of the O atoms of the solvent water molecules and ligands,and weak C-H…O interactions between the neighboring ligands,including interactions of C4-H4…O3C with thedistanceof0.241 58 nm and C7-H7…O3C (Symmetry code:C:2-x,1-y,1-z)with the distanc of 0.254 39 nm.In addition,2D layer structure ifurther extended to a 3D supramolecular network by C-H…π interactions(Fig.3b).The C-H…π interactions play important roles in the structural stabilization.The crystal structures analysis reveal that aromatic C-H…π interactions exist between benzene and pyridine rings,including interactions of C13-H13…Cg7(Cg7 is the centroid of the benzene ring C17,C18,C19B,C17B,C18B,C19 with the distance of 0.284 78 nm,Symmetry code:B:x,-1+y,z)and C19-H19…Cg4(Cg4 is the centroid of the pyridine ring N1,C1,C2,C3,C4,C5 with the distance of 0.287 65 nm).

Fig.3 (a)2D supramolecular network of 1;(b)A view of the packing of the organic groups

Fig.4 (a)Crystal structure of complex 2 with 30% thermal ellipsoids;(b)Dimer Cu units sharing one edge of their polyhedron

Fig.5 1D chain structure of complex 2
2.2Structure description of 2
Thesingle-crystalX-raydiffractionanalysis reveals that 2 belongs to monoclinic system,space group P21/c.Each Cu(Ⅱ) ion adopts a six-coordinated manner by chelating to three nitrogen atoms(N1,N2 and N3)from one L ligand,two oxygen atoms(O1 and O2)from other two L ligands,and with an oxygen atom(O3)fromClO4-toformadistorted octahedral geometry(Fig.4b).The Cu-N bond lengths range from 0.194 8(3)to 0.206 8(3)nm,and Cu-O bond lengths range from 0.194 0(2)nm to 0.237 7(3)nm.The complex 2 displays a 1D chain structure by the coordination of ligands and metal ions(Fig.5).
The crystal structure of complex 2 exhibits four inter-chain hydrogen bonds.Selected bond lengths and angles of the hydrogen bonds are listed in Table 4.Adjacent chains were connected into a 2D layers structurebyhydrogenbondinteractions(Fig.6). Intermolecular face-to-face interactions between the neighboring pyridine rings were observed with the Cg4…Cg4 distance of 0.350 4(2)nm(Cg4 is the centroid of the benzene ring N2,C6,C7,C8,C9,C10).
2.3TG analysis of complexes
The TG analyses were carried out from room temperature to 800℃in a nitrogen atmosphere with a heating rate of 10℃·min-1.As shown in Fig.7,the first weight loss of complex 1 started at 153.4℃ and completed at 161.4℃,corresponding to the loss of one lattice water molecule(Obsd.5.24%,Calcd.4.09%). With the increase of temperature,the second weight loss of 40.02%(Calcd.39.41%)from 292.7 to 313.9℃results from the decomposition of the tp.The organic groups start to decompose gradually in the range of 315 to 800℃.Complex 2 did not show gradual weight losses before the decomposition of the framework at about 360℃ indicating non-existing of solvent molecules and being in agreement with the result of the crystal structure(Fig.7).With the increase of temperature,the sharp weight loss from 365.1 to 373.3℃corresponds to the decomposition o L ligand and the ClO4-with the weight loss of 84.23% (Calcd.85.57%).

Fig.6 2D layer structure formed by hydrogen bonds in complex 2

Fig.7 TG curves of complex 1 and complex 2

Fig.8 UV-Vis absorption spectra of complexes 1 and 2
2.4Fluorescent property
The UV-Vis spectra of the complexes 1 and 2 in DMSO solutions with the concentration of 10-4mol·L-at room temperature are shown in Fig.8.Complex 1 shows an intense absorption band at 278 nm and weak one at 340 nm,and complex 2 shows intens absorption band at 298 nm and 334 nm.The former i owing to the π-π*transition of the coordination between the ligand and the metal ions.
The fluorescent spectra of complexes 1 and 2 were measured in DMSO solution with the concentration of 10-4mol·L-1at room temperature,as depicted in Fig.9.When the exciting radiation was set at 365 nm complex 2 exhibits only a weak emission band at 463nm.While complex 1 exhibits a weak emission at 452 nm,and a much stronger emission at 563 nm(Fig.9).

Fig.9 Fluorescent emission spectra of complexes 1 and 2 at room temperature
3 Conclusions
Two Cu(Ⅱ) complexes based on 4′-carboxy-2,2′∶6′,2″-terpyridine have been synthesized and characterized in detail by X-ray crystal structure analyses.For the title complexes,the 1D chains of complex 1 are assembled into 3D network by inter-chains hydrogen bond interactions and C-H…π interactions;whereas a 2D layer structure of complex 2 is assembled by hydrogen bonds between chains.Complex 2 shows remarkablethermalstabilities.TheFluorescence propertiesshowthatcomplex1exhibitshigher fluorescence intensity than 2.The observed strong photoluminescence of complex 1 indicates that the compoundcanhavepotentialapplicationasa photoactive material.
References:
[1]Robin A Y,Fromm K M.Coord.Chem.Rev.,2006,250:2127 -2157
[2]Zhao D,Timmons D J,Yuan D Q,et al.Acc.Chem.Res.,2011,44:123-133
[3]Gu Z Y,Yang C X,Chang N,et al.Acc.Chem.Res.,2012,45:734-745
[4]Férey G.Chem.Soc.Rev.,2008,37:191-214
[5]Kitagawa S,Kitaura R,Noro S.Angew.Chem.,Int.Ed.,2004,43:2334-2375
[6]Ni W X,Li M,Zhan S Z,et al.Inorg.Chem.,2009,48:1433-1441
[7]Yin X J,Zhou X H,Gu Z G,et al.Inorg.Chem.Commun.,2009,12:548-551
[8]Zhu X D,Lü J,Li X J,et al.Cryst.Growth Des.,2008,8: 1897-1901
[9]Yoshida J,Nishikiori S,Kuroda R.Chem.Lett.,2007,36: 678-679
[10]Liu C,Ding Y B,Shi X H,et al.Cryst.Growth Des.,2009,9:1275-1277
[11]Liang X Q,Xiao H P,Liu B L,et al.Polyhedron,2008,27: 2494-2500
[12]Yang F L,Li B,Hanajima T,et al.Dalton Trans.,2010,9: 2288-2292
[13]Liang X Q,Zhou X H,Chen C,et al.Cryst.Growth Des.,2009,9:1041-1053
[14]Sauvage J P,Collin J P,Chambron J C,et al.Chem.Rev.,1994,94:993-1019
[15]Medlyeott E A,Hanan G S.Chem.Soc.Rev.,2005,34:133-142
[16]Puntoriero F,Campagna S,Stadler A M,et al.Coord.Chem. Rev.,2008,252:2480-2492
[17]Yuan F,Zhu Q E,Hu H M,et al.Inorg.Chim.Acta,2013,397:117-123
[18]Yuan F,Shen S S,Hu H M,et al.Inorg.Chim.Acta,2015,430:17-23
[19]Yuan F,Xie J,Hu H M,et al.CrystEngCommun,2013,15: 1460-1467
[20]Cooke M W,Tremblay P,Hanan G S.Inorg.Chim.Acta,2008,361:2259-2269
[21]Wang B C,Wu Q R,Hu H M,et al.Inorg.Chem.Commun.,2010,13:715-719
[22]Sheldrick G M.SHELX-97,Programs for Crystal Structure Analysis(Release 97-2),University of Göttingen,Germany,1998.
中图分类号:O614.121文献识别码:A
文章编号:1001-4861(2016)05-0864-07
DOI:10.11862/CJIC.2016.108
收稿日期:2015-11-27。收修改稿日期:2016-03-28。
Syntheses,Crystal Structures and Properties of Two Cu(Ⅱ)Complexes Based on 4′-Carboxy-2,2′∶6′,2″-terpyridine
FAN YanWANG Chen-MinQU Zhi-Rong*
(Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education,Hangzhou Normal University,Hangzhou 311121,China)
Abstract:Two new Cu(Ⅱ)coordination compounds,{[Cu2(L)2(tp)]·2H2O}n(1)and[Cu(L)(ClO4)]n(2)(L=4′-carboxy-2,2′∶6′,2″-terpyridine,H2tp=terephthalic acid),were synthesized under hydrothermal conditions.The structures were characterized by single-crystal X-ray diffraction analyses.The results show that both the complex 1 and 2 display 1D chain structure composed of ligands and metal ions.Complex 1 belongs to triclinic system,space group P1,and it self-assembles into a 3D netlike structure by the inter-chain hydrogen bond interactions and CH…π interactions;whereas a 2D layer structure of complex 2 is assembled by hydrogen bonds between the chains.Thermal stability and fluorescent properties of 1 and 2 were also investigated.CCDC:1428254,1;1438989,2.
Keywords:4′-carboxy-2,2′∶6′,2″-terpyridine;crystal structure;thermal stability,fluorescent property
