双氢青蒿素对C2株蓝氏贾第鞭毛虫Delta giardin抑制作用的研究
2015-05-04刘阿倩林志强张亚粉田喜凤
刘阿倩,王 洋,林志强,张亚粉,田喜凤,余 源
·实验研究·
双氢青蒿素对C2株蓝氏贾第鞭毛虫Delta giardin抑制作用的研究
刘阿倩,王 洋,林志强,张亚粉,田喜凤,余 源
目的 建立实时荧光定量RT-PCR( real- time quantitative, RT- PCR)检测C2株蓝氏贾第鞭毛虫(Giardialamblia)Delta giardin基因mRNA表达量的方法,分析双氢青蒿素(Dihydroartemisinin, DHA)对体外C2株蓝氏贾第鞭毛虫Delta giardin基因mRNA表达水平的影响。方法 分别采用100 μg/mL、200 μg/mL的双氢青蒿素改良型TYI-S-33培养基培养C2株蓝氏贾第鞭毛虫,以不含药物组作为阴性对照,分别培养2 h、4 h、8 h、12 h,提取总RNA,逆转录合成cDNA,实时荧光定量PCR检测Delta giardin基因mRNA表达情况。结果 100 μg/mL双氢青蒿素培养2 h、4 h、8 h、12 h后,Delta giardin基因mRNA相对表达量分别为0.44、0.26、0.25、0.02;200 μg/mL双氢青蒿素培养2 h、4 h、8 h、12 h后,Delta giardin基因mRNA相对表达量分别为0.30,0.26,0.11,0.02。药物对照组中C2株蓝氏贾第鞭毛虫Delta giardin基因mRNA表达量明显低于阴性对照组。结论 双氢青蒿素对C2株蓝氏贾第鞭毛虫Delta giardin基因mRNA的表达具有明显的抑制作用,提示双氢青蒿素对蓝氏贾第鞭毛虫具有明显的防治效果。
C2株蓝氏贾第鞭毛虫; 双氢青蒿素; 实时荧光定量RT-PCR; Delta giardin
蓝氏贾第鞭毛虫(Giardialamblia,简称贾第虫)是一种世界性分布的胃肠道寄生虫,主要寄生于人和多种哺乳动物的小肠内并引起以腹泻为主要症状的蓝氏贾第鞭毛虫病(giardiasis, 简称贾第虫病)[1]。贾第虫双核、具有鞭毛,地理分布广泛,有多种动物宿主,因此可通过粪便、被污染的水源、食物进行传播。本病已被列为全世界危害人类健康的十种主要寄生虫病之一,在发达国家和发展中国家均有广泛流行[2-5]。有研究表明蓝氏贾第鞭毛虫能够通过其腹吸盘吸附于宿主肠上皮细胞,是其致病的关键因素。Crossley和Holberton[6-7]首次提出贾第素是贾第虫细胞骨架的特有成分,主要分为4大类, 即α、β、γ和δ贾第素[8-10]。腹吸盘的主要成分包括骨架蛋白,因此,贾第虫的骨架结构与致病性密切相关,针对蓝氏贾第鞭毛虫骨架蛋白进行新药物的研发对贾第虫病的有效防治具有重要的意义。前期研究采用蛋白质组学技术观察到双氢青蒿素对体外培养的蓝氏贾第鞭毛虫滋养体蛋白质具有明显的损伤作用,其中包括细胞骨架蛋白[11]。为了进一步研究双氢青蒿素对蓝氏贾第鞭毛虫Delta giardin的抑制作用,本研究采用含有双氢青蒿素的改良TYI-S-33培养基培养C2株蓝氏贾第鞭毛虫,实时荧光定量RT-PCR检测双氢青蒿素对C2株蓝氏贾第鞭毛虫Delta giardin的抑制作用。
1 材料与方法
1.1 材料 双氢青蒿素粉剂(原药批号010904)为北京丰台科技园生物技术公司藤海宁女士惠赠;C2株蓝氏贾第鞭毛虫由本实验室液氮保存;M-MLV 逆转录酶,RNase inhibitor,Random Primers购自Promega公司;dNTP,SYBR®Premix Ex TaqTMReal-Time PCR试剂盒购自TaKaRa(大连)公司;引物由生工生物工程(上海)公司合成;组织/细胞基因组RNA提取试剂盒购自Invitrogen;焦碳酸二乙酯(DEPC)购自上海生工生物技术有限公司。
1.2 方法
1.2.1 蓝氏贾第鞭毛虫复苏培养 将液氮内冻存的C2株蓝氏贾第鞭毛虫复苏,置含改良TYI-S-33培养基的4 mL硼酸硅培养管内,于37 ℃培养。48~72 h后,虫体即呈对数生长期。选取虫体生长旺盛的培养管,置4 ℃冰浴,15 min后取出,在双手掌间多次滚搓培养管,使贴壁生长的虫体完全自管壁脱落。用血球计数板计数虫数,再用培养基将虫液浓度调为6×106~10×106个滋养体/mL[12],传代培养。
1.2.2 药物培养 培养48 h后选取虫体贴满瓶壁的培养管,药物组培养基内分别加入100 μg/mL 、200 μg/mL双氢青蒿素,对照组培养基内不加药;37 ℃分别培养2 h、4 h、8 h、12 h后,收集虫体,用血球计数板计数虫数,用培养基将虫液浓度调至1×107个滋养体/mL;取1mL虫液,用PBS(pH7.4)清洗3次,离心10 min(4 000 r/min),弃上清,留沉淀。
1.2.3 总RNA提取及cDNA的合成 离心分离的虫体细胞中加入细胞裂解液Trizol(Invitrogen)1 mL,反复颠倒后迅速吸入经焦碳酸二乙酯(DEPC)处理过的eppendorf离心管中,按照说明书提取总RNA,紫外分光光度法鉴定总RNA的纯度。
建立20 μL反转录体系:RNA模板,5 μL,M-MLV 1 μL,Random Primers(100 ng/μL) 1 μL,RNase inhibitor 1 μL,10 mmol/L dNTP 1 μL,5×反应缓冲液4 μL,DEPC水7 μL;反转录条件:70 ℃变性5 min,42 ℃反转录60 min,即得cDNA,-80 ℃保存备用。
1.2.4 引物设计 NCBI网站检索蓝氏贾第鞭毛虫Delta giardin(XM_001707397.1)和GAPDH (XM_001704991.1)基因序列(http://www.ncbi.nlm.nih.gov/gene/5700349),采用Primer Premier 5软件分别设计合成蓝氏贾第鞭毛虫Delta giardin(ATCC 50803)及GAPDH(内参基因)特异性引物 (表1)。

表1 Delta giardin和GAPDH引物序列
1.2.5 实时荧光定量PCR检测Delta giardin基因mRNA的相对表达量 采用Corbett实时荧光定量PCR仪,分别以Delta giardinS/Delta giardinA; GAPDHS/GAPDHA为引物进行实时荧光定量PCR检测。药物培养组及阴性对照组每个样品均做2个重复,以各组逆转录合成的cDNA作为模板,磷酸甘油醛脱氢酶基因(GAPDH)为内参基因,建立20 μL反应体系: 2×SYBR MIX 10 μL,cDNA 1 μL,Prime S 1 μL,Prime A 1 μL,DEPC H2O 7 μL,反应条件:95 ℃变性15 s,60 ℃复性15 s, 72 ℃延伸30 s。Delta Delta CT法分析检测结果,确定Delta giardin基因mRNA的相对表达量。
2 结 果
2.1 总RNA提取结果 严格按照试剂盒操作说明进行,利用Trizol提取蓝氏贾第鞭毛虫总RNA,各组RNAA260nm/A280nm值均在1.8~2.0范围内,总RNA提取质量符合实验要求。
2.2 Real-Time PCR检测结果 分别得到Delta giardin基因Real-time PCR扩增曲线、熔解曲线(图1-2)和GAPDH基因Real-time PCR扩增曲线、熔解曲线(图3-4),融解曲线只有单峰值, 排除了非特异性扩增。Delta Delta CT法分析各组Delta giardin基因mRNA的相对表达量。△循环阈值(cycle threshold, Ct) =样品Ct均值-内参照Ct均值,△△Ct = △Ct-(随机阴性对照样品Ct均值-该样品内参照Ct均值),以2-△△Ct表示样品中目的基因初始cDNA相对表达量[13]。研究结果表明(表2),培养基内加入浓度为100 μg/mL的双氢青蒿素,分别培养蓝氏贾第鞭毛虫2 h、4 h、8 h、12 h后,Delta giardin基因mRNA相对表达量为0.44,0.26,0.25,0.02;培养基内加入浓度为200 μg/mL的双氢青蒿素,分别培养蓝氏贾第鞭毛虫2 h、4 h、8 h、12 h后,Delta giardin基因mRNA相对表达量为0.30,0.26,0.11,0.02。药物组Delta giardin基因mRNA均低于对照组,表明双氢青蒿素对C2株蓝氏贾第鞭毛虫Delta giardin基因表达具有明显的抑制作用,抑制作用随着药物浓度的增高和作用时间的延长而增强。
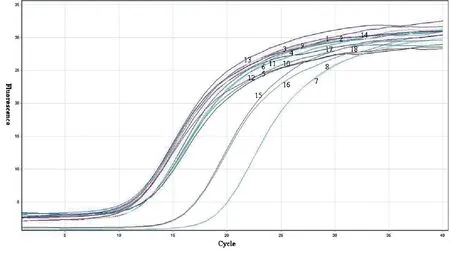
1-2: DHA 200 μg/mL for 2 h; 3-4: DHA 200 μg/mL for 4 h; 5-6: DHA 200 μg/mL for 8 h; 7-8: DHA 200 μg/mL for 12 h; 9-10: DHA 100 μg/mL for 2 h; 11-12: DHA 100 μg/mL for 4 h; 13-14: DHA 100 μg/mL for 8 h; 15-16: DHA 100 μg/mL for 12 h; 17-18: Negative control.
图1 Delta giardin mRNA Real-time PCR扩增曲线
Fig.1 Amplification curve of Delta giardin mRNA
3 讨 论

1-2: DHA 200 μg/mL for 2 h; 3-4: DHA 200 μg/mL for 4 h; 5-6: DHA 200 μg/mL for 8 h; 7-8: DHA 200 μg/mL for 12 h; 9-10: DHA 100 μg/mL for 2 h; 11-12: DHA 100 μg/mL for 4 h; 13-14: DHA 100 μg/mL for 8 h; 15-16: DHA 100 μg/mL for 12 h; 17-18: Negative control.
图2 Delta giardin mRNA Real-time PCR熔解曲线
Fig.2 Melting curve of Delta giardin mRNA

1-2: DHA 200 μg/mL for 2 h; 3-4: DHA 200 μg/mL for 4 h; 5-6: DHA 200 μg/mL for 8 h; 7-8: DHA 200 μg/mL for 12 h; 9-10: DHA 100 μg/mL for 2 h; 11-12: DHA 100 μg/mL for 4 h; 13-14: DHA 100 μg/mL for 8 h; 15-16: DHA 100 μg/mL for 12 h; 17-18: Negative control.
图3 GAPDH mRNA Real-timePCR扩增曲线
Fig.3 Amplification curve of GAPDH mRNA
1-2: DHA 200 μg/mL for 2 h; 3-4: DHA 200 μg/mL for 4 h; 5-6: DHA 200 μg/mL for 8 h; 7-8: DHA 200 μg/mL for 12 h; 9-10: DHA 100 μg/mL for 2 h; 11-12: DHA 100 μg/mL for 4 h; 13-14: DHA 100 μg/mL for 8 h; 15-16: DHA 100 μg/mL for 12 h; 17-18: Negative control.
图4 GAPDH mRNA Real-time PCR熔解曲线
Fig.4 Melting curve of GAPDH mRNA
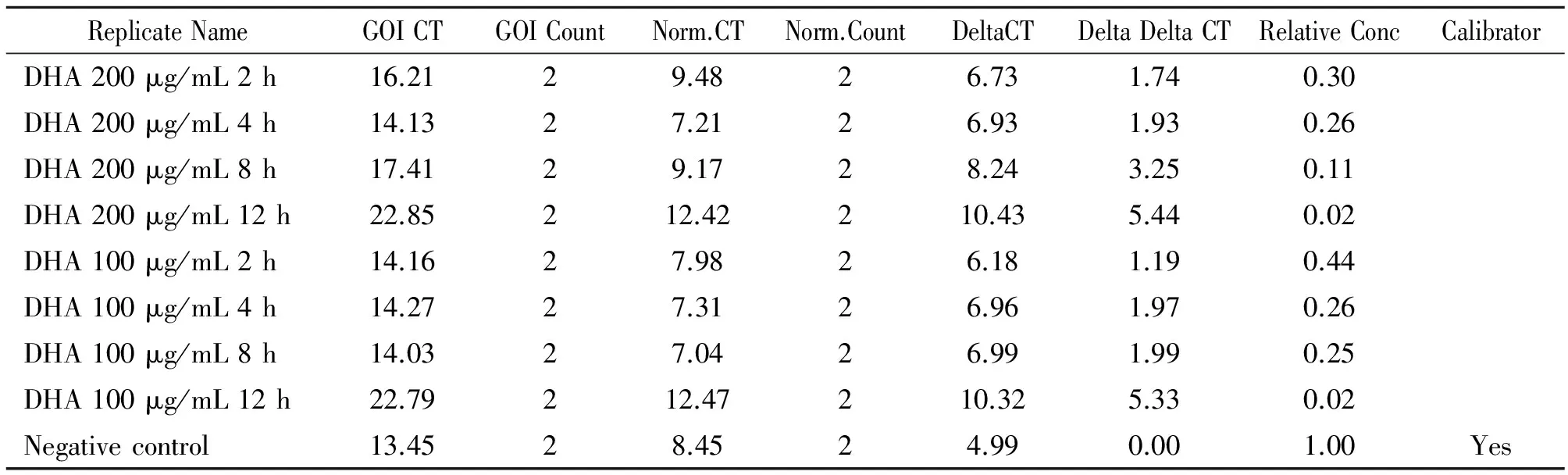
表2 DHA作用后Delta giardin基因mRNA表达量分析结果
青蒿素(artemisinin)是从菊科蒿属黄花蒿茎叶中提取的一种含过氧基团的倍半萜内酯, 经过人工化学修饰、改进后发展了多种衍生物,主要包括青蒿琥酯(artesunate) 、双氢青蒿素(dihydroartemisinin)及蒿甲醚(artemether) 等[14]。双氢青蒿素是青蒿素及其衍生物蒿甲醚和青蒿琥酯在体内的有效活性代谢产物,为广谱抗寄生虫药物,对多种寄生性原虫有良好的杀灭作用[15-16]。在抗疟疾、弓形虫、肺孢子虫肺炎、肿瘤,以及调节免疫和抗孕等方面具有广泛的应用[17-18]。
实时荧光定量PCR技术( real-time fluorescent quantitative PCR, FQ-PCR) 于1996年由美国Applied Biosystems公司推出,该技术在PCR反应体系中加入荧光基团,利用荧光信号积累实时监测整个PCR进程[19-20]。实时荧光定量RT-PCR ( real-time fluorescent quantitative reverse transcription-polymerase chain reaction, FQ RT-PCR )将实时荧光定量PCR与逆转录技术相结合,能够实时检测记录PCR扩增产物的增加,从而准确检测相应mRNA的含量[21-22]。
虽然有研究表明双氢青蒿素对疟原虫、弓形虫、血吸虫、肺孢子虫等多种寄生虫均有显著的损伤效果,但其作用机制鲜有报道,为了进一步研究其药理机制,本研究采用实时荧光定量RT-PCR检测双氢青蒿素对C2株蓝氏贾第鞭毛虫Delta giardin的抑制作用。研究结果表明,双氢青蒿素对蓝氏贾第鞭毛虫Delta giardin基因的表达具有明显的抑制作用,抑制作用与药物浓度和作用时间成正比。此结果与蓝氏贾第鞭毛虫骨架蛋白双向电泳质谱结果一致[23]。蓝氏贾第鞭毛虫能够通过其腹吸盘吸附于宿主肠上皮细胞,其骨架结构与致病性密切相关。Delta giardin是蓝氏贾第鞭毛虫骨架蛋白的主要成分,是其致病的关键因素,因此双氢青蒿素对贾第虫Delta giardin基因的抑制作用可能会影响蓝氏贾第鞭毛虫的感染过程,对蓝氏贾第鞭毛虫的防治起到一定的效果,本研究也为进一步阐明其药理机制提供了有价值的参考资料。
[1]Adam RD. Biology ofGiardialamblia[J]. Clin Microbiol Rev, 2001, 14(3): 447-475.
[2]Gardner TB, Hill DR. Treatment of giardiasis[J]. Clin Microbiol Rev, 2001, 14(1): 114-128.
[3]Lu SQ. Domestic research ofGiardialamblia[J]. Acta Parasitologica Et Medica Entomologica Sinica, 1999, 6(4): 193-200. (in Chinese) 卢思奇.国内蓝氏贾第鞭毛虫研究[J].寄生虫与医学昆虫报, 1999, 6(4): 193-200.
[4]Sandhu H, Mahajan RC, Ganguly NK. Flow cytometric assessment of the effect of drugs onGiardialambliatrophozoitesinvitro[J]. Mol Cell Biochem, 2004, 265: 151-160.
[5]Tian XF, Lu SQ. Cytoskeleton ofGiardialamblia[J]. World Chin J Digestol, 2005, 13(12): 1434-1436. (in Chinese) 田喜凤, 卢思奇.蓝氏贾第鞭毛虫的细胞骨架[J].世界华人消化杂志, 2005, 13(12): 1434-1436.
[6]Holberton DV, Ward AP. Isolation of the cytoskeleton fromGiardia. Tubulin and a low-molecular-weight protein associated with microribbon structures[J]. J Cell Sci, 1981, 47: 139-166.
[7]Crossley R, Holberton DV. Characterization of proteins from the cytoskeleton ofGiardialamblia[J]. J Cell Sci, 1983, 59: 81-103.
[8]Kim J, Goo SY, Chung HJ, et al. Interaction of beta giardin with the Bop1 protein inGiardialamblia[J]. Parasitol Res, 2006, 98(2): 138-144. DOI: 10.1007/s00436-005-0040-8
[9]Jenkins MC, O'Brien CN, Murphy C, et al. Antibodies to the ventral disc protein delta-giardin preventinvitrobinding ofGiardialambliatrophozoites[J]. J Parasitol, 2009, 95(4): 895-899. DOI: 10.1645/GE-1851R.1
[10]Nohria A, Alonso RA, Peattie DA. Identification and characterization of gamma-giardin and the gamma-giardin gene fromGiardialamblia[J]. Mol Biochem Parasitol, 1992, 56: 27-37. DOI: 10.1016/0166-6851(92)90151-9
[11]Tian XF, Lu SQ, Liu YM, et al. Effect of dihydroartemisinin on ultra structure ofGiardiaLambliainvitro[J]. Chin J Parasitol Parasit Dis, 2005, 23(5): 292-295. (in Chinese) 田喜凤,卢思奇,刘业民, 等.双氢青蒿素对体外蓝氏贾第鞭毛虫的损伤[J].中国寄生虫学与寄生虫病杂志, 2005, 23(5): 292-295.
[12]He B, Liu GW, Cao L, et al.Invitroidentification of the cytoskeleton proteins ofGiardialambliausing a MS technique[J]. J Pathog Biol, 2010, 5(12): 898-900. (in Chinese) 何冰, 刘广伟, 曹蕾,等.质谱技术鉴定体外蓝氏贾第鞭毛虫的细胞骨架蛋白[J].中国病原生物学杂志, 2010, 5(12): 898-900.
[13]Si JL, Qi YQ, Zhou CH, et al. Detection of peripheral blood human telomerase reverse transcriptase mRNA in colorectal cancer with real-time fluorescent quantitative RT-PCR and its clinical significance[J]. World Chin J Digestol, 2008, 16(36): 4067-4070. (in Chinese) 司君利,亓玉琴,周长宏,等.实时荧光定量RT-PCR检测结直肠癌中外周血端粒酶逆转录酶mRNA的表达及其临床意义[J]. 世界华人消化杂志, 2008, 16(36):4067-4070.
[14]Mu D, Zhang W, Chu D, et al. The role of calcium, P38M APK in dihydroartemisinin-induced apoptosis of lung cancer PC-14 cells[J]. Cancer Chemother Pharmacol, 2008, 61(4): 639-645. DOI: 10.1007/s00280-007-0517-5
[15]Li HJ, Wang W, Liang YS. Advances in research of dihydroartemisinin against parasitic diseases[J]. Chin J Schisto Ctrl, 2011, 23(4): 460-463. (in Chinese) 李洪军, 汪伟, 梁幼生.双氢青蒿素抗寄生虫作用研究进展[J].中国血吸虫病防治杂志, 2011, 23(4): 460-463.
[16]Xiang LX. Research progress of dihydroartemisinin[J]. Guangxi J Light Industry, 2010(3): 7-8. (in Chinese) 相丽欣.双氢青蒿素研究进展[J].广西轻工业, 2010(3): 7-8.
[17]Ru WW, Liang YS. Progress of research on artemisine against parasitic diseases[J]. Chin J Schisto Ctrl, 2006, 18(1): 78-80. (in Chinese) 茹炜炜, 梁幼生.青蒿素类药物抗寄生虫作用研究进展[J].中国血吸虫病防治杂志, 2006, 18(1): 78-80.
[18]Li W, Shi CR. Research progress of Artemisinin[J]. China Pharm, 2003, 14(2): 118-119. (in Chinese) 李伟, 石崇荣.双氢青蒿素研究进展[J].中国药房, 2003, 14(2): 118-119.
[19]Ouyang SY, Yang D, Ouyang HS, et al. Real-time fluorescent quantitative PCR and application[J]. Chem Life, 2004, 24(1): 74-76. (in Chinese) 欧阳松应, 杨冬, 欧阳红生, 等.实时荧光定量PCR技术及其应用[J].生命的化学, 2004, 24(1): 74-76.
[20]Zhao HY, Bao JF. The principle and application research progress of real-time fluorescent quantitative PCR[J]. Chin J Histochem Cytochem, 2007, 16(4): 492-497. (in Chinese) 赵焕英, 包金风.实时荧光定量PCR技术的原理及其应用研究进展[J].中国组织化学与细胞化学杂志, 2007, 16(4): 492-497.
[21]Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays[J]. J Mol Endocrinol, 2000, 25 (2): 169-193.
[22]Giulietti A, Overbergh L, Valckx D, et al. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression[J]. Methods, 2001, 25(4): 386-401. DOI: 10.1006/meth.2001.1261
[23]Yu Y, Chen Y, Yang ZH, et al. Effects of dihydroartemisinin on protein ofGiardialambliatrophozoites[J]. Chin J Zoonoses, 2010, 26(11): 995-997. (in Chinese) 余源, 陈阳, 杨志宏, 等.双氢青蒿素对蓝氏贾第鞭毛虫滋养体蛋白质的损伤[J].中国人兽共患病学报, 2010, 26 (11): 995-997.
Yu Yuan,Email:yuyuan5188@163.com
Dihydroartemisinin inhibition on Delta giardin in C2Giardialamblia
LIU A-qian,WANG Yang,LIN Zhi-qiang,ZHANG Ya-fen,TIAN Xi-feng,YU Yuan
(NorthChinaUniversityofScienceandTechnology,Tangshan063000,China)
We established real-time fluorescent quantitative reverse transcriptase polymerase chain reaction (RT-PCR) for determining the expression of Delta giardin mRNA to explore effects of dihydroartemisinin (DHA) on the expression level of Delta giardin mRNA in C2Giardialamblia.Giardialambliarespectively cultivated for 2, 4, 8, and 12 h respectively with modified TYI-S-33 medium which contained 100 μg/mL and 200 μg/mL DHA, while the negative control group performed in the same experimental condition without DHAs. All of the RNAs were extracted and cDNA were synthesized. The relative expressive quantity of Delta giardin mRNA was determined by real-time PCR and the results were 0.44, 0.26, 0.25, and 0.02 whenGiardialambliarespectively cultivated for 2 h, 4 h, 8 h, and 12 hours which contained 100 μg/mL DHA. The relative expression quantities of Delta giardin mRNA were 0.30, 0.26, 0.11, and 0.02 whenGiardialambliarespectively cultivated for 2 h, 4 h, 8 h, and 12 h which contained 200 μg/mL DHA. The expressive quantities of Delta giardin mRNA with DHA were significantly lower than that in the control group. It suggests that dihydroartemisinin has obvious inhibitory effects on the expression level of Delta giardin mRNA in C2Giardialamblia, and DHA has significant prevention and cure function to C2Giardialamblia.
C2Giardialamblia; dihydroartemisinin; real-time reverse transcription PCR; Delta giardin
国家自然科学基金(No. 31471954)、河北省青年科学基金(No. C2012401039)、河北联合大学培养基金(No.GP201308)、唐山市科技支持计划项目(No.12140209A-33)联合资助
余源,Email:yuyuan5188@163.com
华北理工大学生命科学学院,唐山 063000
Supported by the National Natural Science Foundation of China (No. 31471954), the Youth Science Fund Project in Hebei Province (No. C2012401039), the Hebei United University Training Fund (No. GP201308), and the Scientific and Technological Support Projects from Tangshan (No. 12140209A-33)
10.3969/cjz.j.issn.1002-2694.2015.06.006
R382.2
A
1002-2694(2015)06-0522-05
2014-07-21;
2014-10-30
