Application of Protein Feed Processed by Microbial Fermentation to Dairy Cow
2014-03-07SunZheLiuYingPanHongbaoandGaoXuejun
Sun Zhe, Liu Ying , Pan Hong-bao, and Gao Xue-jun
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
Application of Protein Feed Processed by Microbial Fermentation to Dairy Cow
Sun Zhe, Liu Ying , Pan Hong-bao, and Gao Xue-jun*
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
Methionine (Met) and lysine (Lys) have been reported as the first two limiting amino acids (AA) for maximum milk yield and milk protein production. Supplying these AA may improve microbial protein synthesis and therefore improve milk production without adding excess N to the environment. This observation utilized fermented soybean meal (SBM), cottonseed meal (CSM), rapeseed meal (RSM) and corn by Bacillus subtilis 168 and Leuconostoc mesenteroides as core feedstuffs to produce special biological protein feed for dairy cow. The results showed that the milk production, milk protein percentage, milk fat percentage and milk DM percentage of test groups in trial period were significantly more than those of the control group (P<0.01), the results showed that adding fermenting protein feed in dairy cow diets could significantly improve milk yield, milk protein and milk fat content. The economic benefits of actual application were analyzed, the group of 0.5% was the best compared with the other groups.
soybean meal, cottonseed meal, rapeseed meal, corn, fermentation; Bacillus subtilis 168, Leuconostoc mesenteroides, methionine, lysine
Introduction
Amino acid nutrition of dairy cows has received much attention over the last decade resulting in several nutritional models, which allow for diet formulation on the basis of amino acids (AA). Although it is difficult to estimate the ratio of metabolizable AA in the small intestine, methionine (Met) and lysine (Lys) have been identified as one of the first and second limiting AA in popular diets for lactating dairy cows (NRC, 2001). Lys and Met supply is often limiting for milk protein production in dairy cows. Based on the results of Schwab (1995) and others, it was concluded that optimal use of metabolizable protein (MP) for the combined functions of maintenance and milk protein production requires concentrations of Lys and Met in MP that approximate 7.2% and 2.4%, respectively (NRC, 2001). Numerous experiments have studied the benefits of adding different forms of synthetic Lys or Met to lactating cow rations. Responses have included increased milk yield, milk fat content, milk protein content, and protein yield (Noftsger et al., 2005; Rode et al., 1998; Piepenbrinket et al., 2004; Socha et al., 1994). However, optimal concentrations of AA for milk protein synthesis are not readily achieved when using only conventional feedstuffs. The practical way to reach these ratios of AA is dietary supplementation with the protein feed processed by microbial fermentation.
Soybean meal (SBM) is recognized as an important source of protein in the poultry industry due to its high protein content and widespread availability. However, the price of SBM has increased dramatically in recentyears, resulting in an urgent need for alternative protein sources (Laudadio and Tufarelli, 2010). Cottonseed meal (CSM), a by-product obtained from the process of extracting the oil from cotton seed that contains approximately 22% to 56% crude protein is a potential alternative to SBM in China (Nagalakshmi et al., 2007). Among plant protein sources, and due to its high protein content, reasonable price and steady supply, soybean meal, cottonseed meal and rapeseed meal are the most frequently studied ingredients as an FM replacer in diets for many terrestrial animals and fish (Storebakken et al., 2000; Yue et al., 2008; Wang et al., 2010). Soybean meal, cottonseed meal and rapeseed meal are coproducts from oil extraction of the seed kernel (after removal of the hull). Due to its high protein content, the value has been largely ascribed to its value as protein supplement.
Fermentation has been proven to improve the nutritional value of protein feed by increasing the bioavailability of nutrients and reducing antinutritional factors. An in vitro study indicated that fermentation of soybean resulted in an increase in nutrient solubility and digestibility (Kiers et al., 2003). One result indicate that FSBM may not only improve growth performance, feed intake, and health conditions during the preweaning period, but also alleviate stress responses, which is indicated by reduced induction of stress hormone, proinflammatory cytokines, and acute phase proteins in Holstein calves after weaning (Sun et al., 2012). Nevertheless, data on the effect of FSBM on milk yield in cow is scarce. The objective of this study was to increase essential amino acids (Lys and Met) by fermentation process with Bacillus subtilis 168 and Leuconostoc mesenteroides and enzymatic process. In addition, the suitability of a commercially available fermented feeds was gained to replace non-fermented feeds in livestock diets.
Materials and Methods
Chemicals and equipments
An HPLC system consisted of a LC-10AT VP high efficiency liquid chromatography (Shi Mad Zu); SPD-10A VP UV-VIS detector (Shi Mad Zu); chromatographic column (Dikma Technologies); Ultrafilter (Cole-Parmer Instrument Company); transferpettor (X96316, Finnpipette); HZQ-C Airbath Alternator (Haerbin Dongming Medical Treatment Instruction Limited Company). All the reagents used were of analytic degree.
Microorganism and growth conditions
Leuconostoc mesenteroides ATCC 8293 (ATCC8293) and Bacillus subtilis 168 (AL009126.3) were stored in our laboratory; soybean meal, cottonseed meal, rapeseed meal and corn were offered from Fubang Company, Harbin, China.
LB medium: 1% w/v peptone, 0.5% w/v yeast extract, 1% w/v NaCl (pH 7.0).
MRS medium: 0.5% soybean peptone, 0.5% poly peptone, 0.05% ascorbic acid, 0.5% yeast extract, 0.5% lactose, 1.1% β-glycerol phosphate disodium, Tween 80 1 mL, 1 mol • L-1MgSO4• 7H2O 1 mL, autoclave to sterilize (pH 6.2-6.4).
Fermentation medium: soybean meal, cottonseed meal, rapeseed meal and corn meal were mixed by 11 : 3 : 3 : 3, 0.1 mol • L-1phosphate buffer, and 3 mL salt solution.
All media were autoclaved to sterilize.
Pretreatment of raw materials
The substrate was purchased from Fubang Company, Harbin, China. The raw material was ground to 0.63 mm particle size using a disintegrator, and later thoroughly mixed and stored in plastic bags.
Enzymatic hydrolysis
Ten grams of substrate were added to 100 mL of distilled water, in the presence of phosphate buffer. pH of the suspension was corrected to 7.7 with saturation Ca(OH)2. The experiments were carried out at 55℃, 100 r • min-1. After sterilization (121℃, 15 min), protease was added at concentration of 0.5% (protein/ protein) and hydrolyzed for 4.5 h. At the end of thehydrolysis, pH of the suspension was measured and again corrected to 6.5-7.0.
Substrate fermentation
Leuconostoc mesenteroides and Bacillus subtilis 168 were previously isolated from the cereals. The isolation of microorganisms and cultivation was carried out in Petri dishes using LB and MRS media at 37℃. All the stock cultures were stored at 4℃.
After enzymatic hydrolysis and sterilization (121℃, 15 min), the substrate solution was inoculated with Bacillus subtilis 168 and fermented in climatic chamber at 38.0℃ for 24 h, next after sterilization (121℃, 15 min) the fermented solution was further inoculated with Leuconostoc mesenteroides and fermented in climatic chamber at 29.0℃ for 18 h. The final liquid was dried at 40℃.
Lys and Met contents detected by HPLC
Determination of Lys and Met contents was carried out by acid hydrolysis, and HPLC quantification as described in the standard method GB/T 18246-2000. Protein hydrolysis was carried out with 6 mol • L-1HCl for 24 h at 110℃ in a vacuum closed vial. Hydrolysates were dried under vacuum and rinsed twice with water. CDNB (2, 4-dinitrochlorobenzene 99%, Aldrich) were used for amino acid derivatization. The hydrolyzed sample was filtered through a 0.22 um membrane and analyzed by HPLC. Lys and Met contents were determined by measuring the ratios of the chromatographic peaks of the ions derived from the analyses (Lys and Met) to those derived from the external standards. Samples were injected in a Diamonsil C18 (5u 250 mm×4.6 mm) column, maintained at 20℃. The flow rate was 800 uL • min-1. Each experiment was duplicated.
Animals, diets, and experiment design
The use of the animals was approved by Dongfanghong Farm, Harbin, China. Total 15 multiparous Chinese Holstein cows in mid-lactation were used in a 3-treatment randomized block design for 15 days. Fifteen cows with the similar age, parity and milk production were randomly assigned to three treatments: 0% (control), 0.5% (treatment 1) and 1% (treatment 2). Milk production and feed intake were measured daily; milk samples from the afternoon milking were taken everyday and analyzed for detection of contents fat, protein, lactose and milk DM.
Statistical analysis
Diets were assigned by completely randomized design. Data were analyzed by one-way analysis of variance (ANOVA) in SPSS version 11.0. Differences in mean values were made with Duncan's multiple range tests when ANOVA identified differences among groups. Statistical significance was determined by setting the aggregate type I error at 5% (P<0.05) for each set of comparisons. Data presented as mean±SD. Percentage data were arcsine transformed before statistical analysis.
Results
Lys and Met standard samples and standard curves
Using standards, relative retention time and spectral characteristics, Lys and Met in fermentation samples were identified. HPLC result of derived standard Lys liquid of 80 ug • mL-1was shown, the retention time was 15.145 min (same as the standard samples of CDNB-lys). The selection of solvent system was made of 25% methanol in the sodium acetate buffer (pH 5.2). The chromatogram of Lys from fermentation feed was shown. The retention time of standard Met sample was 6.533 min.
Linearity was assessed over concentrations varying from 2 ug • mL-1to 80 ug • mL-1for Lys and Met standard. The calibration plots for Lys and Met were fit to linear equations of slope and intercept (Table 1). The correlation coefficients were in excess of 0.99 in case.
Determination of contents of Lys and Met of fermented samples
The protein concentration and Lys and Met contentsof rough material ingredients were listed in Table 2. Detection of unfermented rough material revealed a protein level of 39.80% dry matter in accordance to levels reported in the literature. The fermentation process brought about a significant increase in total protein, after rough material was naturally fermented. Rough materials were fermented with Leuconostoc mesenteroides and Bacillus subtilis 168, and its protein level was increased to 52.4%. Lys content raised to 13.56% (CP %), whereas methionine was only 7.87% (CP%).

Table 1 Regression equations, correlation coefficients and detection limits for samples

Table 2 Contents of Lys and Met and CP analysis in feed protein
Milk production and milk quality
The results showed that the milk production, milk protein percentage, milk fat percentage and milk DM percentage of test groups in trial period were significantly more than those of control group (P<0.01), the milk production raised 11.0% and 13.7%, respectively; milk protein percentage raised 5.6% and 6.6%, respectively; milk fat percentage raised 7.0% and 7.5%, respectively, lactose contents raised by a certain degree (Table 3). The results showed that adding fermenting peptides of rich limiting essential amino acids in dairy cow diets could significantly improve milk yield, milk protein and milk fat content.

Table 3 Influence of fermenting protein feed on milk performance
Economic analysis of feeding fermenting forage to dairy cows
After cost-profit calculating, each dairy cow in treatment 1 group earned pure profit 4.23 Yuan/day, the treatment 2 group earned 4.95 Yuan/day, and the ratios of input-output were 1:10.1 and 1:6.1, respectively (Table 4). The group of 0.5% was the best compared with the other two groups.
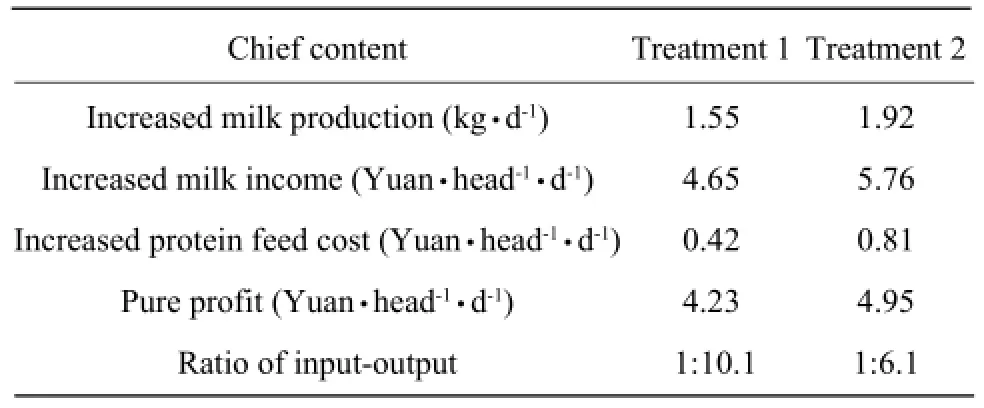
Table 4 Economic analysis of feeding fermenting protein feed to dairy cows
Discussion
Feed cost is the major factor that influences the productivity of the livestock industry. In livestock diet composition, dietary protein accounts for the most expensive proportion among all other nutrients. Digested dietary protein supplies essential AA (EAA), non-essential AA (NEAA), N, and peptides for animal physiological uses. In high-producing dairy cows, AA supplys from microbial protein alone can not meet the requirement for milk production (Volden et al., 1998), and there is a need to supple these cows with protein sources high in ruminally undegraded feed protein (RUP). Under most commercial settings, Met and Lys have been reported as the two first-limiting AA for maximum milk yield and milk protein production (Schwab et al., 1992). However, dietary protein of a conventional diet varies and differs from microbial and milk protein in AA composition (Schwab, 1995). Common feeds, such as alfalfa (11.1% Lys and 3.8% Met), corn silage (7.5% Lys and 4.8% Met), yellow corn (7% Lys and 5% Met), and soybean meal (13.7% Lys and 3.1% Met) are often deficient in Lys and Met contents as compared to milk (16% Lys and 5.5% Met) and ruminal bacteria (15.8% Lys and 5.2% Met) in their Lys and Met profiles (Schwab, 1995). To meet the animal's MP requirement and increase average daily gain (ADG), not only quantities of MP supply but also the limiting AA of MP in the diet should be considered. Feeding dairy cows with diets containing imbalanced AA could reduce milk production and N utilization efficiency.
Soybean meal (SBM), cottonseed meal (CSM), and rapeseed meal (RSM) are the most nutritious of all plant protein sources. For example, soybean meal is the leading oilseed crop produced globally (Gatlin et al., 2007). Because of its high protein content, high digestibility, relatively well-balanced amino acid profile, reasonable price, and steady supply, SBM is widely used as a cost-effective feed ingredient for many animals (Storebakken et al., 2000), and it is currently the most commonly used plant protein source in fish feeds. With regard to cottonseed, the annual production is more than 6 million tons in China (Luo et al., 2006). It has been used in diets for both terrestrial animals and fish due to its high protein content. Two experiments were conducted to test the feeding value of fermented cottonseed meal (FCSM) in broilers and the results showed that appropriate dietary inclusion of FCSM could improve cecal microflora, intestinal morphology, and digestive enzyme activity in yellow-feathered broilers. Thus, fermented CSM could be used as a promising source of protein in feed (Sun et al., 2012).
In this study, we provided the use of a fermentation process and enzymatic process as a strategy to reduce the detriment factor of proteins feed and consequently avoid detrimental effects in Chinese Holstein cows. The enzymatic process is an alternative for the protein extraction and offers advantages. However, the efficiency of fermentation to reduce proteins feed immunoreactivity and gossypol content depends on many factors such as starter culture, type of fermentation, fermentation time, raw material particle size(Kiers et al., 2000; Song et al., 2008; Frias et al., 2008). We confirmed that fermentation hydrolyzed proteins into smaller peptides (data not reported), changed the amino acid composition, reduced immunoreactivity and gossypol content. Depending on the degree and type of microbial hydrolysis, fermentation could potentially decrease or even eliminate allergenic reactions. This work further verified that rough material fermented by bacteria or yeast be excellent sources of proteins for human and animal diets, as a feasible method of fermentation, it can effectively improve the nutritional quality of SBM, and FSBM product as a source of feed protein with high quality for younger animals could be valuable in terms of nutrition and resources, especially when a high import pressure of 77% of annual consumption of SBM is imposed on animal husbandry in China (Teng et al., 2012).
Frias J, Song Y S, Martinez C, et al. 2008. Immunoreactivity and amino acid content of fermented soybean products. Food Chem, 56: 99-105.
Gatlin D M, Barrows F T, Brown P, et al. 2007. Expanding the utilization of sustainable plant products in aquafeeds. Res, 38: 551-579.
Kiers J L, Meijer J C, Nout M J R, et al. 2003. Effect of fermented soya beans on diarrhea and feed efficiency in weaned piglets. Microbiol, 95: 545-552.
Kiers J L, Laeken E V, Rombouts F M, et al. 2000. In vitro digestibility of bacillus fermented soya bean. Food Microbiol, 60: 163-169.
Laudadio V, Tufarelli V. 2010. Growth performance and carcass and meat quality of broiler chickens fed diets containing micronizeddehulled peas (Pisum sativum cv. Spirale) as a substitute of soybean meal. Poultry Science, 89: 1537-1543.
Luo L, Xue M, Wu X, et al. 2006. Partial or total replacement of fish meal by solvent-extracted cottonseed meal in diets for juvenile rainbow trout (Oncorhynchus mykiss). Nutr, 12: 418-424.
Nagalakshmi D, Rao S V R, Panda A K, et al. 2007. Cottonseed meal in poultry diets. Journal of Poultry Science, 44: 119-134.
Noftsger S, St-Pierre N R, Sylvester J T. 2005. Determination of rumen degradability and ruminal effects of three sources of methionine in lactating cows. Dairy Sci, 88: 223-237.
NRC. 2001. Nutrient requirements of dairy cattle. Natl Acad Sci, Washington. pp. 14-17.
Piepenbrink M S, Marr A L, Waldron M R, et al. 2004. Feeding 2-hydroxy-4 (methylthio)-butanoic acid to periparturient dairy cows improves milk production but not hepatic metabolism. Dairy Sci, 87: 1071-1084.
Rode L M, Knight C D, Andrews K A, et al. 1998. Effects of pre- and post-partum Alimet supplementation on milk production of dairy cows. Dairy Sci, 81(Suppl): 294-296.
Schwab C G, Bozak C K, Whitehouse N L, et al. 1992. Amino acids limitation and flow to the duodenum at four stages of lactating, sequence of lysine and methionine limitation. Dairy Sci, 75: 3486-3502.
Schwab C G. 1995. Protected proteins and amino acids for ruminants. In: Biotechnology in animal feeds and animal feeding. VCH Press, Germany. pp. 115-141.
Song Y, Martinez V S, Mejia C, et al. 2008. Quantification of human IgE immunoreactive soybean proteins commercial soy ingredients and products. Food Sci, 73: 90-99.
Socha M T. 1994. Determining the methionine requirements of lactating dairy cows. University of New Hampshire, Durham.
Storebakken T, Refstie S, Ruyter B. 2000. Soy products as fat and protein sources in fish diets for intensive aquaculture. Federation of Animal Science Societies, Champaign. pp. 127-170.
Sun H, Tang J W, Yao X H, et al. 2013. Effects of dietary inclusion of fermented cottonseed mealon growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop Anim Health Prod, 45: 987-993.
Teng D, Gao M Y. 2012. Bio-modification of soybean meal with Bacillus subtilis or Aspergillus oryzae. Biocatalysis and Agricultural Biotechnology, 1: 32-38.
Volden H W, Velle O M, Harstad A, et al. 1998. Apparent ruminal degradation and rumen escape of lysine, methionine, and threonine administered intraruminally in mixtures to high-yielding cows. Dairy Sci, 76: 1232-1240.
Wang R H, Sharman S M, Godoy L C, et al. 2010. Bioconversion of rapeseed meal for the production of a generic microbial feedstock. Enzyme and Microbial Technology, 47: 77-83.
Yue Y R, Zhou Q C. 2008. Effect of replacing soybean meal with cottonseed meal on growth, feed utilization, and hematological indexes for juvenile hybrid tilapia. Aquaculture, 7: 185-189.
S816.6
A
1006-8104(2014)-01-0039-06
Received 26 August 2013
Supported by "863" Project of Ministry of Science and Technology of China (2013AA102504-03)
Sun Zhe (1980-), female, engaged in the research of husbandry probiotics. E-mail: sunzhe1998@163.com
* Corresponding author. Gao Xue-jun, professor, supervisor of Ph. D student, engaged in the research of molecular biology. E-mail: gaoxj5390@sina.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Relationship Between Cucumber Germplasm and Propamocarb Residue Using Subjective Rating Technique
- Cloning and Expression Analysis of Mlo Gene from Pericallis hybrida B. Nord.
- Simulation of in situ Root Decomposition of Two Barley Cultivars
- Vertical Migrating and Cluster Analysis of Soil Mesofauna at Dongying Halophytes Garden in Yellow River Delta
- Effect of MSTN Propeptide and shRNA Co-expression Vector on Proliferation of Skeletal Muscle Satellite Cells
- Cloning and Sequence Analysis of Y-box Binding Protein Gene in Min Pig
