3,6-二(N-咪唑/苯并咪唑基)哒嗪配体配合物的合成、结构、荧光及光催化性能
2016-07-22李金萍范建中王多志新疆大学化学化工学院乌鲁木齐830046
李金萍 范建中 王多志(新疆大学化学化工学院,乌鲁木齐 830046)
3,6-二(N-咪唑/苯并咪唑基)哒嗪配体配合物的合成、结构、荧光及光催化性能
李金萍范建中王多志*
(新疆大学化学化工学院,乌鲁木齐830046)
摘要:用溶剂热法设计、合成了4个金属-有机配合物[Mn(L1)4(OH)2](1),{[MnL1(H2O)4]SO4}n(2),[CdL2(NO3)2]n(3)和{[Co(L2)2](PF6)2}(4),(L1=3,6-二(N-咪唑基)哒嗪,L2=3,6-二(N-苯并咪唑基)哒嗪),并通过元素分析、红外、X射线单晶衍射对配合物结构进行了表征,测试结果表明配合物1具有单核结构,2为一维链结构,配合物3和4均为二维网状结构。此外,对配合物3和4的固态荧光性能及光催化的性能做了进一步研究。
关键词:配合物;晶体结构;荧光性能。
0 Introduction
In the past decades,a growing interest in the study of metal-organic coordination polymers(MOFs)has attracted much attention owing to their various structural and potential applications in the area of catalysis, fluorescence, chemicalsensors, ion exchange,gas storage[1-5].For the construction of meta -organiccoordinationpolymers,themostcritica strategy is the rational design and selection of the appropriate ligands,which possess versatile coordina tion modes and strong coordination abilities to meta ions[6-8].Up to now,a great number of MOFs with variousstructuressuchaszero-dimensional(0D
国家自然科学基金(No.21361026)资助项目。
*通信联系人。E-mail:wangdz@xju.edu.cn;会员登记号:S06N9862M1207。clusters[9],1Dchains[10],2Dlayers[11]and3Dframeworks[12]have been reported.At the same time,to analysis topology of MOFs is not only an effective tool for simplifying the complicated structures but also play an important role in the deliberate design of certain MOFs with specific properties[13].However,it is still a greatchallengetoconstructtargetcoordination polymerswithdesiredstructuresandfunctional properties[14].
So far,humanity is faced with serious environmental pollution including atmospheric pollution,soil pollution,water pollution and so on.The most serious is the water pollution,which not only could poison creatures living in the water but also damage people′s health.Considerable efforts have been made in treating waste water with many methods such as adsorption and separation,chemical treatment,photocatalysis[15]. In addition,MOFs have attracted much attention because of their photocatalysis properties in purifying wastewaterbythoroughlydecomposingorganic pollutants[16].This is a convenient and recyclable approach to solve the problem of water pollution.
In recent years,a series of N-containing heterocyclic ligands containing bis(imidazolyl/benzimidazolyl)have attracted much attention owing to their remarkable features as follows:(a)The ligands have an identical ring size and a similar set of donor atoms[17]. (b)They contain two imidazole rings,which nitrogen atoms can act as hydrogen bond donors to build hydrogen bond interaction and their rings can form ππ stacking interaction[18].(c)They exhibit a wide variety of pharmacological activities like fungicides or anti-helminthics[19].Therefore,a large number of metal coordination compounds with bis(imidazolyl/benzimidazolyl)about luminescent properties and photocatalytic activity for(methylene blue)MB dye degradation have been reported[20].
In this work,we carried out research on the coordinationchemistryofpyridazinecontaining ligand.We designed and synthesized ligands 3,6-bis (N-imidazolyl)pyridazine(L1)and 3,6-bis(N-benzimidazolyl)pyridazine(L2)(Chart 1)and successfully prepared four novel complexes,namely,[Mn(L1)4(OH)2](1),{[MnL1(H2O)4]SO4}n(2),[CdL2(NO3)2]n(3),{[Co(L2)2](PF6)2}n(4).We also discuss the syntheses,crystal structures of complexes 1~4 and analysis the topology of complex 4.Furthermore,the luminescent properties and photocatalytic activities of the complexes 3 and 4 have been investigated and discussed.

Chart 1 Structures of the ligands L1and L2
1 Experimental
1.1Materials and general methods
All reagents were purchased commercially and used without further purification.Elemental analyses (C,H,N)were performed on a Thermo Flash EA 1112-NCHS-O analyzer.IR spectra were measured on a Brucker Equinox 55 FT-IR spectrometer with KBr Pellets in the range of 4 000~400 cm-1.1H NMR data were collected using an INOVA-400 NMR spectrometer. TGA of complexes 1 and 2 were performed on a DSC200F3 analyzer heated from 30 to 1 000℃,and TGA of complexes 3 and 4 were performed on a Perkin-Elmer TG-7 analyzer heated from 30 to 700 ℃.The X-ray powder diffraction patterns(XRPD)of 1~4 were recorded on a Bruker D8 automated diffractometer,operated at 40 kV and 100 mA,using a Cutargettubeandagraphitemonochromator(Kα radiation,λ=0.154 18 nm).Fluorescence spectra were performedusingaHitachiF-4500Fluorescence Spectrophotometer with a Xe arc lamp as the light source and bandwidths of 2.5 nm at room temperature. UV-Vis absorption spectra were obtained using a Hitachi UV-3010 UV-Vis spectrophotometer.
1.2Synthesis of ligand L1
Imidazole(3.4 g,49.9 mmol),sodium(1.2 g,52.2 mmol)and 80 mL THF was added to a 250 mL threenecked flask.The reaction mixture was refluxed for 40 min.A solution of 3,6-dichloropyridazine(3.7 g,24.8 mmol)in 10 mL THF was added to the mixture within 40 min.The mixture was refluxed for 4 h under N2.The reaction mixture was poured into icewater after cooled to room temperature.The earthy yellow solid of L1was obtained after filtering.Pure ligandL1wasobtainedbyrecrystallizationfrom ethanol as white crystals.Yield:80%.m.p.281~282 ℃.1H NMR(DMSO-d6):δ 8.69(s,2H,imidazole-2),8.58(s,2H,pyridazine),8.11(s,2H,imidazole-5),7.23 (s,2H,imidazole-4);Anal.Calcd.for C10H8N6(%):C,56.60;H,3.79;N,39.60;Found(%):C,56.75;H,3.75;N,39.54.IR(KBr,cm-1):3 145(m),3 109(m),3 047(m),3 018(m),1 645(w),1 577(s),1 519(s),1 490(s),1 459(s),1 371(m),1 321(s),1 277(m),1 237(m),1 163(m),1 106(m),1 059(m),1 031(s),962(m),903(m),861(s),823(s),766(s),749(s),645(s),613(m),507(w),487(m).
1.3Synthesis of ligand L2
Ligand L2was synthesized similarly as L1by using benzimidazole instead of imidazole.Yield:76%. m.p.283~284℃.1H NMR(DMSO-d6):δ 9.130(s,2H,imidazole-2),8.653(s,2H,pyridazine),7.388~8.384 (m,8H,benzene);Anal.Calcd.for C18H12N6(%):C,69.21;H,3.87;N,26.91;Found(%):C,69.34;H,3.56;N,27.41.IR(KBr,cm-1):3 082(m),3 058(m),1 669(w),1 609(m),1 590(m),1 558(s),1 497(s),1 459(s),1 359(m),1 338(m),1 308(m),1 279(m),1 238(m),1 190(s),1 163(m),1 138(m),1 035(m),979(m),939(m),884(m),830(m),769(s),753(s),732(s),623(m),575(m),486(m),427(m).
1.4Synthesis of complex 1
A mixture of Mn(BF4)2·4H2O(90 mg,0.3 mmol),L1(63 mg,0.3 mmol)and 5 mL water was sealed in a 25 mL Teflon-lined stainless steel vessel and heated at 140℃for 48 h,then cooled to room temperature at a rate of 10℃·h-1.The colorless block crystals were obtained(ca.36%yield based on L1).Anal.Calcd.for C40H34MnN24O2(%):C,51.22;H,3.65;N,35.84.Found (%):C,52.48;H,3.97;N,36.76.IR (KBr,cm-1):3 600 (m),3 387(m),3 119(m),1 580(m),1 522(s),1 481 (s),1 319(s),1 242(m),1 098(m),1 064(s),1 033 (s),968(m),918(m),838(m),738(m),651(m),615 (m),492(m).
1.5Synthesis of complex 2
2 was synthesized similarly as 1 by using MnSO4· H2O instead of Mn(BF4)2·4H2O.The colorless block crystals were obtained(ca.36%yield based on L1)Anal.Calcd.for C10H16MnN6O8S(%):C,27.59;H 3.70;N,19.30.Found(%):C,27.43;H,3.89;N 19.57.IR(KBr,cm-1):3 167(m),3 130(m),1 643 (m),1 581(m),1 495(s),1 456(s),1 303(s),1 258 (m),1 073(s),1 044(s),966(m),924(m),823(m)773(m),728(m),643(m),616(m),597(m),494(m.
1.6Synthesis of complex 3
A mixture of Cd(NO3)2·4H2O(30 mg,0.1 mmol)L2(31 mg,0.1 mmol)and 5 mL methanol was sealed in a 25 mL Teflon-lined stainless steel vessel and heated at 140℃ for 48 h,then cooled to room temperature at a rate of 10℃·h-1.The colorless block crystals were obtained(ca.36%yield based on L2)Anal.Calcd.for C18H12CdN8O6(%):C,39.39;H,2.20 N,20.41.Found(%):C,40.48;H,2.88;N,23.74.IR (KBr,cm-1):3 116(m),3 104(m),3 050(m),1 611 (m),1 584(m),1 562(m),1 504(s),1 462(s),1 442 (s),1 399(m),1 378(m),1 349(m),1 322(s),1 298 (s),1 248(s),1 232(s),1 044(m),1 037(m),1 013 (m),909(m),884(m),855(m),808(m),703(m),739 (s),619(m).
1.7Synthesis of complex 4
4 was synthesized similarly as 3 by using Co(PF6)·6H2O(46 mg,0.1 mmol)instead of Cd(NO3)2·4H2O The purple block crystals were obtained(ca.45% yield based on L2).Anal.Calcd.for C36H24CoF12N12P (%):C,44.41;H,2.48;N,17.26.Found(%):C,45.02 H,2.56;N,18.37.IR(KBr,cm-1):3 147(w),3 073(w)1 569(m),1 504(s),1 465(s),1 439(s),1 298(m)1 232(s),1 156(m),1 092(s),1 042(m),1 014(m)915(m),837(m),780(m),748(s),612(m),448(m)417(m).
1.8Determination of photocatalytic activity
50 mg of complexes 3 and 4 were mixed togethe with 50 mL of an aqueous solution of MB(10 mg·L-1)Then the solution was exposed to UV irradiation under an Hg lamp(300 W)for 4 h and kept stirring during irradiation.Samples of 5 mL were taken away every 30 min for UV measurement.
1.9Structure determination
Experimental reflections were collected with a Bruker APEX Ⅱ Smart CCD diffractometer usingMo Kα radiation(λ=0.071 073 nm)(Table 1).The program SAINT[21]was used for integration of the diffraction profiles.The structures of 1~4 were solved by the direct method and refined by the full-matrix least squares on F2using the SHELXTL[22a].Semiempirical absorption corrections were carried out using SADABS[22b]program.Hydrogen atoms of carbon were located at calculated positions and refined with fixed thermal parameters riding on their parent atoms. All non-hydrogen atoms were refined with anisotropic displacement parameters.The complex 4 exhibited a structuredisorder.Experimentaldetailsforthe structure determination are presented in Table 1.The selected bond distances and angles were listed in Table 2.
CCDC:1444988,1;1444989,2;1022876,3;1022877,4.
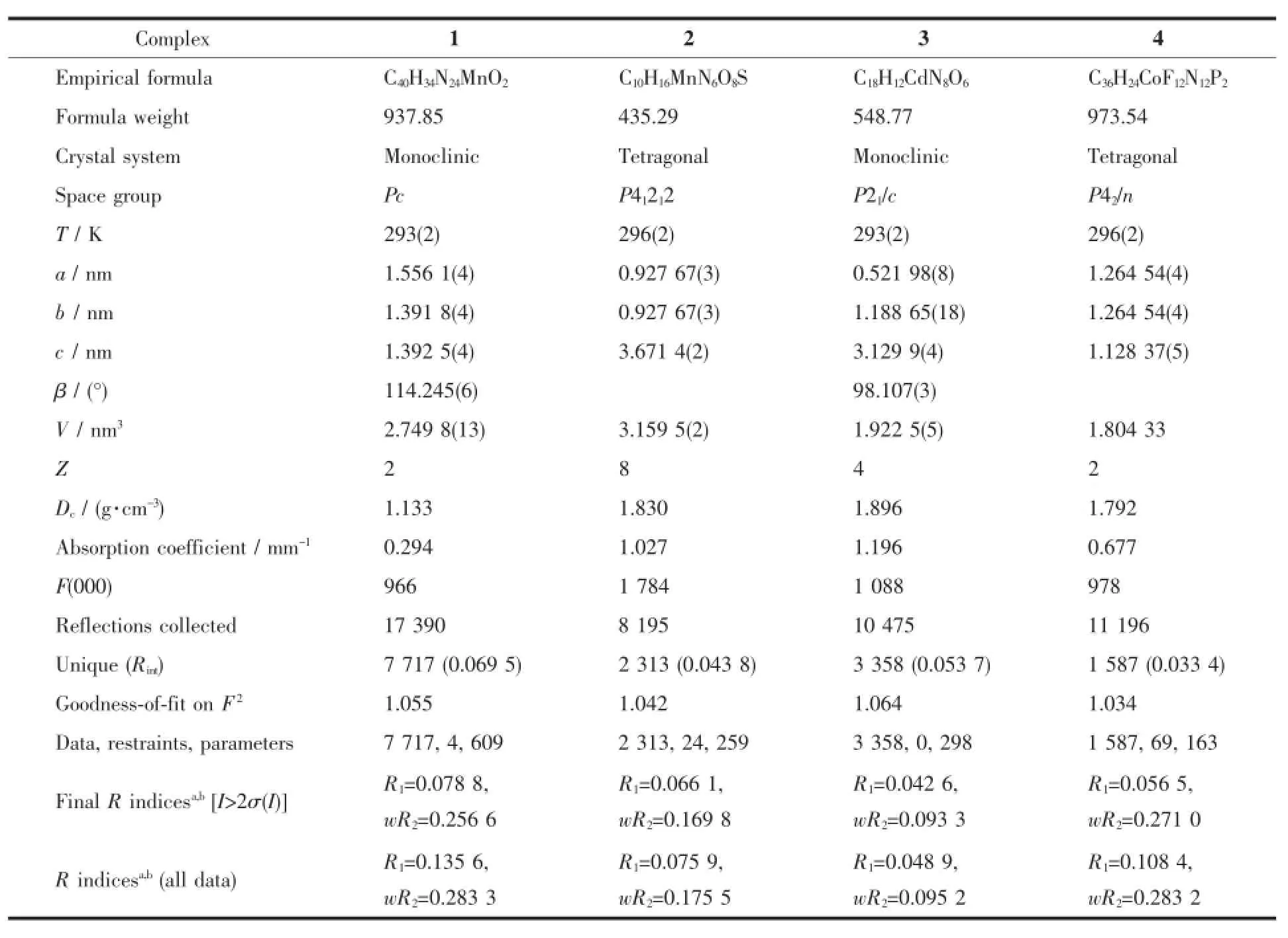
Table 1 Crystallographic data for complexes 1~4

Table 2 Selected bond distances(nm)and angles(°)for complexes 1~4

Continued Table 2
2 Results and discussion
2.1Structure description of 1
Single crystal X-ray diffraction analysis reveals that complex 1 crystallizes in the monoclinic space group Pc,and the selected bond distances and angles are listed in Table 2.Complex 1 has a mononuclear structure,as shown in Fig.1.The structural unit of 1 contains an independent Mn(Ⅱ)cation,four neutral L1ligands and two OH-anions.The Mn(Ⅱ) center is six coordinated to complete a distorted octahedral geometry. The equatorial plane is formed by four N atoms from four distinct L1ligands and the axial position ar occupied by two O atoms from two OH-anions.Th coordination angles around Mn(Ⅱ)center are vary from 86.2(3)°to 179.1(3)°.The Mn-O bond lengths ar 0.214 9(5)nm and 0.216 5(5)nm,respectively.Th lengths of Mn-N bonds are 0.222 8(7),0.225 3(6)0.225 4(7)and 0.226 3(7)nm,respectively.The L1act as a monodentate ligand in complex 1 and two coordinated OH-anions not only participated in coordination but also acts as a counter anion fo charge balance.

Fig.1 View of the coordination environment of Mn(Ⅱ)ion in 1
2.2Structure description of 2
Complex 2 crystallizes in the tetragonal spac group P41212.Crystallographic data and experimenta details for structural analyses were summarized in Table 1.In 2,each Mn(Ⅱ) center adopts a slightl distorted octahedral coordinated by two N atoms from two L1ligands(Fig.2)and four O atoms from wate molecules.The N1i-Mn1-N6 coordination angle i 98.6(2)°.The Mn-N bond distances are 0.226 1(5)and0.226 2(5)nm,respectively.(see Table 2).The Mn-O bond distances are 0.216 5(5),0.218 4(5),0.219 9(5),and 0.222(5)nm(see Table 2),respectively.The two imidazole rings are coplanar with the central pyridazine ring.The L1ligands,acting as a bisconnector,link Mn(Ⅱ)centers to form the 1D chain with the Mn…Mn distance of 1.311 9 nm.From such coordination mode,the[MnL1(H2O)4]2+unit has two positive charges.The SO42-anions don′t show any bonding interaction with Mn(Ⅱ) centers,and only acts as a counteranion for charge balance.

Fig.2 View of 1D chain structure of 2
2.3Structure description of 3
Complex 3 crystallizes in the monoclinic space group P21/c and displays 2D network structure,as shown in Fig.3.The structure unit of 3 contains one Cd(Ⅱ) ion,two L2ligands and three coordinated NO3-anions.As shown in Fig.3a,The Cd(Ⅱ) ion is sevencoordinated to two N atoms from two distinct L2ligands(the Cd-N lengths are 0.225 2(4)and 0.226 3(4)nm,respectively)and five O atoms from distinct three nitrate anions(the bond lengths of Cd1-O1,Cd1-O2ii,Cd1-O3,Cd1-O4andCd1-O6 are 0.2714(3),0.2446(3),0.235 9(3),0.230 8(4)and 0.282 5(5)nm,respectively). Each Cd(Ⅱ) ion demonstrates a distorted monocapped octahedral geometry.The combination of Cd(Ⅱ)centers and rigid ligands L1form an infinite 1D chain,and the 1D chains are further assembled into a 2D network structure through NO3-anions bridging coordination mode,as shown in Fig.3b.The bond angles aroundCd(Ⅱ)centervaryfrom 49.95(10)°to 163.18(14)°. The neighboring non-bonding Cd…Cd distance connected by L2is 1.289 2 nm,and Cd…Cd distance connected by NO3-anion is 0.522 0 nm.

Fig.3 View of the coordination environment of Cd(Ⅱ)ion in 3(a)andthe 2D net of the complex 3(b)

Fig.4 View of the coordination environment of Co(Ⅱ)ion in 4(a),2D network structure of 4(b)and the 44topology of 4(c)
2.4Structure description of 4
Complex 4 crystallizes in the tetragonal with space group P42/n.The structural unit of 4 contains a Co(Ⅱ) ion,four neutral L2ligands,two uncoordinated PF6-.The CoⅡcenter is coordinated to four N atomsfrom four distinct L2ligands(the Co-N bond lengths are 0.199 6(5)nm)to complete a distorted tetrahedral coordinated geometry with the coordination angles around Co(Ⅱ)center being 107.84(14)°and 112.8(3)°,respectively(Fig.4a and Table 2).Each L2ligand links two Co(Ⅱ)centers and in turn each Co(Ⅱ) center connects four ligands forming a layer structure with (4,4)foursquare grid units.Meanwhile,and all the Co(Ⅱ) centers are located in one plane(Fig.4c).As shown in Fig.4b,each(4,4)grid unit is constructed by four ligands acting as four edges and four Co(Ⅱ)centers as four vertexs.All the lengths of the edges are equal(the edges are 1.264 5 nm)and the orthogonal distance is 1.788 3 nm.The uncoordinated PF6-anions locate at the cavities of the sheets to serve as counter anions.
2.5Thermogravimetric analysis
To examine the thermal stabilities of complexes 1~4,the TGA analyses of complexes were carried out at the rate of 10℃·min-1in nitrogen atmosphere(1 and 2:from room temperature to 1 000℃;3 and 4: from room temperature to 700℃)as shown in Fig.5. The TGA study of complex 1 shows a slow weight loss from 30 to 270℃,Then the weight loss occurred continuously within the range of 270~1 000℃.The TGA curve of 2 displays a weight loss of 20.76% (Calcd.16.54% )in the range of 100~164℃corresponding to the loss of four coordinated water molecule.Above 164℃,a rapid weight loss is observed which can be attributed to the decomposing of th organic ligands.The remainder may be MnO2(Obsd 23.38%,Calcd.19.97%).Complexes 3 and 4 los weight in the range of 315~620℃and 332~590℃respectively,corresponding to the decomposition o ligands and the remains are CdO for 3(Obsd.22.08% Calcd.23.39%),CoO for 4(Obsd.9.22%,Calcd 7.69%).

Fig.5 TGA curves of complexes 1~4
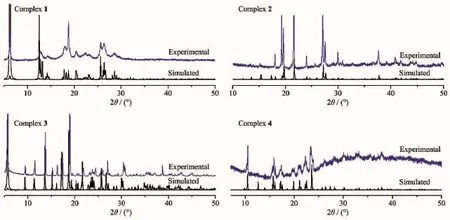
Fig.6 XRPD patterns of 1~4
2.6XRPD patterns
To confirm whether the crystal structures ar truly representative ofthe bulk materials,X-ra powder diffraction(XRPD)experiments were carried out for complexes 1~4.The XRPD experimental and computer-simulatedpatternsofthecorrespondin complexesareshowninFig.6.Althoughthexperimentalpatternshaveafewunindexed diffraction lines and some are slightly broadened in comparison with those simulated from the single crystal modes,it still can be considered favorably that the bulk synthesized materials and the as-grown crystals were homogeneous for 1~4.
2.7Luminescent properties
Luminescent materials are of great interest due to their various applications in chemical sensor,photochemistry and light-emitting diodes(LEDs).Hence photoluminescence properties of 3,4 and ligand L2are investigated in the solid state at room temperature,as shown in Fig.7.Upon excitation with 370 nm light,the free ligand L2present emission bands at 469 nm. Complexes 3 and 4 all exhibitfluorescent emission with the maxima at 469 and 407 nm,upon excitation at 370 and 250 nm,respectively.The emission of the ligand may be assigned to π*→n or π*→π transitions of the intra-ligands[23].The emission peak of 3 is similar to the free ligand.It should be pointed out that the emission of complex 3 is neither metal-toligand charge transfer(MLCT)nor ligand-to-metal charge transfer(LMCT)in nature since the Cd(Ⅱ) is difficult to oxidize or to reduce due to its d10configuration[13].Therefore they are mainly based on the intra-ligandtransitions.However,themaximum emission wavelength of complex 4 is blue-shifted,which is probably due to intra-ligand charge transitions[24].The differences of the peaks for 3 and 4 may result from different coordination and conformations[25].

Fig.7 Emission spectra of 3,4 and L2in the solid state at room temperature
2.8Photocatalytic activity
Photocatalystshaveattractedmuchattention because of their potential application in purifying water by thoroughly decomposing organic pollutants. In this work,the photocatalytic activities of complexes 3 and 4 were investigated with the photodegradation of MB.The decomposition of MB was monitored by the characteristic absorption band at 664 nm.The final results showed that the degradation rate of MB added the complex 4 and added no complex are almost equal,but the degradation rate of MB is 79% when added complex 3 after 4 h under UV irradiation,as shown in Fig.8.The results showed that 3 is efficientcatalystsforMBdegradation,however,complex 4 is nearly inefficient catalysts for MB.The different photocatalytic performances of complexes 3 and 4 may be due to their different components and structures[26].
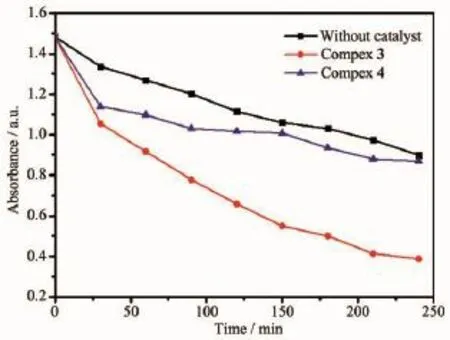
Fig.8 Curves of absorbance of the MB solution degraded by 3,4 under UV light
3 Conclusions
In conclusion,we have successfully synthesized and characterized four new complexes based on the ligands 3,6-bis(N-imidazolyl)pyridazine(L1)and 3,6-bis(N-benzimidazolyl)pyridazine(L2).X-ray diffraction analyses reveal that complex 1 has a mononuclear structure and complex 2 features a 1D structure. Complexes 3 and 4 exhibit 2D network structures. Moreover,the fluorescence properties of ligand L2and complexes 3 and 4 display blue fluorescent emission at room temperature.The photocatalytic activities of complexes 3 and 4 proved that 3 may be photocatalystsfor degradation of organic dyes in a certain extent.
Supporting information is available at http://www.wjhxxb.cn
References:
[1]Li B,Tian F Y,Qin C,et al.Russ.J.Coord.Chem.,2015,41:706-714
[2]Qin L,Xiao S L,Ma P J,et al.Transition Met.Chem.,2013,38:627-633
[3]An H Y,Wang L,Hu Y,et al.CrystEngComm,2015,17: 1531-1540
[4]Guo F,Zhu B Y,Liu M L,et al.CrystEngComm,2013,15: 6191-6198
[5]He H M,Song Y,Sun F X,et al.Cryst.Growth Des.,2015,15:2033-2038
[6]Liang G R,Liu Y R,Zhang X,et al.Synth.React.Inorg. Met.-Org.Chem.,2016,46:251-256
[7]Tomono K,Otani E,Ikeda R,et al.J.Inclusion Phenom. Mol.Recognit.Chem.,2011,70:241-247
[8]Li X J,Yu Z J,Guan T,et al.Cryst.Growth Des.,2015,15: 278-290
[9]Li G B,He J R,Pan M,et al.Dalton Trans.,2012,41:4626-4633
[10]Zhang X Y,Zhang Y H,Liu S Z,et al.Inorg.Chem.Commun.,2014,46:289-294
[11]Liu L,Huang C,Zhang L,et al.Cryst.Growth Des.,2015,15:2712-2722
[12]Chen J Q,Cai Y P,Fang H C,et al.Cryst.Growth Des.,2009,9:1605-1613
[13]Chen L,Zhang L,Li S L,et al.CrystEngComm,2013,15 8214-8221
[14]Li H,Xu G C,Zhang L,et al.Polyhedron,2013,55:209-215
[15]Shao Z C,Huang C,Han X,et al.Dalton Trans.,2015,44 12832-12838
[16]Wang X L,Sha X T,Liu G C,et al.CrystEngComm,2015 17:7290-7299
[17]Payra P,Hung S C,Kwok W H,et al.Inorg.Chem.,2001 40:4036-4039
[18]Wang X X,Ma Y J,Li H H,et al.Transition Met.Chem. 2015,40:99-108
[19]Li S L,Lan Y Q,Ma J C,et al.Cryst.Growth Des.,2010 10:1161-1170
[20]Chen L,Xu G J,Shao K Z,et al.CrystEngComm,2010,12 2157-2165
[21]SAINT Software Reference Manual,Bruker AXS,Madison WI,1998.
[22](a)Sheldrick G M.SHELXS 97,Program for Solution an Refinemen of Crystal Structures,University of Göttingen Germany,1997. (b)Sheldrick G M.SADABS,Program for Empirical Absor ption Correction of Area Detector Data,University o Göttingen,Germany,1997.
[23]Liu K,Ma B H,Guo X L,et al.CrystEngComm,2015,17 5054-5065
[24]Zhang M D,Qin L,Yang H T,et al.Cryst.Growth Des. 2013,13:1961-1969
[25]Cao L H,Wei Y L,Yang Y,et al.Cryst.Growth Des.,2014 14:1827-1838
[26]Wang X L,Chen N L,Liu G C,et al.Inorg.Chim.Acta 2015,432:128-135
中图分类号:O614.71+1;O614.24+2 O614.81+2
文献标识码:A
文章编号:1001-4861(2016)05-0753-09
DOI:10.11862/CJIC.2016.093
收稿日期:2016-01-05。收修改稿日期:2016-03-01。
Metal-Organic Complexes Based on 3,6-Bis(N-imidazolyl/benzimidazolyl)Pyridazine:Syntheses,Structures,Emission and Photocatalytic Properties
LI Jin-PingFAN Jian-ZhongWANG Duo-Zhi*
(College of Chemistry and Chemical Engineering,Xinjiang University,Urumqi 830046,China)
Abstract:Four new metal-organic coordination polymers namely,[Mn(L1)4(OH)2](1),{[MnL1(H2O)4](SO4)}n(2)[CdL2(NO3)2]n(3),{[Co(L2)2](PF6)2}n(4)(L1=3,6-bis(N-imidazolyl)pyridazine,L2=3,6-bis(N-benzimidazolyl)pyridazine have been synthesized under solvothermal conditions and characterized by elemental analyses,IR spectra as well as single-crystal X-ray diffraction analysis.The analysis reveals that complex 1 has a mononuclear structure,and complex 2 features a 1D structure.Complexes 3 and 4 exhibit 2D network structures.The fluorescence and photocatalytic activities of the complexes 3 and 4 have been investigated and discussed.CCDC:1444988,1 1444989,2;1022876,3;1022877,4.
Keywords:complex;crystal structure;luminescent property
