Biogenesis of Plant MicroRNAs
2014-03-07KongWenwenWangHongboandLiJing
Kong Wen-wen, Wang Hong-bo, and Li Jing
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
Biogenesis of Plant MicroRNAs
Kong Wen-wen, Wang Hong-bo, and Li Jing*
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
microRNAs (miRNAs) play important regulatory roles in eukaryotic gene expression, predominantly at the posttranscriptional level. Elaborate and diverse biogenesis pathways have evolved to produce miRNAs. miRNA biogenesis is a multistep process including transcription, precursor slicing, methylation, nuclear export, and RNA-induced silencing complex assembly. In the decade, since the first discovery of plant miRNAs, many enzymes and regulatory proteins involved in miRNA biogenesis in plants have been uncovered and a basic picture of miRNA processing is emerging gradually. In this article, we summarized the current study of plant miRNA biogenesis and discussed the multiple integrated steps and diverse pathways of miRNA processing.
miRNA, biogenesis, Arabidopsis thaliana, pathway
Introduction
MicroRNAs (miRNAs) are 18-25 nt long, singlestranded, regulatory non-coding RNAs derived from hairpin-like precursors. These small molecules regulate gene expression through mRNA cleavage, translation repression, and DNA methylation (Llave et al., 2002; Mallory et al., 2008; Brodersen et al., 2008; Wu et al., 2010; Sun, 2012). In plants, miRNAs play important roles in diverse regulatory pathways and are involved in almost all the developmental events such as leaf shape, floral transition (Liu et al., 2006; Park et al., 2002) as well as mediating responses to both abiotic (drought, temperature, salinity) (Wang et al., 2012; Feng et al., 2013; Ni et al., 2013; Sunkar et al., 2007) and biotic (bacteria, viruses) (Brotman et al., 2012; Fagard et al., 2007) environmental conditions. To further dissect the miRNA regulation network, it is important to understand how miRNA genes (MIRs) themselves are generated and regulated.
The first miRNA was discovered in C. elegans in 1993 by Lee et al. (1993) and the first plant miRNA was reported in Arabidopsis in 2002 (Reinhart et al. 2002). The miRNAs biogenesis pathways in animals and plants are now basically established after more than twenty and ten years investigation, respectively. Despite both animal and plant miRNAs perform important roles during their life cycle, there are many different details in animal and plant miRNA biogenesis procedures. In animals, MIRs are transcribed by polymerase II (POL II) similar to plant MIRs transcription, while some MIR genes are transcribed by polymerase III (POL III) (Bartel, 2004; Chen et al. 2004). Then, the transcripts are sliced twice, and the first slicing in animals is the nuclear cleavage of the pri-miRNA, which liberates a stem loop intermediate, known as the pre-miRNA by the Drosha RNase III endonuclease (Lee et al., 2003). By Ran-GTP and Exportin-5, this pre-miRNA is transported to the cytoplasm and slicedby another RNase III endonuclease Dicer for the second time and formed the miRNA/miRNA* duplex. (Lund et al., 2004; Lee et al., 2003; Bernstein et al., 2001). In plants, MIRs are only transcribed by RNA polymerase II (Pol II) to form stem-loop structured primary microRNA (pri-miRNA) (Xie et al., 2005). The Dicer-like1(DCL1) complex then slices the primiRNA twice similar to in animals, while both of them occurred in nucleus. The base of the stem is cut off in the first slicing and precursor miRNA (pre-miRNA) is produced, while the loop is removed in the second slicing and a duplex of miRNA/miRNA* is formed (Reinhart et al., 2002). After the miRNA/miRNA* duplex formation, the miRNA strand (guide strand) is selected and incorporated into the ARGONAUTE1 (AGO1) component of the RNA-induced silencing complex (RISC), which then mediates the repression of gene expressions (Song et al., 2004). Recently, increasing numbers of new proteins involved in plant miRNA biogenesis are being discovered. Here, we focused on current advancements in plant miRNA studies, and the integrated processes and diverse pathways of plant miRNA biogenesis were discussed in details.
Transcription of MIR Genes
The majority of MIR genes are located in intergenic loci between protein-coding genes, while others are found in intragenic loci, in either introns or exons (Rajagopalan et al., 2006; Cui et al., 2009). MIR genes are transcribed by Pol II in plants (Xie et al., 2005) (Fig. 1). As the important gene expression regulator, MIR genes transcribed are under precision regulated. Recent study found that introns of plant pri-miRNAs can enhance miRNA biogenesis (Bielewicz et al. 2013). Otherwise, the transcriptional co-activator mediator, a conserved protein known to promote transcription of protein-coding genes, is required to recruit Pol II to transcript factor-binding sites in MIR promoters to regulate MIRs transcript (Kim et al., 2011) (Fig. 1). A pair of NOT2 proteins, NOT2a and NOT2b (previously known as At-Negative on TATA-less2 [NOT2] and VIRE2-INTERACTING PROTEIN2, respectively), was recently found to promote this transcription through their interaction with Pol II (Fig. 1). NOT2s also interact with DCL1 and some other slicing factors and thus are functional in subsequent processing steps (Wang et al., 2013). The transcripts are modified by adding a 5' cap and a 3' polyadenylate tail like other coding genes (Xie et al., 2005; Lee et al., 2004; Jones-Rhoades et al., 2006).
Some MIR genes contain introns, which have to be removed before the formation of the stem-loop structure (Aukerman and Sakai, 2003; Nikovics et al., 2006). STABILIZED1 (STA1), a protein functioning in pre-mRNA processing in Arabidopsis, was identified to be responsible for the intron splicing of miRNA transcripts (Ben et al., 2013) (Fig. 1). A dramatic reduction in the number of mature miRNAs and an accumulation of unspliced pri-miRNAs was detected in sta1. In addition, the transcript level of DCL1 was reduced in sta1, indicating that STA1 is responsible for splicing the pre-mRNAs of DCL1 (Ben et al., 2013). These results suggested that STA1 was involved in miRNA biogenesis directly by functioning in pri-miRNA splicing and indirectly by modulating the DCL1 transcript level (Ben et al., 2013).
Based on its own compliment sequence, the transcribed RNA will fold back into a stem-loop structured pri-miRNA. The length of pri-miRNAs can be variable and ranges from hundreds to several kilobases (Szarzynska et al., 2009). Generally, one primiRNA produces one mature miRNA, but it has been found that pri-miRNAs containing multiple stemloop structures can produce more than one miRNA (Merchan et al., 2009; Zhang et al., 2010; Lacombe et al., 2008).
Slicing of pri- and pre-miRNA
The pri-miRNA undergoes two slicing steps to cut off the loop and the stem separately. The slicing is a complicated process and a series of proteins are involved.
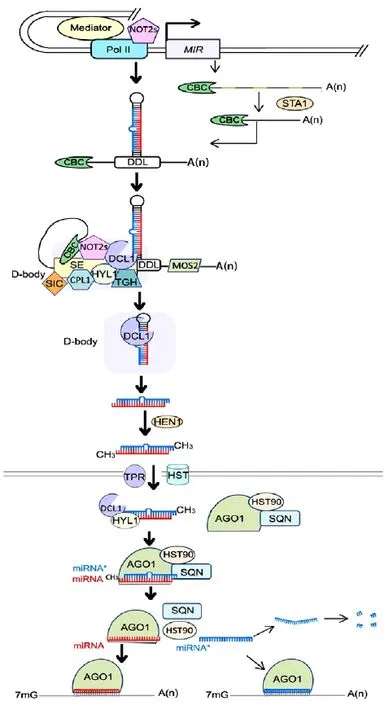
Fig. 1 Biogenesis of plant miRNAs
Proteins Involved in Slicing
DCL and DRB family
The DCL and double-stranded RNA-binding (DRB) families are very important in plant miRNA production. Several members in each family are involved in miRNA biogenesis and the relationships of the members within and between families result in the diversity and complexity of miRNA biogenesis.
In animals, the slicing of miRNA is catalyzed by Drosha (Bartel, 2004; Lee et al., 2003), while in plants, it is performed by a homolog of Drosha, DCL1, which is a key protein in plant miRNA biogenesis (Park et al., 2002). Loss of DCL1 function results in severely defective phenotypes. In Arabidopsis, dcl1 is embryo lethal (Golden et al., 2002). DCL1 belongs to a multidomain ribonuclease III (RNase III)-like family that contains four members, DCL1, DCL2, DCL3, and DCL4 in Arabidopsis (Voinnet, 2009). DCL proteins contain a DExD/H-box RNA helicase domain, a DUF283 RNA-binding domain, a small RNA-binding PAZ domain, two tandem RNase III domains, and two tandem dsRNA-binding domains (dsRBD) (Margis et al., 2006; Liu et al., 2006). The length of small RNAs sliced by DCLs is based on the distance between the PAZ and RNase III domains (Macrae et al., 2006), consequently DCL enzymes themselves are molecular rulers controlling the length of the small RNAs they produce. DCL1 can produce 18-21 nt long RNAs while DCL2, DCL3, and DCL4 mainly produce 22, 24, and 21 nt products, respectively (Voinnet, 2009; Takanashi et al., 2011).
DCL1 is believed to be the slicing enzyme for most plant miRNAs (Park et al., 2002; Reinhart et al., 2002) (Figs. 1 and 2a), while the other DCLs were originally characterized as proteins that function in other small RNA biogenesis pathways (Xie et al., 2004; Gasciolli et al., 2005). However, DCL4, previously identified as an enzyme for biogenesis of trans-acting small-interfering RNAs (ta-siRNAs), was found to be involved in miRNA processing (Fig. 2d). A few miRNAs like miR822, miR839, and miR869 are sliced primarily by DCL4, possibly due to the highly complementary fold-backs in these pri-miRNAs (Rajagopalan et al., 2006; Ben et al., 2009).
In Arabidopsis, besides the canonical 21-nt miRNAs, a minor fraction of 23-25 nt miRNAs are generated independently from the same miRNA precursors by DCL3 for a large number of MIR genes (Vazquez et al., 2008). In rice, dozens of MIR genes producing only 24 nt long miRNAs or both canonical and long miRNAs have been found (Wu et al., 2010; Wu et al., 2009; Zhu et al., 2008). These DCL3-dependent long miRNAs were demonstrated to be loaded into the AGO4-containing RISC complex and direct DNA methylation at their loci of origin as well as in trans at their target genes (Wu et al., 2010) (Fig. 2c). The generation of long miRNAs is usually dependent on the spatial or temporal expression of DCL3, implying possible competition among DCL proteins for miRNA precursor slicing under certain conditions (Vazquez et al., 2008).
DRB proteins have widespread functions in RNA metabolism (Eamens et al., 2012). In Arabidopsis, the DRB family contains five closely related members, DRB1-DRB5, all of which are involved in small RNA biogenesis. DRB1, also called HYL1 (HYPONASTIC LEAVES1), facilitates pri-miRNA processing and was verified to associate with DCL1 both in vitro (Lu and Fedoroff, 2000) and in vivo (Kurihara et al., 2006) (Fig. 1, 2a). In hyl1, both spliced and intron-containing pri-miRNAs are accumulated (Vazquez et al., 2004; Han et al., 2004) and DCL1 cleavage sites are misplaced, resulting in a lower level of functional mature miRNA (Vazquez et al., 2004). Therefore, HYL1 is required for efficient and precise cleavage of primiRNA. However, there are still some functional mature miRNAs in the hyl1 null mutant, suggesting that additional factors or alternative DRB proteins are involved in accurate DCL slicing (Kurihara et al., 2006; Han et al., 2004; Yang et al., 2010).
DRB2 was reported to be involved in the maturation of subsets of miRNAs. Therefore, another DRB2-medi-ated non-canonical miRNA pathway was proposed (Eamens et al., 2012) (Fig. 2b). In this pathway, the pri-miRNA transcript is recognized alternatively by a DCL1/DRB2 pairing complex and is sliced to form a miRNA/miRNA* duplex. The selected miRNA strand is associated either with an AGO1-catalyzed RISC or with an alternate RISC complex containing an unknown AGO protein, DRB3 and DRB5 instead (Fig. 2b). In the subsequent step, AGO1-catalyzed RISC mediates mRNA cleavage and the DRB3 and DRB5-containing RISC causes translational repression of the target mRNA (Eamens et al., 2012).

Fig. 2 Biogenesis pathways of plant miRNAs
DRB4 was previously identified to play an important role in small interfering RNA biogenesis by associating with DCL4 (Hiraguri et al., 2005; Nakazawa et al., 2007) (Fig. 2d). DCL4-dependentmiRNA accumulation is strongly reduced in drb4, indicating that DRB4 is required in DCL4-dependent miRNA biogenesis (Fukudome et al., 2011; Pélissier et al., 2011). Furthermore, northern blot hybridization showed that accumulation of miR839 was reduced in drb2 and drb4, indicating that DRB2 is also required for DCL4-mediated miRNA accumulation (Vazquez et al., 2010) (Fig. 2d).
Other slicing factors
Serrate (SE) is a C2H2-zinc finger protein localized in the nucleus (Laubinger et al., 2008). The phenotypes of se show a lot of similarities to other miRNA-processing enzyme mutants (Lu and Fedoroff, 2000; Prigge and Wagner, 2001; Bezerra et al., 2004; Clarke et al., 1999; Bollman et al., 2003). It was revealed that most pri-miRNAs are accumulated while mature miRNAs are reduced in se, and the decreased levels of mature miRNAs are correlated with increased levels of their target gene transcripts (Lobbes et al., 2006). SE enhances the accuracy of pri-miRNA cleavage by DCL1 both in vitro (Dong et al., 2008) and in vivo (Manavella et al., 2012). Thus, SE is required for proper slicing of pri-miRNA (Fig. 1). SE was found to be able to react with most of the pri-miRNA slicing proteins like DCL1 and HYL1 (Lobbes et al., 2006; Machida et al., 2011), and was therefore suggested to work as a scaffold-like protein capable of binding both proteins and RNA to guide the positioning of miRNA precursors toward the DCL1 catalytic site (Machida et al., 2011).
C-TERMINAL DOMAIN PHOSPHATASELIKE1 (CPL1), containing TFIIF-interacting CTD phosphatase1-like phosphatase and dsRBD domains, is a recently identified protein that affects the accuracy of miRNA maturation (Fig. 1). Loss of CPL1 function results in the accumulation of miRNA target mRNAs and inaccurate miRNAs without changing the total miRNA level. It was discovered that CPL1 is recruited by SE to the DCL1 complex and dephosphorylates HYL1. Dephosphorylation of HYL1 was found to be important for accurate miRNA processing and strand selection of the miRNA duplex (Manavella et al., 2012).
DAWDLE (DDL), a nuclear RNA-binding protein, was shown to stabilize pri-miRNA in miRNA biosynthesis (Yu et al., 2008) (Fig. 1). In Arabidopsis, mature miRNA accumulation is greatly reduced in the ddl mutant. DDL is capable of binding pri-miRNA and interacting with DCL1 in vitro. Therefore, it was suggested that DDL could improve the stability of primiRNA either by binding to the end of the stem-loop or by the indirect effect of DCL1 recruitment (Yu et al., 2008; Rogers and Chen, 2013). DDL binding is general to RNAs and not specific to pri-miRNA; thus, it is involved in other RNA metabolism, which might explain why the ddl mutant showed more serious developmental deficiencies than those of the dcl1 mutants (Yu et al., 2008).
CAP BINDING Complex (CBC) is a heterodimer that contains two subunits, CBP80 and CBP20 (Laubinger et al., 2008; Gregory et al., 2008), and binds to the 5' cap structure of Pol II transcripts. PrimiRNA is accumulated while mature miRNA is reduced in cbp80 and cbp20, indicating that CBC plays a role in the pri-miRNA slicing process (Gregory et al., 2008) (Fig. 1). CBP20 binds to SE in vivo and in vitro, suggesting that CBC might be required for the formation of a pri-miRNA processing center by interacting with SE (Wang et al., 2013). Like DDL, CBC does not function only in miRNA biogenesis, as mRNA intron slicing is also suppressed in cbp80 and cbp20, suggesting that CBC is required in intron slicing (Laubinger et al., 2008; Gregory et al., 2008; Kim et al., 2008).
NOT2s are required in pri-miRNA slicing in addition to promoting MIR gene transcription by interacting with Pol II. NOT2s associate with some key miRNA processing factors, including DCL1, CBC, and SE (Fig. 1). Impairment of NOT2s leads to mislocalization of DCL1, suggesting that NOT2 proteins facilitate efficient recruitment of DCL1 and other processing factors in miRNA biogenesis. These results explain why inactivation of NOT2s decreases theaccumulation of both pri-miRNA and mature miRNA (Wang et al., 2013).
TOUGH (TGH) contains G-patch and SWAP domains (Suppressor-of-White-APricot), which often exist within RNA metabolism-related proteins (Calderon-Villalobos et al., 2005), and was found to be a component of the DCL1 slicing complex (Fig. 1). In tgh, the accumulation of both DCL1- and DCL4-mediated miRNAs is reduced and the transcript levels of their pri-miRNAs are increased (Ren et al., 2012). TGH binds ssRNA but not dsRNA in vitro, so it possibly functions by binding to the loop or the bulge of pri- or pre-miRNAs. Unlike HYL1 and SE, TGH does not affect miRNA precision, as evidenced by the low ratio of imprecise miRNAs in tgh.
Sickle (SIC), a hydroxyproline-rich glycoprotein, was recently discovered to be involved in miRNA biogenesis (Fig. 1). Immunolocalization revealed that SIC and HYL1 were colocalized in nuclear bodies. sic exhibited some common phenotypes of mutants defective in miRNA biogenesis and accumulated lower levels of a subset of miRNAs and higher levels of corresponding pri-miRNAs than the wild type (Zhan et al., 2012). It remains unknown how SIC functions in pri-miRNA processing. SIC has a function in sliced intron decay (Zhan et al., 2012), suggesting a possible common mechanism for the cleavage of pri-miRNA hairpins and the decay of sliced introns.
MOS2 encodes a G-patch and KOW domaincontaining RNA-binding protein and was previously identified to be essential for innate immunity in Arabidopsis (Zhang et al., 2005). MOS2 is required for efficient processing of pri-miRNA through facilitating the recruitment of pri-miRNA by the dicing complex, as evidenced by the greatly reduced association between HYL1 and pri-miRNA in mos2. Interestingly, MOS2 binds pri-miRNA both in vitro and in vivo, but does not interact with HYL1, SE, or DCL1 and is not localized in the D-body which is nuclear processing center discussed in the following (Fig. 1). Furthermore, MOS2 is also involved in the biogenesis of ta-siRNA, which is dependent on DRB4 and DCL4, indicating its role in regulating the DRB4 and DCL4-containing dicing complex (Wu et al., 2013).
Direction of slicing
In animals, the rules for miRNA excision from the precursor are simple because of the uniform size and shape of the pri-miRNA. In contrast, the stemloops of pri-miRNAs in plants are quite variable in both length and secondary structure. Accordingly, the mechanism for miRNA precursor recognition by the DCL1 complex is more complicated and less well understood. Most plant pri-miRNAs are sliced in the stem-to-loop direction like in animals (Breakfield et al., 2012). Analysis of pre-miR172 processing showed that the initial cut by the DCL1 complex is located –15 nt from the base of the stem, while the loop is removed in the second slicing (Mateos et al., 2010; Song et al., 2010; Werner et al., 2010).
Unlike most plant miRNAs, miR159 and miR319 are sliced in the loop-to-base direction (Bologna et al., 2013). Both miR159 and miR319 have long transcripts and can fold-back into a long double-stranded backbone. Evidence from detailed mutagenesis experiments showed that pre-miR159 and pre-miR319 processing begins with a cleavage near the loop (Bologna et al., 2009; Naqvi et al., 2012). DCL1 then continues to cut the precursor three more times at 20-22 nt intervals until the miRNA is finally released (Bologna et al., 2009; Addo-Quaye et al., 2009). Interestingly, the long precursors of miR319 and miR159 are highly conserved in plants, indicating that this processing mechanism is ancient (Bologna et al., 2009).
Methylation of miRNA/miRNA* duplex
HUA ENHANCER1 (HEN1) is a protein containing a dsRNA-binding motif and a C-terminal methyltransferase domain and therefore is able to bind dsRNA and deposit a methyl group on to the 2'-OH of the 3'-terminal nucleotide (Yang et al., 2006). HEN1 plays an important role in miRNA maturation because of its ability to stabilize the miRNA/miRNA* duplex(Chen, 2005; Li et al., 2005; Yu et al., 2005) (Fig. 1). After being sliced from the pre-miRNA, the miRNA/ miRNA* duplex is methylated by HEN1 and thus is protected from degradation. The hen1 mutant shows a similar phenotype to dcl1 and the 3'-ends of miRNAs are found to have additional nucleotides, primarily uridines instead of methylated ends. It was discovered that the uridylation of unmethylated miRNAs in hen1 is performed by a DNA polymerase β family protein, HEN1 SUPPRESSOR1 (HESO1), and that the uridylation leads to degradation (Ren et al., 2012; Zhao et al., 2012). Furthermore, the methylation of miRNA duplexes may also act as an export signal to the cytoplasm (Park et al., 2005).
Transportation and selection of mature miRNA
In mammals, Exportin 5 (Exp5) exports pre-miRNAs to the cytoplasm before the mature miRNA is generated (Lund et al., 2004). In plants, the corresponding transportation process is still not clear and both the transporter and the transported molecules remain unknown. HASTY (HST), a nuclear shuttle protein that is homologous to Exp5, was suggested to function by exporting methylated miRNA/miRNA* duplexes or single-stranded miRNA to the cytoplasm (Bollman et al., 2003; Park et al., 2005) (Fig. 1). Reduced accumulation of many miRNAs in hst mutants indicates a role for HST in miRNA biogenesis. However, the similarly reduced levels of these miRNAs in both the nucleus and cytoplasm do not seem to support the hypothesis that HST functions in miRNA export (Park et al., 2005). Several species of miRNA are exported to the cytoplasm by an HST-independent mechanism and it was suggested that mRNA transport proteins are possibly involved in this process (Park et al., 2005). In addition, the nuclear pore complex might play a role in miRNA transportation as evidenced by the reduced accumulation of some miRNAs in mutants of the nuclear pore component TRANSLOCATED PROMOTER REGION (TPR) (Jacob et al., 2007) (Fig. 1). Before mature miRNA is generated, one strand of the methylated miRNA/miRNA* duplex has to be selected as miRNA (guide strand) while the other strand (passenger strand/miRNA*) undergoes degradation. The determinant for this selection was discovered by bioinformatic analysis and confirmed by artificial miRNA experiments (Rajagopalan et al., 2006). The majority of Arabidopsis miRNAs are preferentially selected over their miRNA* strands because of the asymmetric thermodynamic stability of the miRNA/ miRNA* duplex terminus, and the strand with a weaker 5'-terminus is preferentially chosen as the miRNA guide strand (Rajagopalan et al., 2006; Eamens et al., 2009).
In addition, DRB1 (HY1) is required for strand selection as evidenced by the higher level of miRNA* in hyl1 mutants (Eamens et al., 2009). Consistently, cpl1 mutants accumulate higher levels of miRNA*, indicating that the dephosphorylation of HYL1 by CPL1 is also required for proper guide strand selection (Manavella et al., 2012). DRB1 may bind to or interact with the more thermodynamically stable end of the miRNA duplex, and then DRB1 alone, or in a heterodimer with DCL1, directionally loads the miRNA duplex to the critical miRNA machinery protein AGO1 (Eamens et al., 2009; Matranga et al., 2005; Tomari et al., 2004; Baumberger et al., 2005) where the guide strand is selected (Fig. 1).
RlSC assembly
The assembly of RISC involves the loading of the miRNA/miRNA* duplex into AGO complex and subsequent removal of the miRNA* strand. HEAT SHOCK PROTEIN 90 (HSP90) was found to be involved in the assembly process as a molecular chaperone that binds to AGO1 and facilitates the loading of the dsRNA duplex (Iki et al., 2010) (Fig. 1). HSP90 mediates substrate peptide association in response to ATP binding and releases the substrate on ATP hydrolysis by changing its conformation (Iki et al., 2010).
SQUINT (SQN), a member of the immunophilin family, facilitates RISC assembly and is able to form a complex with HSP90, AGO1, and small RNAduplexes (Fig. 1). Expression of SQN can rescue the phenotype of sqn null alleles, while expression of a mutated SQN showing reduced interaction with HSP90 was unable to rescue the phenotype, indicating that interaction with HSP90 is required for proper SQN function (Iki et al., 2010; Earley et al., 2011; Smith et al., 2009). ATP hydrolysis induces dissociation of HSP90 and SQN from the complex, followed by unwinding and release of the miRNA*. Finally, a mature RISC that contains AGO1 and the guide strand miRNA is formed (Fig. 1). Once the mature RISC is formed, the miRNA can guide the RISC to repress the expression of target genes (Voinnet, 2009).
Subcellular organization of miRNA biogenesis
The subcellular organization of miRNA biogenesis is not very clear so far. Fang and Spector (2007) showed that DCL1 and HYL1 colocalize in discrete nuclear bodies, which are refered as nuclear dicing bodies (D-bodies). Since several proteins essential for miRNA processing and pri-miRNAs are also associated with D-bodies, they suggested that D-bodies are the nuclear sites for the dicing reaction of DCL1 and proteinprotein interaction of the respective proteins (Fig. 1). HEN1 and AGO1 localize both in the nucleus and the cytoplasm, and in nucleus a large nucleoplasmic signal is observed in additional to a low level localization to D-bodies (Fang and Spector, 2007). Therefore, the methylation of miRNA/miRNA* duplexes and the loading of miRNA can be either in cytoplasm or in nucleus, with the possibility in nucleoplasm. The molecules that are transported through the nuclear membrane and the proteins responsible for the transporting are still kept unknown. It is of great interest to further discover the detailed cellular basis of miRNA biogenesis.
miRNA*
MiRNA* strand is originally considered to be nonfunctional and simply removed and degraded (Rajagopalan et al., 2006; Eamens et al., 2009). Interestingly, recent studies indicate that miRNA* can be active in gene silencing in Drosophila melanogaster, humans, and plants (Ghildiyal et al., 2010; Manavella et al., 2013; Meng et al., 2011) (Fig. 1). Large-scale sequencing research demonstrates that some miRNA*s are quite abundant and enriched in AGO1 complexes and miRNA* guided mRNA cleavage products are detected (Devers et al., 2011). In Arabidopsis miR171a* was verified to be abundant and trigger the silencing of SU (VAR) 3-9 HOMOLOG8 by association with the AGO1 complex possibly under tissuespecific control (Manavella et al., 2013). Meng et al. (2011) predicted all of the potential miRNA* target pairs in rice and Arabidopsis based on degradome sequencing data. Their results suggested widespread miRNA*-mediated gene regulation in plants, but little is known about the exact mechanism.
Conclusions
In the decade since the first report of plant miRNAs, considerable advances have been made in our understanding of the biogenesis of miRNAs in plants and a general picture of plant miRNA biogenesis has emerged. However, there are still some hazy parts. The nuclear export of miRNA in plants is not as clear as in their metazoan counterparts, with both the transporter and transported molecules remaining unknown; the mechanisms of some processing factors are also not yet clear; the generation of functional miRNA* is waiting to be discovered.
Some key protein family (e.g., DCL and DRB) mem-bers that were originally identified as being involved in other small RNA pathways have been found to take part in miRNA processing and can cooperate or compete with their miRNA-producing relatives. The intertwined relationships among these processing proteins have revealed the diversity and complexity of miRNA biogenesis in plants.
Considering its complexity and the large number of participating proteins, understanding the regulation of miRNA biogenesis, especially for spatially andtemporally specific miRNAs, can be quite challenging. However, deep sequencing (Friedländer et al., 2008) and tiling array (Liu et al., 2008) technologies have allowed the examination of precursor, mature, and intermediate miRNA molecules at an unprecedented global level and provide unlimited potential for further study of plant miRNA.
Addo-Quaye C, Snyder J A, Park Y B, et al. 2009. Sliced microRNA targets and precise loop-first processing of MIR319 hairpins revealed by analysis of the Physcomitrella patens degradome. RNA, 15(12): 2112-21.
Aukerman M J, Sakai H. 2003. Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell, 15(11): 2730-41.
Bartel D P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116(2): 281-97.
Baumberger N, Baulcombe D C. 2005. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci USA, 102(33): 11928-33.
Ben Amor B, Wirth S, Merchan F, et al. 2009. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res, 19(1): 57-69.
Ben Chaabane S, Liu R, Chinnusamy V, et al. 2013. STA1, an Arabidopsis pre-mRNA processing factor 6 homolog, is a new player involved in miRNA biogenesis. Nucleic Acids Res, 41(3): 1984-97.
2011年江西省评选的第六届特级教师共233名,本研究随机抽取其中150人为被试,回收有效问卷116份。随机抽取江西省上饶市中小学普通教师246人为比较被试,回收有效问卷197份。特级教师中,男62人,女54人;小学教师46人,初中教师25人,高中教师45人。普通教师中,男106人,女91人;小学教师48人,初中教师34人,高中教师115人。
Bernstein E, Caudy A A, Hammond S M, et al. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature, 409(6818): 295-296.
Bezerra I C, Michaels S D, Schomburg F M, et al. 2004. Lesions in the mRNA cap-binding gene ABA HYPERSENSITIVE 1 suppress FRIGIDA-mediated delayed flowering in Arabidopsis. Plant J, 40(1): 112-119.
Bielewicz D, Kalak M, Kalyna M, et al. 2013. Introns of plant primiRNAs enhance miRNA biogenesis. EMBO Rep, 14(7): 622-8.
Bollman K M, Aukerman M J, Park M Y, et al. 2003. HASTY, the Arabidopsis ortholog of exportin 5/MSN5, regulates phase change and morphogenesis. Development, 130(8): 1493-504.
Bologna N G, Mateos J L, Bresso E G, et al. 2009. A loop-to-base processing mechanism underlies the biogenesis of plant microRNAs miR319 and miR159. EMBO J, 28(23): 3646-56.
Bologna N G, Schapire AL, Palatnik J F. 2013. Processing of plant microRNA precursors. Brief Funct Genomics, 12(1): 37-45.
Breakfield N W, Corcoran D L, Petricka J J, et al. 2012. High-resolution experimental and computational profiling of tissue-specific known and novel miRNAs in Arabidopsis. Genome Res, 22(1): 163-76.
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, et al. 2008. Widespread translational inhibition by plant miRNAs and siRNAs. Science, 320(5880): 1185-90.
Brotman Y, Lisec J, Méret M, et al. 2012. Transcript and metabolite analysis of the Trichoderma induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology, 158(pt1): 139-46.
Chen CZ, Li L, Lodish H F, et al. 2004. MicroRNAs modulate hematopoietic lineage differentiation. Science, 303(5654): 83-6.
Chen X. 2005. MicroRNA biogenesis and function in plants. FEBS Lett, 579(26): 5923-31.
Clarke JH, Tack D, Findlay K, et al. 1999. The SERRATE locus controls the formation of the early juvenile leaves and phase length in Arabidopsis. Plant J, 20(4): 493-501.
Cui X, Xu S M, Mu D S, et al. 2009. Genomic analysis of rice microRNA promoters and clusters. Gene, 431(1-2): 61-6.
Devers EA, Branscheid A, May P, et al. 2011. Stars and symbiosis: microRNA- and microRNA*-mediated transcript cleavage involved in arbuscular mycorrhizal symbiosis. Plant Physiol, 156(4): 1990-2010.
Dong Z, Han M H, Fedoroff N. 2008. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc Natl Acad Sci USA, 105(29): 9970-5.
Eamens A L, Smith N A, Curtin S J, et al. 2009. The Arabidopsis thaliana double-stranded RNA binding protein DRB1 directs guide strand selection from microRNA duplexes. RNA, 15(12): 2219-35.
Eamens A L, Wook Kim K, Waterhouse P M. 2012. DRB2, DRB3 and DRB5 function in a non-canonical microRNA pathway in Arabidopsis thaliana. Plant Signal Behav, 7(10): 1224-9.
Earley K W, Poethig R S. 2011. Binding of the cyclophilin 40 ortholog SQUINT to Hsp90 protein is required for SQUINT function in Arabidopsis. J Biol Chem, 286(44): 38184-9.
Fagard M, Dellagi A, Roux C, et al. 2007. Arabidopsis thaliana expresses multiple lines of defense to counterattack Erwiniachrysanthemi. Mol Plant Microbe Interact, 20(7): 794-805.
Fang Y, Spector D L. 2007. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr Biol, 17(9): 818-823.
Feng H, Zhang Q, Wang Q, et al. 2013. Target of tae-miR408, a chemocyanin-like protein gene (TaCLP1), plays positive roles in wheat response to high-salinity, heavy cupric stress and stripe rust. Plant Mol Biol [Epub ahead of print].
Friedländer M R, Chen W, Adamidi C, et al. 2008. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol, 26(4): 407-415.
Fukudome A, Kanaya A, Egami M, et al. 2011. Specific requirement of DRB4, a dsRNA-binding protein, for the in vitro dsRNA-cleaving activity of Arabidopsis Dicer-like 4. RNA, 17(4): 750-760.
Gasciolli V, Mallory A C, Bartel D P, et al. 2005. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr Biol, 15(16): 1494-1500.
Ghildiyal M, Xu J, Seitz H, et al. 2010. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. RNA, 16(1): 43-56.
Golden TA, Schauer S E, Lang J D, et al. 2002. SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. Plant Physiol, 130(2): 808-822.
Gregory B D, O'Malley R C, Lister R, et al. 2008. A link between RNA metabolism and silencing affecting Arabidopsis development. Dev Cell, 14(6): 854-866.
Han M H, Goud S, Song L, et al. 2004. The Arabidopsis doublestranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc Natl Acad Sci USA, 101(4): 1093-8.
Hiraguri A, Itoh R, Kondo N, et al. 2005. Specific interactions between dicer-like proteins and HYL1/DRB-family dsRNA-binding proteins in Arabidopsis thaliana. Plant Mol Biol, 57(2): 173-188.
Iki T, Yoshikawa M, Nishikiori M, et al. 2010. In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol Cell, 39(2): 282-291.
Jacob Y, Mongkolsiriwatana C, Veley K M, et al. 2007. The nuclear pore protein AtTPR is required for RNA homeostasis, flowering time, and auxin signaling. Plant Physiol, 144(3): 1383-1390.
Jones-Rhoades M W, Bartel D P, Bartel B. 2006. MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol, 57(10): 19-53.
Kim S, Yang J Y, Xu J, et al. 2008. Two cap-binding proteins CBP20 and CBP80 are involved in processing primary MicroRNAs. Plant Cell Physiol, 49(11): 1634-1644.
Kim Y J, Zheng B, Yu Y, et al. 2011. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J, 30(5): 814-822.
Kurihara Y, Takashi Y, Watanabe Y. 2006. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA, 12(2): 206-212.
Lacombe S, Nagasaki H, Santi C, et al. 2008. Identification of precursor transcripts for 6 novel miRNAs expands the diversity on the genomic organization and expression of miRNA genes in rice. BMC Plant Biol, 8(7): 123-142.
Laubinger S, Sachsenberg T, Zeller G, et al. 2008. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc Natl Acad Sci USA, 105(25): 8795-800.
Lee Y, Ahn C, Han J, et al. 2003. The nuclear RNase III Drosha initiates microRNA processing. Nature, 425(6956): 415-419.
Lee Y, Kim M, Han J, et al. 2004. MicroRNA genes are transcribed by RNA polymerase II. EMBO J, 23(20): 4051-4060.
Li J, Yang Z, Yu B, et al. 2005. Methylation protects miRNAs and siRNAs from a 3'-end uridylation activity in Arabidopsis. Curr Biol, 15(16): 1501-1507.
Liu B, Li P C, Li X, et al. 2005. Loss of function of OsDCL1 affects microRNA accumulation and causes developmental defects in rice. Plant Physiol, 139(1): 296-305.
Liu C, Axtell M J, Fedoroff N V. 2012. The helicase and RNaseIIIa domains of Arabidopsis dicer-Like1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol, 159(2): 748-758.
Liu H H, Tian X, Li Y J, et al. 2008. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA, 14(5): 836-843.
Llave C, Xie Z, Kasschau K D, et al. 2002. Cleavage of scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science, 297(5589): 2053-2056.
Lobbes D, Rallapalli G, Schmidt D D, et al. 2006. SERRATE: a new player on the plant microRNA scene. EMBO Rep, 7(10): 1052-1058.
Lu C, Fedoroff N. 2000. A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell, 12(12): 2351-2366.
Lund E, Güttinger S, Calado A, et al. 2004. Nuclear export of micro-RNA precursors. Science, 303(5654): 95-98.
Machida S, Chen HY, Adam Yuan Y. 2011. Molecular insights into miRNA processing by Arabidopsis thaliana SERRATE. Nucleic Acids Res, 39(17): 7828-7836.
Macrae I J, Zhou K, Li F, et al. 2006. Structural basis for doublestranded RNA processing by dicer. Science, 311(5758): 195-8.
Mallory AC, Bouché N. 2008. MicroRNA-directed regulation: to cleave or not to cleave. Trends Plant Sci, 13(7): 359-367.
Manavella PA, Hagmann J, Ott F, et al. 2012. Fast-forward genetics identifies plant CPL phosphatases as regulators of miRNA processing factor HYL1. Cell, 151(4): 859-870.
Manavella PA, Koenig D, Rubio-Somoza I, et al. 2013. Tissue-specific silencing of Arabidopsis SU(VAR)3-9 HOMOLOG8 by miR171a. Plant Physiol, 161(2): 805-812.
Margis R, Fusaro A F, Smith N A, et al. 2006. The evolution and diversification of Dicers in plants. FEBS Lett, 580: 2442-2450.
Mateos J L, Bologna N G, Chorostecki U, et al. 2010. Identification of microRNA processing determinants by random mutagenesis of Arabidopsis MIR172a precursor. Curr Biol, 20(1): 49-54.
Matranga C, Tomari Y, Shin C, et al. 2005. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell, 123(4): 607-620.
Meng Y, Shao C, Gou L, et al. 2011. Construction of microRNA- and microRNA*-mediated regulatory networks in plants. RNA Biol, 8(6): 1124-1148.
Merchan F, Boualem A, Crespi M, et al. 2009. Plant polycistronic precursors containing non-homologous microRNAs target transcripts encoding functionally related proteins. Genome Biol, 10(12): R136.
Nakazawa Y, Hiraguri A, Moriyama H, et al. 2007. The dsRNA-binding protein DRB4 interacts with the dicer-like protein DCL4 in vivo and functions in the trans-acting siRNA pathway. Plant Mol Biol, 63(6): 777-785.
Naqvi AR, Sarwat M, Hasan S, et al. 2012. Biogenesis, functions and fate of plant microRNAs. J Cell Physiol, 227(9): 3163-3168.
Nikovics K, Blein T, Peaucelle A, et al. 2006. The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell, 18(11): 2929-2945.
Ni Z, Hu Z, Jiang Q, et al. 2013. GmNFYA3, a target gene of miR169, is a positive regulator of plant tolerance to drought stress. Plant Mol Biol, 82(1-2): 113-129.
Park MY, Wu G, Gonzalez-Sulser A, et al. 2005. Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA, 102(10): 3691-3696.
Park W, Li J, Song R, et al. 2002. CARPEL FACTORY, a dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol, 12(17): 1484-1495.
Pélissier T, Clavel M, Chaparro C, et al. 2011. Double-stranded RNA binding proteins DRB2 and DRB4 have an antagonistic impact on polymerase IV-dependent siRNA levels in Arabidopsis. RNA, 17(8): 1502-1510.
Prigge M J, Wagner D R. 2001. The Arabidopsis SERRATE gene encodes a zinc-finger protein required for normal shoot development. Plant Cell, 13(6): 1263-1279.
Rajagopalan R, Vaucheret H, Trejo J, et al. 2006. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev, 20(24): 3407-3425.
Reinhart B J, Weinstein E G, Rhoades M W, et al. 2002. MicroRNAs in plants. Genes Dev, 16(13): 1616-1626.
Ren G, Xie M, Dou Y, et al. 2012. Regulation of miRNA abundance by RNA binding protein TOUGH in Arabidopsis. Proc Natl Acad Sci USA, 109(31): 12817-12821.
Rogers K, Chen X. 2013. microRNA Biogenesis and Turnover in Plants. Plant Cell [Epub ahead of print].
Smith MR, Willmann MR, Wu G, et al. 2009. Cyclophilin 40 is required for microRNA activity in Arabidopsis. Proc Natl Acad Sci USA, 106(13): 5424-5429.
Song J J, Smith S K, Hannon G J, et al. 2004. Crystal structure of Argonaute and its implications for RISC slicer activity. Science, 305(5689): 1434-1437.
Song L, Axtell M J, Fedoroff N V. 2010. RNA secondary structural determinants of miRNA precursor processing in Arabidopsis. Curr Biol, 20(1): 37-41.
Sun G. 2012. MicroRNAs and their diverse functions in plants. Plant Mol Biol, 80(1): 17-36.
Sunkar R, Chinnusamy V, Zhu J, et al. 2007. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci, 12(7): 301-309.
Szarzynska B, Sobkowiak L, Pant B D, et al. 2009. Gene structures and processing of Arabidopsis thaliana HYL1-dependent pri-miRNAs. Nucleic Acids Res, 37(9): 3083-3093.
Takanashi H, Ohnishi T, Mogi M, et al. 2011. DCL2 is highly expressed in the egg cell in both rice and Arabidopsis. Plant Signal Behav, 6(4): 604-606.
Tomari Y, Matranga C, Haley B, et al. 2004. A protein sensor for siRNA asymmetry. Science, 306(5700): 1377-1380.
Vazquez F, Blevins T, Ailhas J, et al. 2008. Evolution of Arabidopsis MIR genes generates novel microRNA classes. Nucleic Acids Res, 36(20): 6429-6438.
Vazquez F, Gasciolli V, Crété P, et al. 2004. The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr Biol, 14(4): 346-351.
Vazquez F, Legrand S, Windels D. 2010. The biosynthetic pathways and biological scopes of plant small RNAs. Trends Plant Sci, 15(6): 337-345.
Voinnet O. 2009. Origin, biogenesis, and activity of plant microRNAs. Cell, 136(4): 669-87.
Wang L, Song X, Gu L, et al. 2013. NOT2 proteins promote polymerase II-dependent transcription and interact with multiple MicroRNA biogenesis factors in Arabidopsis. Plant Cell, 25(2): 715-727.
Werner S, Wollmann H, Schneeberger K, et al. 2010. Structure determinants for accurate processing of miR172a in Arabidopsis thaliana. Curr Biol, 20(1): 42-48.
Wang Y, Sun F, Cao H, et al. 2012. TamiR159 directed wheat TaGAMYB cleavage and its involvement in anther development and heat response. PLoS One, 7(11): e48445.
Wu L, Zhang Q, Zhou H, et al. 2009. Rice MicroRNA effector complexes and targets. Plant Cell, 21(11): 3421-3435.
Wu L, Zhou H, Zhang Q, et al. 2010. DNA methylation mediated by a microRNA pathway. Mol Cell, 38(3): 465-475.
Wu X, Shi Y, Li J, et al. 2013. A role for the RNA-binding protein MOS2 in microRNA maturation in Arabidopsis. Cell Res, 23(5): 645-657.
Xie Z, Allen E, Fahlgren N, et al. 2005. Expression of Arabidopsis MIRNA genes. Plant Physiol, 138(4): 2145-2154.
Xie Z, Johansen L K, Gustafson A M, et al. 2004. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol, 2(5): E104.
Yang SW, Chen HY, Yang J, et al. 2010. Structure of Arabidopsis HYPONASTIC LEAVES1 and its molecular implications for miRNA processing. Structure, 18(5): 594-605.
Yang Z, Ebright YW, Yu B, et al. 2006. HEN1 recognizes 21-24 nt small RNA duplexes and deposits a methyl group onto the 2' OH of the 3' terminal nucleotide. Nucleic Acids Res, 34(2): 667-675.
Yu B, Bi L, Zheng B, et al. 2008. The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc Natl Acad Sci USA, 105(29): 10073-10078.
Yu B, Yang Z, Li J, et al. 2005. Methylation as a crucial step in plant microRNA biogenesis. Science, 307(5711): 932-935.
Zhang W, Gao S, Zhou X, et al. 2010. Multiple distinct small RNAs originate from the same microRNA precursors. Genome Biol, 11(8): R81.
Zhang Y, Cheng Y T, Bi D, et al. 2005. MOS2, a protein containing G-patch and KOW motifs, is essential for innate immunity in Arabidopsis thaliana. Curr Biol, 15(21): 1936-1942.
Zhan X, Wang B, Li H, et al. 2012. Arabidopsis proline-rich protein important for development and abiotic stress tolerance is involved in microRNA biogenesis. Proc Natl Acad Sci USA, 109(44): 18198-18203.
Zhao Y, Yu Y, Zhai J, et al. 2012. The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Curr Biol, 22(8): 689-694.
Zhu Q H, Spriggs A, Matthew L, et al. 2008. A diverse set of micro-RNAs and microRNA-like small RNAs in developing rice grains. Genome Res, 18(9): 1456-1465.
Q74
AArticle lD:1006-8104(2014)-01-0084-13
Received 6 May 2013
Supported by the National Natural Science Foundation of China (31070265)
Kong Wen-wen (1990-), male, Ph. D, engaged in the research of plant molecular biology. E-mail: wenwendadi@126.com
* Corresponding author. Li jing, professor, supervisor of Ph. D student, engaged in the research of plant molecular biology. E-mail: lijing@neau.edu.cn
猜你喜欢
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Relationship Between Cucumber Germplasm and Propamocarb Residue Using Subjective Rating Technique
- Cloning and Expression Analysis of Mlo Gene from Pericallis hybrida B. Nord.
- Simulation of in situ Root Decomposition of Two Barley Cultivars
- Vertical Migrating and Cluster Analysis of Soil Mesofauna at Dongying Halophytes Garden in Yellow River Delta
- Effect of MSTN Propeptide and shRNA Co-expression Vector on Proliferation of Skeletal Muscle Satellite Cells
- Application of Protein Feed Processed by Microbial Fermentation to Dairy Cow
