黄酒多酚对同型半胱氨酸诱导的血管平滑肌细胞增殖和迁移的影响及其机制研究
2016-06-16刘龙斌孟立平许富康池菊芳郭航远
刘龙斌,孟立平,季 政,许富康,池菊芳,郭航远
312000浙江省绍兴市人民医院心内科 浙江大学绍兴医院
·论著·
黄酒多酚对同型半胱氨酸诱导的血管平滑肌细胞增殖和迁移的影响及其机制研究
刘龙斌,孟立平,季 政,许富康,池菊芳,郭航远
312000浙江省绍兴市人民医院心内科 浙江大学绍兴医院
【摘要】目的验证黄酒多酚(YWPC)是否具有抑制同型半胱氨酸(Hcy)诱导的大鼠血管平滑肌细胞(VSMCs)增殖和迁移的作用以及其产生作用的信号通路。方法采用组织贴块法培育SD大鼠胸主动脉VSMCs,取第4~7代细胞用于实验。细胞分为5组,分别为对照组,Hcy组(500 μmol/L Hcy),YWPC 1 mg/L组(500 μmol/L Hcy+1 mg/L YWPC),YWPC 10 mg/L组(500 μmol/L Hcy+10 mg/L YWPC),YWPC 100 mg/L组(500 μmol/L Hcy+100 mg/L YWPC)。采用MTT法检测VSMCs增殖情况;细胞划痕实验和Transwell法检测VSMCs迁移和侵袭情况;Western blotting法检测p-AKT/AKT表达情况。为验证YWPC是否经Pi3K/AKT通路发挥抑制VSMCs的增殖和迁移,分别加入LY-294002和胰岛素生长因子1(IGF-1)用来抑制和激活Pi3K/AKT通路,细胞被分为Hcy组,YWPC组,LY-294002+Hcy组,LY-294002+YWPC组;Hcy组,YWPC组,IGF-1+Hcy组,IGF-1+YWPC组,再分别采用上述方法检测VSMCs增殖、迁移和侵袭情况。结果培养大鼠胸主动脉VSMCs,2周左右细胞融合可以传代,经SM-actin细胞免疫荧光鉴定及DAPI核染,确定细胞纯度在99%以上。24、48 h时,5组OD值比较,差异有统计学意义(F=52.575,P=0.016;F=83.756,P=0.014);其中Hcy组OD值高于对照组和YWPC组,且与YWPC呈剂量依赖性(P<0.05)。24、48、72、96 h时,5组VSMCs迁移面积比较,差异均有统计学意义(P<0.05);其中Hcy组VSMCs迁移面积多于对照组(P<0.05);YWPC组VSMCs迁移面积低于Hcy组,且与YWPC呈剂量依赖性(P<0.05)。48 h时,5组迁移细胞数比较,差异有统计学意义(F=79.354,P=0.001);其中Hcy组穿透Transwell膜细胞数高于对照组(P<0.05);YWPC组穿透Transwell膜细胞数低于Hcy组,且与YWPC呈剂量依赖性(P<0.05)。5组p-AKT/AKT比较,差异有统计学意义(F=56.723,P=0.002);其中Hcy组p-AKT/AKT高于对照组(P<0.05);YWPC组p-AKT/AKT低于Hcy组,且与YWPC呈剂量依赖性(P<0.05)。加入LY-294002:24 h时,各组OD值、VSMCs迁移面积比较,差异有统计学意义(F=46.875,P=0.004;F=67.723,P=0.002);其中LY-294002+Hcy组OD值、VSMCs迁移面积低于Hcy组(P<0.05);LY-294002+Hcy组与LY-294002+YWPC组OD值、VSMCs迁移面积比较,差异无统计学意义(P>0.05)。48 h时,各组VSMCs穿透Transwell膜细胞数、p-AKT/AKT比较,差异有统计学意义(F=52.904,P=0.003;F=87.904,P=0.001);其中LY-294002+Hcy组VSMCs穿透Transwell膜细胞数、p-AKT/AKT低于Hcy组(P<0.05);LY-294002+Hcy组与LY-294002+YWPC组VSMCs穿透Transwell膜细胞数、p-AKT/AKT比较,差异无统计学意义(P>0.05)。加入IGF-1:24 h时,各组OD值、VSMCs迁移面积比较,差异有统计学意义(F=48.052,P=0.007;F=63.085,P=0.002);其中IGF-1+YWPC组OD值、VSMCs迁移面积高于YWPC组(P<0.05);IGF-1+Hcy组与IGF-1+YWPC组OD值、VSMCs迁移面积比较,差异无统计学意义(P>0.05)。48 h时,各组VSMCs穿透Transwell膜细胞数、p-AKT/AKT比较,差异有统计学意义(F=55.941,P=0.008;F=65.011,P=0.005);其中IGF-1+YWPC组VSMCs穿透Transwell膜细胞数、p-AKT/AKT高于YWPC组(P<0.05);IGF-1+Hcy组与IGF-1+YWPC组VSMCs穿透Transwell膜细胞数、p-AKT/AKT比较,差异无统计学意义(P>0.05)。结论YWPC通过抑制Pi3K/AKT信号通路抑制Hcy诱导的VSMCs增殖和迁移。
【关键词】多酚类;肌,平滑,血管;Pi3K/AKT
刘龙斌,孟立平,季政,等.黄酒多酚对同型半胱氨酸诱导的血管平滑肌细胞增殖和迁移的影响及其机制研究[J].中国全科医学,2016,19(14):1676-1683.[www.chinagp.net]
Liu LB,Meng LP,Ji Z,et al.Influence of yellow wine polyphenol compounds on homocysteine induced VSMCs proliferation and migration and its mechanism[J].Chinese General Practice,2016,19(14):1676-1683.
黄酒作为我国的特产,是世界上最古老的酿造酒种之一。黄酒以红粬、糯米和水为原料,以人工自然发酵酿制而成。现已证实黄酒中含有丰富的多酚、氨基酸、多肽、维生素、低聚糖、有机酸以及矿物质等有益于心脑血管健康的成分[1]。笔者前期研究已经证实黄酒不但可以抑制大鼠血管平滑肌细胞(VSMCs)增殖和迁移,而且可以抑制低密度脂蛋白受体基因敲除(LDLR-/-)小鼠动脉粥样硬化形成[2-4]。
随着“地中海饮食”以及“法国悖论”等概念的提出,大量的研究证实规律适量的饮用红酒具有心血管保护作用[5-6],并且已经阐明红酒的心血管保护作用主要源于其中的多酚类成分[7]。绍兴黄酒由糯米发酵而成,同样富含没食子酸、儿茶素等多酚类物质,所以推测,黄酒中主要也是多酚类物质在发挥其心脑血管保护作用。本研究主要验证黄酒多酚(YWPC)是否具有抑制VSMCs增殖和迁移的作用以及是否通过抑制Pi3K/AKT途径实现这一作用。
1材料与方法
1.1实验材料(1)实验动物:SPF级SD大鼠,雌雄不限,出生约60 d,体质量150~180 g(购自浙江省医学科学院实验动物中心);遗传背景为C57BL/6J 10代后雄性LDLR-/-小鼠(购于南京医科大学实验动物中心);(2)实验试剂:YWPC(由上海中药制药技术有限公司分离提取,纯度约60%);乙醚、甲醇、75%乙醇、95%乙醇、无水乙醇、甲醛(杭州化学试剂有限公司),DMEM高糖培养基、磷酸盐缓冲液(PBS)、0.25%胰蛋白酶-EDTA、青霉素和链霉素(杭州吉诺生物医药技术有限公司);同型半胱氨酸(Hcy)、雷帕霉素(Sigma公司),二甲基亚砜(DMSO)(MP Biomedicals公司),胎牛血清(GIBCO公司),MTT(Emresco公司);DAPI(Rcohe公司);兔抗p70S6K、p-p70S6K多克隆抗体、兔抗大鼠平滑肌肌动蛋白(SM-actin)单克隆抗体(ABCOM公司);辣根过氧化酶标记的山羊抗兔或抗鼠二抗、异硫氰酸荧光素(FITC)标记的山羊抗兔IgG(Jackson公司);Western blotting相关试剂(江苏碧云天生物技术研究所)。
1.2实验方法
1.2.1VSMCs原代细胞培养以及鉴定采用组织贴块法培育大鼠胸主动脉VSMCs,采用SM-actin免疫荧光鉴定VSMCs,并通过SM-actin与DAPI核染之间的关系鉴定VSMCs的纯度[8]。取第4~7代细胞用于后续实验,细胞加干预因素时采用含2.5%胎牛血清的培养基。
1.2.2实验分组
1.2.2.1验证不同浓度YWPC是否可以抑制Hcy诱导的VSMCs增殖和迁移细胞分为5组,分别为对照组,Hcy组(500 μmol/L Hcy),YWPC 1 mg/L组(500 μmol/L Hcy+1 mg/L YWPC),YWPC 10 mg/L组(500 μmol/L Hcy+10 mg/L YWPC),YWPC 100 mg/L组(500 μmol/L Hcy+100 mg/L YWPC)。
1.2.2.2验证YWPC是否经Pi3K/AKT通路抑制VSMCs的增殖和迁移(1)抑制Pi3K通路部分:待细胞生长到70%~80%后,血清饥饿使细胞同步化,加入20 nmol/L LY-294002孵育12 h以阻断Pi3K通路,再加入Hcy和YWPC干预VSMCs,观察Pi3K通路被抑制后YWPC对VSMCs的作用,细胞分为4组,分别为:Hcy组、YWPC组、LY-294002+Hcy组和LY-294002+YWPC组。(2)激活Pi3K通路部分:待细胞生长到70%~80%后,血清饥饿使细胞同步化,加入3 μg/L 胰岛素生长因子1(IGF-1)孵育12 h以激活Pi3K通路,再加入Hcy和YWPC干预VSMCs,观察Pi3K通路被激活后YWPC对VSMCs的作用,细胞分为4组,分别为:Hcy组、YWPC组、IGF-1+Hcy组和IGF-1+YWPC组。
1.2.3MTT法检测VSMCs增殖取第4~7代培养细胞,0.25%胰蛋白酶消化单层VSMCs,用含10%胎牛血清的DMEM高糖培养基配成细胞悬液并计数,以6×103个细胞/孔接种于96孔培养板中。待细胞贴壁后,无血清培养基培养24 h使细胞同步化。各组设4个复孔,2个不加细胞的空白对照孔。细胞于37 ℃、5% CO2孵箱培养48 h。培养结束,每孔加入MTT液20 μl,37 ℃继续孵育4~6 h,终止培养,小心弃去培养上清液,每孔加入150 μl DMSO,震荡10 min,选择490 nm波长于酶联免疫检测仪测定各孔吸光度(OD值)并记录。以时间为横轴,OD值为纵轴绘制VSMCs生长曲线。
1.2.4细胞划痕实验观察VSMCs迁移取第4~7代培养细胞,0.25%胰蛋白酶消化单层VSMCs,用含10%胎牛血清的DMEM高糖培养基配成细胞悬液并计数,以105个细胞/孔接种于6孔培养板中,每孔体积2 ml。细胞铺满板底后,无血清培养基培养24 h使细胞同步化,加入1.8 mmol/L羟基脲作用12 h抑制细胞增殖。用100 μl黄色枪头垂直于孔板制造细胞划痕,吸去细胞培养液,用PBS冲洗孔板3次,洗去划痕产生的细胞碎片。加入各组相应的干预因子,拍照记录0、12、24、48、72、96 h图片,用Image-Pro Plus 6.0分析计算细胞迁移的面积,细胞迁移的面积与最开始的划痕面积之间的比值表示迁移的距离。
1.2.5Transwell法检测VSMCs侵袭能力取第4~7代培养细胞,血清饥饿使细胞同步化,加入1.8 mmol/L羟基脲作用12 h抑制细胞增殖,0.25%胰蛋白酶消化单层VSMCs,用含有上述各组干预因子的DMEM高糖培养基(无血清)配成细胞悬液并计数(3×104/ml)。各组24孔板配套的Transwell小室(0.8 μm)上室加入200 μl细胞悬液,下室加入含有10%胎牛血清的DMEM高糖培养基500 μl,培养48 h。干预结束后以棉签轻轻擦去上层未穿透膜的VSMCs,取下Transwell半透膜,PBS洗涤3次,3.7%多聚甲醛溶液室温固定5 min,流水冲洗后,用2 mg/L DAPI染核,PBS冲洗后,荧光显微镜下随机取5个视野计数穿膜细胞数并记录。
1.2.6Western blotting测定VSMCs中p-AKT/AKT表达各组干预因子干预48 h后,裂解VSMCs提取蛋白,BCA法蛋白定量,十二烷基硫酸钠(SDS)-聚丙烯酰氨凝胶电泳(PAGE)每孔加入20 μl样品,80 V进行蛋白浓聚30 min,120 V进行凝胶电泳2 h,并用孔径0.45 μm硝酸纤维素膜250 mA进行转膜90 min。转膜完毕经常温下TBST洗膜3次,脱脂奶粉封闭2 h后,以1∶1 000浓度加入兔抗鼠p-AKT/AKT一抗,4 ℃孵育过夜,常温下TBST洗膜3次,辣根过氧化酶标记的羊抗兔二抗孵育后ECL化学发光法检测目标蛋白。暗室柯达胶片显影,Quantity one软件定量分析。

2结果
2.1VSMCs原代培养及鉴定采用组织贴块法培养大鼠胸主动脉VSMCs,约8d组织块周围有细胞爬出,2周左右细胞融合可以传代。传代后细胞呈典型“峰谷”状排列生长,用SM-actin细胞免疫荧光鉴定VSMCs,DAPI核染之后确定细胞纯度在99%以上(见图1,本文彩图详见本刊网站www.chinagp.net电子期相应文章附件)。
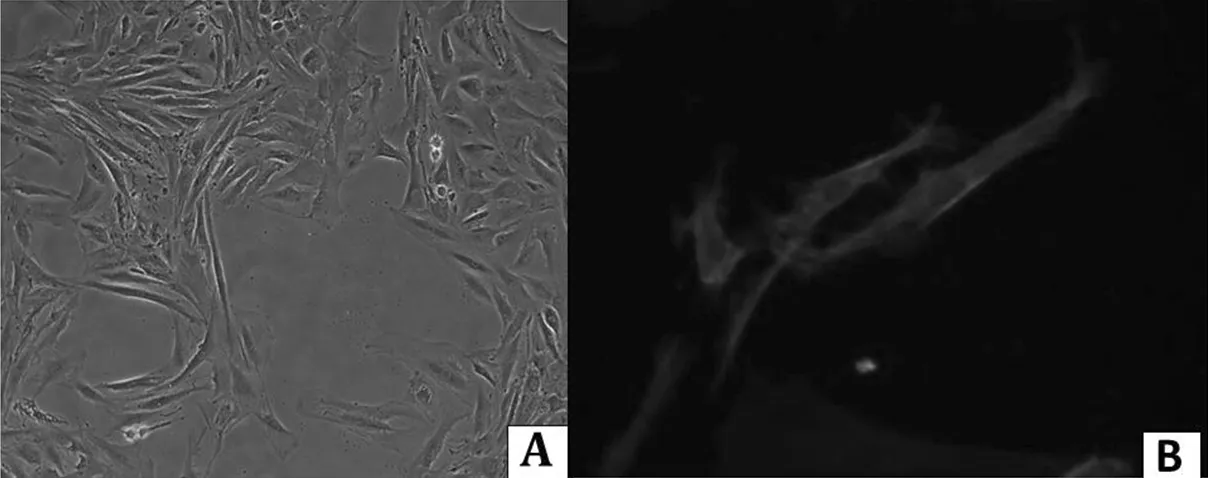
注:A=VSMCs形态学(×100),B=细胞免疫荧光鉴定(×400)
图1大鼠胸主动脉VSMCs形态学及细胞免疫荧光鉴定
Figure1IdentificationoftherataorticVSMCsbymorphologyandimmunocytochemistry
2.25组VSMCs不同时间增殖情况比较12 h时,5组OD值比较,差异无统计学意义(F=2.147,P=0.455)。24 h时,5组OD值比较,差异有统计学意义(F=52.575,P=0.016);其中Hcy组OD值高于对照组和YWPC组,且与YWPC呈剂量依赖性(P<0.05)。48 h时,5组OD值比较,差异有统计学意义(F=83.756,P=0.014);其中Hcy组OD值高于对照组和YWPC组,且与YWPC呈剂量依赖性(P<0.05,见图2)。

注:Hcy=同型半胱氨酸,YWPC=黄酒多酚
图2不同时间点各组VSMCs增殖情况
Figure 2Status of VSMCs proliferation of each group at different time points
2.35组VSMCs迁移面积比较12 h时,5组VSMCs迁移面积比较,差异无统计学意义(P>0.05)。24、48、72、96 h时,5组VSMCs迁移面积比较,差异均有统计学意义(P<0.05);其中Hcy组VSMCs迁移面积大于对照组,差异有统计学意义(P<0.05);YWPC组VSMCs迁移面积小于Hcy组,且与YWPC呈剂量依赖性(P<0.05,见表1)。
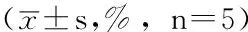
表1 不同时间点各组VSMCs迁移面积比较
注:与对照组比较,aP<0.05;与Hcy组比较,bP<0.05;Hcy=同型半胱氨酸,YWPC=黄酒多酚
2.45组VSMCs侵袭能力比较5组VSMCs 48 h穿透Transwell膜细胞数分别为(46±6)、(277±36)、(194±21)、(126±10)、(69±5),5组迁移细胞数比较,差异有统计学意义(F=79.354,P=0.001);其中Hcy组穿透Transwell膜细胞数多于对照组,差异有统计学意义(P<0.05);YWPC组穿透Transwell膜细胞数少于Hcy组,且与YWPC呈剂量依赖性(P<0.05,见图3)。

图3 Transwell小室培养48 h后各组迁移到下室的细胞DAPI染色图
Figure 3DAPI staining graph of VSMCs migration to lower chamber after 48 h culture in Transwell chamber
2.5YWPC抑制Pi3K/AKT通路的激活5组p-AKT/AKT比较,差异有统计学意义(F=56.723,P=0.002);其中Hcy组p-AKT/AKT高于对照组,差异有统计学意义(P<0.05);YWPC组p-AKT/AKT低于Hcy组,且与YWPC呈剂量依赖性(P<0.05,见图4)。

注:与对照组比较,aP<0.05;与Hcy组比较,bP<0.05
图4Western blotting检测各组VSMCs中AKT和p-AKT的表达
Figure 4Expression of AKT and p-AKT in VSMCs of each group detected by western blotting method
2.6LY-294002对YWPC抑制Hcy诱导的VSMCs增殖和迁移的影响
2.6.1不同时间点各干预因素对VSMCs增殖的影响12 h时,各组OD值比较,差异无统计学意义(F=4.247,P=0.239);24 h时,各组OD值比较,差异有统计学意义(F=46.875,P=0.004);其中LY-294002+Hcy组OD值低于Hcy组,差异有统计学意义(P<0.05);LY-294002+Hcy组与LY-294002+YWPC组OD值比较,差异无统计学意义(P>0.05)。48 h时,各组OD值比较,差异有统计学意义(F=85.736,P=0.001);其中LY-294002+Hcy组OD值低于Hcy组,差异有统计学意义(P<0.05);LY-294002+Hcy组与LY-294002+YWPC组OD值比较,差异无统计学意义(P>0.05,见图5)。
2.6.2不同时间点各干预因素对VSMCs迁移的影响24 h时,各组VSMCs迁移面积比较,差异有统计学意义(F=67.723,P=0.002);其中LY-294002+Hcy组VSMCs迁移面积小于Hcy组,差异有统计学意义(P<0.05);LY-294002+Hcy组与LY-294002+YWPC组VSMCs迁移面积比较,差异无统计学意义(P>0.05,见图6)。
2.6.3不同时间点各干预因素对VSMCs侵袭的影响48 h时,各组VSMCs穿透Transwell膜细胞数比较,差异有统计学意义(F=52.904,P=0.003);其中LY-294002+Hcy组VSMCs穿透Transwell膜细胞数少于Hcy组,差异有统计学意义(P<0.05);LY-294002+Hcy组与LY-294002+YWPC组VSMCs穿透Transwell膜细胞数比较,差异无统计学意义(P>0.05,见图6)。
2.6.4各干预因素对p-AKT/AKT的影响48 h时,各组p-AKT/AKT比较,差异有统计学意义(F=87.904,P=0.001);其中YWPC组、LY-294002+Hcy组、LY-294002+YWPC组p-AKT/AKT低于Hcy组(P<0.05);LY-294002+Hcy组与LY-294002+YWPC组p-AKT/AKT比较,差异无统计学意义(P>0.05,见图7)。
2.7IGF-1对YWPC抑制Hcy诱导的VSMCs增殖和迁移的影响
2.7.1不同时间点各干预因素对VSMCs增殖的影响12 h时,各组OD值比较,差异无统计学意义(F=6.251,P=0.238);24 h时,各组OD值比较,差异有统计学意义(F=48.052,P=0.007);其中IGF-1+YWPC组和IGF-1+Hcy组OD值高于YWPC组,差异有统计学意义(P<0.05);IGF-1+Hcy组与IGF-1+YWPC组OD值比较,差异无统计学意义(P>0.05)。48 h时,各组OD值比较,差异有统计学意义(F=44.052,P=0.003);其中IGF-1+YWPC组和IGF-1+Hcy组OD值高于YWPC组,差异有统计学意义(P<0.05);IGF-1+Hcy组与IGF-1+YWPC组OD值比较,差异无统计学意义(P>0.05,见图8)。

图5 不同时间点各个干预因素对VSMCs增殖的影响
Figure 5Influence of each intervention factor on VSMCs proliferation at different time points

图6 Transwell小室48 h时各组迁移到下室的细胞DAPI染色图
Figure 6DAPI staining graph of VSMCs migration to lower chamber after 48 h culture in Transwell chamber and VSMCs migration after 24 h cell wound scratch assay
2.7.2不同时间点各干预因素对VSMCs迁移的影响24 h时,各组VSMCs迁移面积比较,差异有统计学意义(F=63.085,P=0.002);其中IGF-1+YWPC组VSMCs迁移面积大于YWPC组,差异有统计学意义(P<0.05);IGF-1+Hcy组与IGF-1+YWPC组VSMCs迁移面积比较,差异无统计学意义(P>0.05,见图9)。
2.7.3不同时间点各干预因素对VSMCs侵袭的影响48 h时,各组VSMCs穿透Transwell膜细胞数比较,差异有统计学意义(F=55.941,P=0.008);其中IGF-1+YWPC组VSMCs穿透Transwell膜细胞数多于YWPC组,差异有统计学意义(P<0.05);IGF-1+Hcy组与IGF-1+YWPC组VSMCs穿透Transwell膜细胞数比较,差异无统计学意义(P>0.05,见图9)。
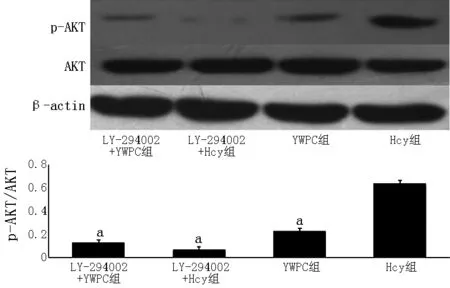
注:与Hcy组比较,aP<0.05
图7Western blotting检测各组VSMCs中p-AKT/AKT的表达
Figure 7Expression of p-AKT/AKT of VSMCs in each group detected by western blotting method
2.7.4各干预因素对p-AKT/AKT的影响48 h时,各组p-AKT/AKT比较,差异有统计学意义(F=65.011,P=0.005);其中Hcy组、IGF-1+Hcy组和IGF-1+YWPC组p-AKT/AKT高于YWPC组,差异有统计学意义(P<0.05);IGF-1+Hcy组与IGF-1+YWPC组p-AKT/AKT比较,差异无统计学意义(P>0.05,见图10)。

注:IGF-1=胰岛素生长因子1
图8不同时间点各个干预因素对VSMCs增殖的影响
Figure 8Influence of each intervention factor on VSMCs proliferation at different time points
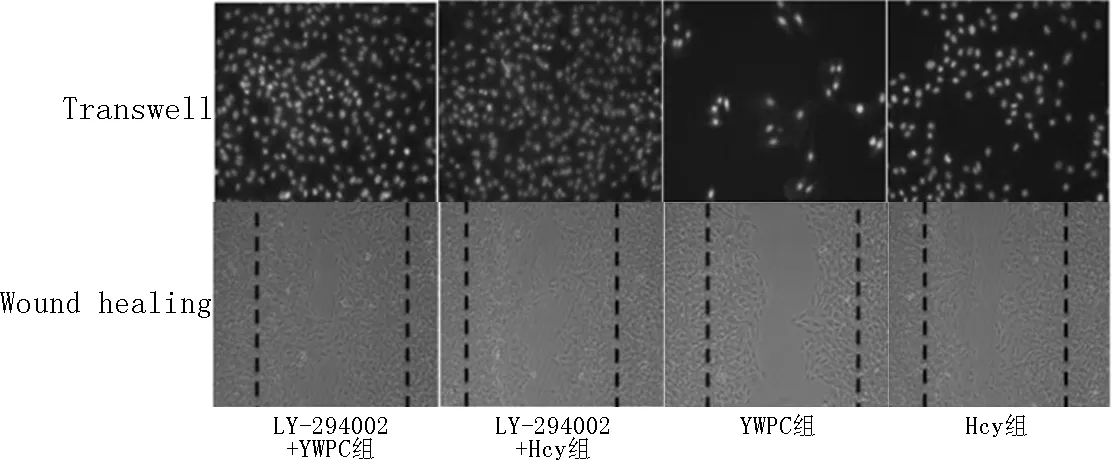
图9 Transwell小室48 h时各组迁移到下室的细胞DAPI染色图
Figure 9DAPI staining graph of VSMCs migration to lower chamber after 48 h culture in Transwell chamber and VSMCs migration after 24 h cell wound scratch assay

注:与YWPC组比较,aP<0.05
图10Western blotting检测各组VSMCs中p-AKT/AKT的表达
Figure 10Expression of p-AKT/AKT of VSMCs detected by western blotting method
3讨论
研究显示规律适量地饮用红酒具有心血管保护作用[5-6],并且已经阐明红酒的心血管保护作用主要是由于其中的多酚类成分[7,9]。红酒多酚由白藜芦醇、儿茶素、花青素等组成,通过调节血脂、改善血管功能、抑制VSMCs增殖和迁移等作用而发挥心血管保护作用。黄酒是一种古老的酿造酒种,以水、红粬、糯米和小麦为原料,采用人工发酵酿制而成。与红酒一样,目前发现绍兴黄酒中也富含儿茶素、没食子酸等多酚类物质(约50 mg/L)[1,10-11]。笔者前期实验已经证明YWPC具有抑制LDLR-/-小鼠动脉粥样硬化的作用,然而其抑制小鼠动脉粥样硬化的具体机制尚未阐明[12]。
VSMCs位于动脉血管的中层,在正常状态下主要发挥收缩血管的功能,当遇到炎性因子等血管损伤因素时,VSMCs可以被激活而增加增殖和迁移的能力[13]。VSMCs大量增殖、迁移、吞噬氧化低密度脂蛋白(ox-LDL)之后形成肌源性泡沫细胞,并进一步分泌更多的炎性因子,使炎性反应级联放大,细胞外基质降解,最终导致粥样斑块的形成以及斑块的破裂。本实验研究结果显示,YWPC组OD值、迁移面积、侵袭能力均低于Hcy组,且p-AKT/AKT低于Hcy组,推测YWPC可能是通过Pi3K/AKT途径发挥抑制Hcy诱导的VSMCs的增殖和迁移,为验证此推测,本实验分别采用Pi3K特异性抑制剂LY-294002抑制VSMCs中的Pi3K/AKT通路和Pi3K激动剂IGF-1激活Pi3K/AKT通路,采用LY-294002干预细胞后,细胞内Pi3K/AKT通路被抑制,与Hcy组比较,LY-294002+Hcy组VSMCs OD值和迁移减少,说明抑制Pi3K/AKT通路可以抑制VSMCs增殖和迁移。在加入LY-294002的基础上再加Hcy或YWPC,此时由于Pi3K/AKT通路已经被抑制,YWPC和Hcy对Pi3K/AKT通路的影响消失,此时实验结果发现LY-294002+Hcy组与LY-294002+YWPC组VSMCs增殖和迁移比较无差异,证实YWPC和Hcy是通过作用在Pi3K/AKT通路发挥其影响VSMCs增殖和迁移的作用。同样,采用IGF-1干预细胞后,细胞内Pi3K/AKT通路被激活,与YWPC组比较,IGF-1+YWPC组VSMCs增殖和迁移增加,说明激活Pi3K/AKT通路可以增加VSMCs增殖和迁移。在加入IGF-1的基础上再加Hcy或YWPC,此时由于Pi3K/AKT通路已经被IGF-1持续激活,YWPC和Hcy对Pi3K/AKT通路的作用消失,此时实验结果发现IGF-1+Hcy组与IGF-1+YWPC组VSMCs增殖和迁移比较无差异,证实YWPC和Hcy是通过作用在Pi3K/AKT通路上发挥其影响VSMCs增殖和迁移的作用。这可能是其具有抗动脉粥样硬化作用的机制之一。
Pi3K/AKT通路在VSMCs增殖和迁移以及表型转化中发挥着重要作用[14-15],p-AKT可以进一步激活mTOR/p70S6K通路,增加p-p70S6K1的表达,最终增加VSMCs增殖和迁移[16]。有研究发现杨梅苷可以通过抑制p-AKT的表达从而抑制血小板衍生生长因子(PDGF)-BB诱导的VSMCs增殖和迁移。同时,Wang等[17]研究结果也显示Apelin通过抑制Pi3K/AKT/FoxO3a/MMP-2通路抑制VSMCs的迁移,本研究结果与上述结果相符,表明YWPC可以抑制Pi3K/AKT通路,减少p-AKT的表达。
本研究在前期已经证实黄酒和YWPC具有抗动脉粥样硬化作用的基础上[2-3],进一步探索证明YWPC具有抗Hcy诱导的VSMCs增殖和迁移的作用,并且证明其作用是通过抑制Pi3K/AKT通路而实现,这可能是其抗动脉粥样硬化的一个重要机制。本实验也存在一定的局限性,黄酒中的多酚类物质是由不同的单体成分组成的混合物,究竟是其中的何种确切成分在发挥作用目前尚不十分清楚,仍需后续实验加以证明。
作者贡献:刘龙斌进行实验设计与实施、资料收集整理、撰写论文、成文并对文章负责;孟立平、季政、许富康、池菊芳进行实验实施、评估、资料收集;郭航远进行质量控制及审校。
本文无利益冲突。
参考文献
[1]Xie GF,Xu R,Fan AP.Analysis of components of Guyue Longshan rice wine benificial to health of heart and blood vessel system[J].Liquor Making,2011,38(5):64-66.(in Chinese)
谢广发,徐榕,樊阿萍.古越龙山黄酒中有益心血管健康的成分分析[J].酿酒,2011,38(5):64-66.
[2]Guo H,Wang P,You B,et al.Chinese yellow wine inhibits production of homocysteine-induced extracellular matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells[J].Clin Nutr,2007,26(3):348-354.
[3]Guo H,Liu L,Shi Y,et al.Chinese yellow wine and red wine inhibit matrix metalloproteinase-2 and improve atherosclerotic plaque in LDL receptor knockout mice[J].Cardiovasc Ther,2010,28(3):161-168.
[4]孟立平,周昌钻,郭艳,等.黄酒中抑制同型半胱氨酸诱导的大鼠血管平滑肌细胞增殖和迁移成分探讨[J].中国应用生理学杂志,2015,31(5):437-442.
[5]Li H,Förstermann U.Red wine and cardiovascular health[J].Circ Res,2012,111(8):959-961.
[6]Arranz S,Chiva-Blanch G,Valderas-Martínez P,et al.Wine,beer,alcohol and polyphenols on cardiovascular disease and cancer[J].Nutrients,2012,4(7):759-781.
[7]Gresele P,Cerletti C,Guglielmini G,et al.Effects of resveratrol and other wine polyphenols on vascular function:an update[J].J Nutr Biochem,2011,22(3):201-211.
[8]Meng LP,Jiang CJ,Zhao F,et al.An improvenment in primary culture of rat vascular smooth muscle cells and its identification[J].Journal of Wenzhou Medical University,2015,45(8):593-596.(in Chinese)
孟立平,蒋承建,赵飞,等.大鼠胸主动脉血管平滑肌细胞原代培养方法的改进[J].温州医科大学学报,2015,45(8):593-596.
[9]Dell′Agli M,Buscialà A,Bosisio E.Vascular effects of wine polyphenols[J].Cardiovasc Res,2004,63(4):593-602.
[10]Yang LN,Zhang PZ.The research progress of nutrient composition and functional components in Chinese rice wine[J].Liquor Making,2009,36(5):9-12.(in Chinese)
杨丽娜,张培正.中国黄酒中营养成分与功能成分的研究进展[J].酿酒,2009,36(5):9-12.
[11]邢杨波,郭航远,王平,等.黄酒对同型半胱氨酸诱导的大鼠血管平滑肌细胞基质分泌金属蛋白酶2表达的影响[J].中华心血管病杂志,2008,36(11):1043.
[12]Zhai X,Chi J,Tang W,et al.Yellow wine polyphenolic compounds inhibit matrix metalloproteinase-2,-9 expression and improve atherosclerotic plaque in LDL-receptor-knockout mice[J].J Pharmacol Sci,2014,125(2):132-141.
[13]Alexander MR,Owens GK.Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease[J].Annu Rev Physiol,2012,74:13-40.
[14]Kaimoto T,Yasuda O,Ohishi M,et al.Nifedipine inhibits vascular smooth muscle cell dedifferentiation via downregulation of Akt signaling[J].Hypertension,2010,56(2):247-252.
[15]Xu H,Dai YR,Fu YR,et al.Coordinated regulation of the proliferation of airway smooth muscle cells by ERK and Pi3K signal pathways in asthmatic rats[J].Chinese General Practice,2015,17(9):1032-1036.(in Chinese)
徐慧,戴元荣,付玉茹,等.磷脂酰肌醇3激酶和细胞外调节蛋白激酶信号通路对支气管哮喘大鼠气管平滑肌细胞增殖的协同调控作用[J].中国全科医学,2015,17(9):1032-1036.
[16]Rotllan N,Wanschel AC,Fernández-Hernando A,et al.Genetic evidence supports a major role for Akt1 in VSMCs during atherogenesis[J].Circ Res,2015,116(11):1744-1752.
[17]Wang C,Wen J,Zhou Y,et al.Apelin induces vascular smooth muscle cells migration via a PI3K/Akt/FoxO3a/MMP-2 pathway[J].Int J Biochem Cell Biol,2015,69:173-182.
(本文编辑:贾萌萌)
Influence of Yellow Wine Polyphenol Compounds on Homocysteine Induced VSMCs Proliferation and Migration and Its Mechanism
LIULong-bin,MENGLi-ping,JIZheng,etal.
DepartmentofCardiology,ShaoxingPeople′sHospital,Shaoxing312000,China
【Abstract】ObjectiveTo verify whether yellow wine polyphenol compounds(YWPC)could inhibit homocysteine(Hcy)induced rat aortic vascular smooth muscle cells(VSMCs)proliferation and migration and its signaling pathway.MethodsTissue-sticking method was used to culture SD rat VSMCs,and 4-7 generations of cells were obtained.The cells were divided into 5 groups,which were control group,Hcy group(500 μmol/L Hcy),YWPC 1 mg/L group(500 μmol/L Hcy+1 mg/L YWPC),YWPC 10 mg/L group(500 μmol/L Hcy+10 mg/L YWPC)and YWPC 100 mg/L group(500 μmol/L Hcy+100 mg/L YWPC).MTT method was used to detect VSMCs proliferation;cell wound scratch assay and Transwell method were used to detect VSMCs migration and invasion;Western blotting method was used to detect p-AKT/AKT expression.In order to verify whether YWPC can inhibit VSMCs proliferation and migration through Pi3K/AKT pathway,LY-294002 and IGF-1 were added respectively to inhibit and activate Pi3K/AKT pathway.The cells added with LY-294002 were divided into Hcy group,YWPC group,LY-294002+Hcy group,and LY-294002+YWPC group;the cells added with IGF-1 were divided into Hcy group,YWPC group,IGF-1+Hcy group,and IGF-1+YWPC group.The above methods were employed again to detect the proliferation,migration and invasion of VSMCs.ResultsRat aortic VSMCs were cultured.Two weeks later,cell fusion and passage occurred,and cell purity was determined to be above 99% by SM-actin cellular immune fluorescent identification and DAPI nuclear staining dye.At 24 h and 48 h,the five groups were significantly different in OD value(F=52.575,P=0.016;F=83.756,P=0.014),and the OD value of Hcy group was higher than that of control group and YWPC group,and in a dose-dependent manner(P<0.05).At 24,48,72 and 96 h,the five groups were significantly different in VSMCs migration area(P<0.05);Hcy group was higher than control group in VSMCs migration area(P<0.05);YWPC group was lower than Hcy group in VSMCs migration area,and in a dose-dependent manner(P<0.05).At 48 h,the five groups were significantly different in the number of migrating cells(F=79.354,P=0.001);Hcy group was higher than control group in the number of cells that penetrated through the transwell membrane(P<0.05);the number of cells that penetrated through the transwell membrane of YWPC group was lower than that of Hcy group,and in a dose-dependent manner(P<0.05).The five group were significantly different in p-AKT/AKT(F=56.723,P=0.002);Hcy group was higher than control group in p-AKT/AKT(P<0.05);the p-AKT/AKT of YWPC group was lower than that of Hcy group,and in a dose-dependent manner(P<0.05).After adding LY-294002 for 24 hours,the four groups were significantly different in OD value,VSMCs migration area(F=46.875,P=0.004;F=67.723,P=0.002);LY-294002+Hcy group was lower than Hcy group in OD value and VSMCs migration area(P<0.05);LY-294002+Hcy group and LY-294002+YWPC group were not significantly different in OD value and VSMCs migration area(P>0.05).After adding LY-294002 for 48 hours,the four groups were significantly different in the number of cells that penetrated through the transwell membrane and p-AKT/AKT(F=52.904,P=0.003;F=87.904,P=0.001);LY-294002+Hcy group was lower than Hcy group in the number of cells that penetrated through the transwell membrane and p-AKT/AKT(P<0.05);LY-294002+Hcy group and LY-294002+YWPC group were not significantly different in the number of cells that penetrated through the transwell membrane and p-AKT/AKT(P>0.05).After adding IGF-1 for 24 hours,the four groups were significantly different in OD value and VSMCs migration area(F=48.052,P=0.007;F=63.085,P=0.002);IGF-1+YWPC group was higher than YWPC group in OD value and VSMCs migration area(P<0.05);IGF-1+Hcy group and IGF-1+YWPC group were not significantly different in OD value and VSMCs migration area(P>0.05).After adding IGF-1 for 48 hours,the four groups were significantly different in the number of cells that penetrated through the transwell membrane and p-AKT/AKT(F=55.941,P=0.008;F=65.011,P=0.005);IGF-1+YWPC group was higher than YWPC group in the number of cells that penetrated through the transwell membrane and p-AKT/AKT(P<0.05);IGF-1+Hcy group and IGF-1+YWPC group were not significantlydifferent in the number of cells that penetrated through the transwell membrane and p-AKT/AKT(P>0.05).ConclusionYWPC inhibits Hcy induced VSMCs proliferation and migration by suppressing Pi3K/AKT signaling pathway.
【Key words】Polyphenols;Muscle,smooth,vascular;Pi3K/AKT
基金项目:浙江省自然科学基金资助项目(LY14H020002);绍兴市科技局项目(2013B70072)
通信作者:郭航远,312000浙江省绍兴市人民医院心内科 浙江大学绍兴医院;E-mail:ghangyuan@hotmail.com
【中图分类号】R 543.31
【文献标识码】A
doi:10.3969/j.issn.1007-9572.2016.14.015
(收稿日期:2015-12-01;修回日期:2016-02-04)
