超低剂量地西他滨联合自体CIK细胞输注治疗骨髓增生异常综合征转化的高龄急性髓细胞白血病1例并文献复习
2014-03-02杨波蔡力力汪海涛朱宏丽迟小华于睿莉杨洋冉海红辛丽君姚善谦卢学春
杨波,蔡力力,汪海涛,朱宏丽,迟小华,于睿莉,杨洋,冉海红,辛丽君,姚善谦,卢学春
急性髓细胞白血病(acute myelocytic leukemia,AML)发病率随年龄增加而升高,发病中位年龄超过65岁,是老年人最常见的恶性血液病类型之一[1]。在绝大部分老年AML中,由于衰老相关的器官功能退化、合并基础疾病多,患者往往不能耐受标准剂量化疗。近来研究显示,去甲基化疗药物地西他滨对骨髓增生异常综合征(myelodysplastic syndrome,MDS)及其转化的AML安全有效[2-3],但常规剂量的地西他滨对老年患者的骨髓毒性作用使其应用受到很大限制。对于老年AML是否存在最适的地西他滨治疗剂量,即既能起到治疗作用又不至于引起严重骨髓抑制,值得进一步研究。我们的前期临床研究发现,自体细胞因子诱导的杀伤细胞(cytokineinduced killer,CIK)能使部分高龄AML患者获得血液学改善甚至是部分缓解[4-7]。考虑到地西他滨通过表观遗传学调控治疗白血病的潜在作用及CIK细胞相对特异性靶向杀伤白血病细胞的作用[8],我们系统观察了超低剂量地西他滨联合自体CIK细胞治疗1例80岁以上高龄MDS转化AML的安全性及疗效,现报道如下。
1 资料与方法
1.1 临床资料 患者,男,83岁,因“乏力、盗汗3个月余”于2006年4月就诊,既往有右肺肺泡癌、冠心病、慢性肾功能不全等病史(表1)。无特殊家族史。查体无明显异常。血常规:白细胞(WBC)3.1×109/L,中性粒细胞(N)34%,淋巴细胞(L)56%,单核细胞(M)5%,血红蛋白(Hb)143g/L,血小板(PLT)149×109/L。红细胞沉降率、血清叶酸、VitB12含量正常。骨髓穿刺见红系发育异常,占12%。铁染色未见环形铁粒幼细胞,诊断为骨髓增生异常综合征–难治性贫血(MDS-RA)。患者从2008年8月开始出现贫血及血小板减少,间断给予氨磷汀联合重组人红细胞生成素β治疗,监测血常规,WBC波动于2.0~3.1×109/L,PLT 70~90×109/L,Hb 90~110g/L。2009年12月15日血常规提示WBC 7.06×109/L,M 16%;外周血涂片发现幼稚细胞(图1A);骨髄穿刺提示原始细胞占10.4%,幼稚单核细胞占2.4%,成熟单核细胞占11.2%(图1B);骨髓单个核细胞多重巢式PCR发现FLT3点突变,HOX11、C-kit、c-myc、GATA2等基因表达中度增高;染色体核型为(46,XY),诊断为慢性粒单细胞白血病(chronic myelocytic mononuclear leukemia,CMML),仍给予氨磷汀联合红细胞生成素治疗。此后,患者外周血单核细胞计数及幼稚细胞比例逐渐增高,2010年2月20日WBC升高至17.62×109/L,其中原始细胞3%,单核细胞31%,骨髓穿刺示原始粒细胞占15%,幼稚单核细胞占23.6%,诊断为急性髓细胞白血病M4型(AML-M4)(图1C)。
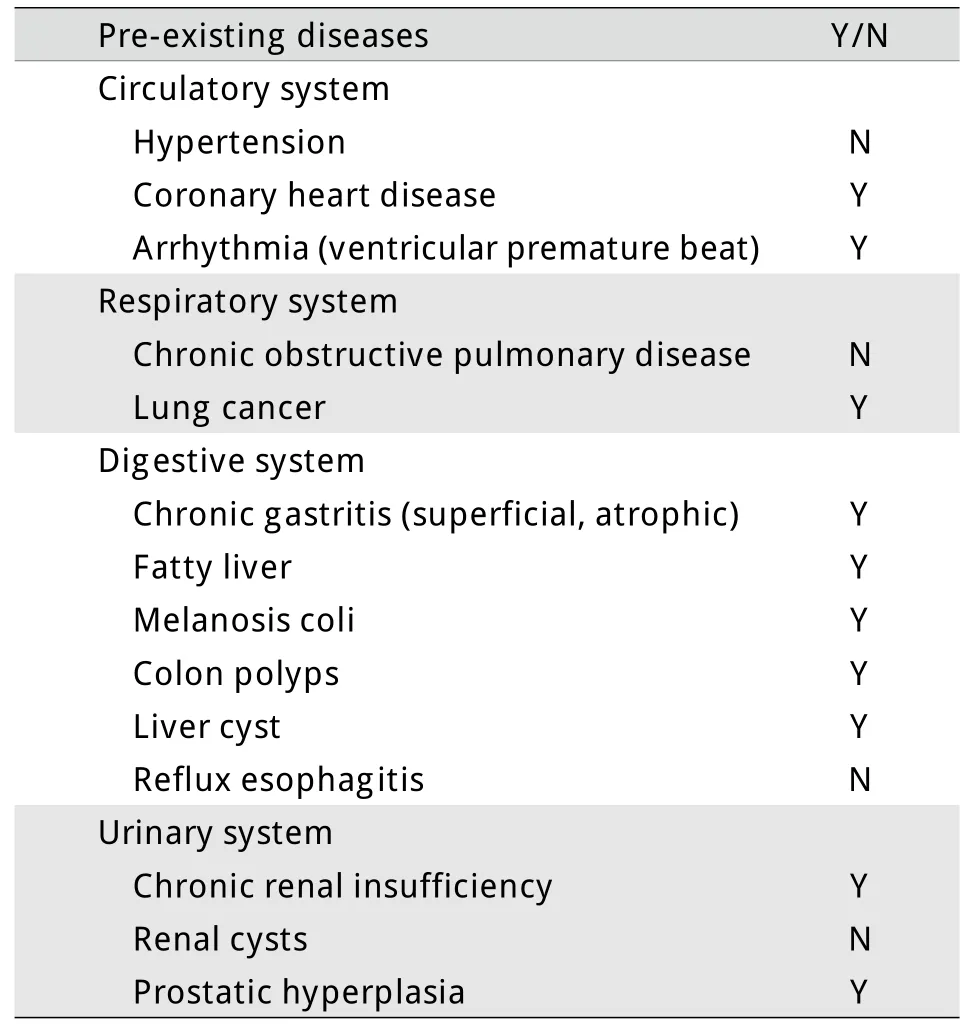
表1 本例既往疾病史Tab.1Retrospective history of the case
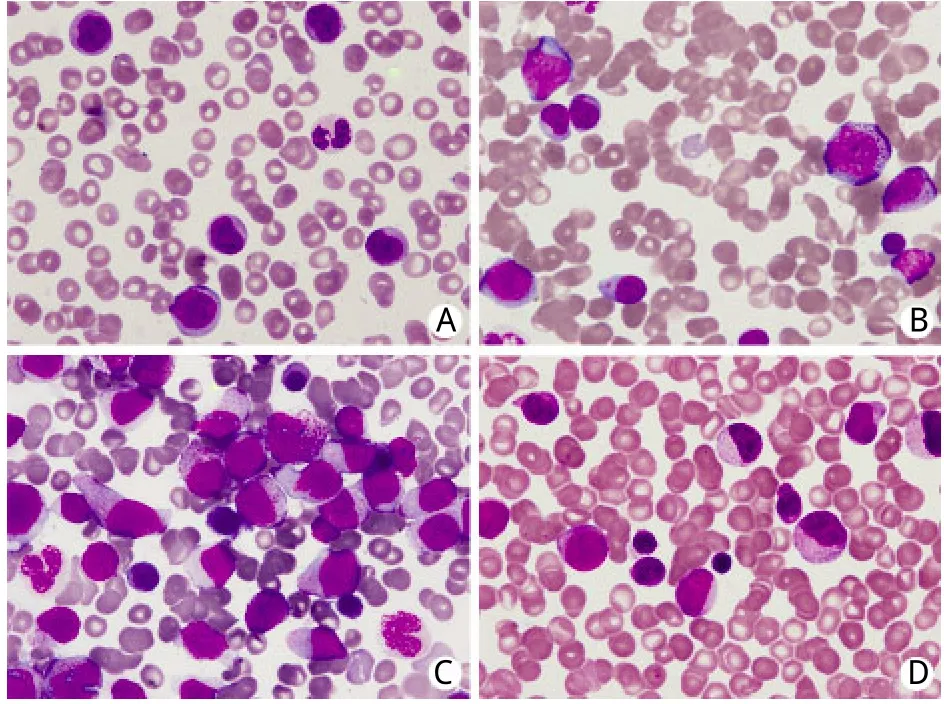
图1 患者外周血涂片及骨髓象Fig.1Peripheral-blood smear and bone marrow hemogram from case
1.2 治疗方案 确诊AML-M4后即开始给予地西他滨(江苏正大天晴制药有限公司)联合自体CIK细胞输注治疗,具体用法:地西他滨10mg d1-5,CIK细胞输注(每次2×109~8×109)d14,rhIL-22mU d15-19,每周期28d(图2)。自体CIK细胞的制备及回输:给予胸腺五肽注射液20mg/d肌内注射,连续1周。第8天晨起空腹采集外周静脉血54ml,在我院基础所免疫室(符合GMP条件)分离单个核细胞(peripheral blood mononuclear cells,PBMC),用无血清培养基调整细胞浓度,加入rhIFN-γ使终浓度达到2000U/ml。将细胞悬液置于透气性培养袋中,37℃、5%CO2条件下悬浮培养,次日加rhIL-21000U/ml,anti-CD3McAb 50ng/ml,培养第4、7、10、13天进行细胞表型分析、调整细胞浓度、补充IL-2,使回输时的CIK细胞表型符合如下标准:CD3+细胞比例大于70%,CD8+细胞比例大于40%,CD3+CD56+细胞比例不低于30%。培养第14天通过静脉将CIK细胞回输给患者。
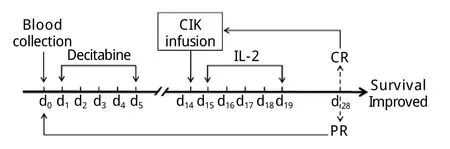
图2 超低剂量地西他滨联合自体CIK细胞治疗方案Fig.2Therapeutic regimen of decitabine combined with autologous CIK cells transfusion
2 结 果
患者共接受8个周期的治疗。化疗期间出现Ⅰ/Ⅱ度骨髓抑制及Ⅰ度胃肠道反应。第2、3、8疗程患者出现高白细胞血症,最高达141.95×109/L,其中原幼细胞49%,单核细胞37%,中性粒细胞8%,淋巴细胞5%。加用依托泊苷(50mg d1-3)治疗,高白细胞血症得到控制。在间断输血治疗下,患者Hb波动于77~138g/L。血小板在第2个疗程开始上升,第6个疗程达到204×109/L(图3)。骨髓象示原始、早幼粒细胞及幼单核细胞占16%。疗效评价达部分缓解(图1D)。2011年12月25日患者死于严重肺部感染引起的多脏器功能衰竭。自诊断AML至死亡总生存22个月。
3 讨 论
老年AML具有自身的临床生物学特点:①脏器功能退化,合并多种慢性基础病,对化疗耐受差[9];②多有前驱血液系统疾病(常见为MDS),造血处于衰竭状态,化疗后血象恢复慢;③染色体异常比例高[10],耐药率高,缓解后容易复发[11]。因此,老年AML治疗难度大,预后差[12]。据文献报道,80岁以上AML患者中位生存期仅6周[13]。Kantarjian等[13]分析了998例老年高危MDS/AML患者的临床资料,共筛选出7个与总生存相关的预后指标,其中,大于3个指标的患者中位总生存期只有1个月。根据此预后评价标准,本例预期生存期为1个月(表2)。
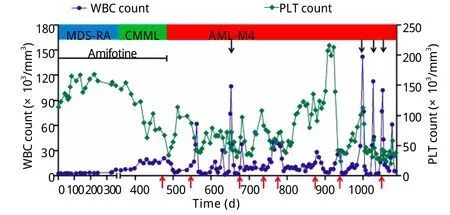
图3 患者病情演变过程及治疗期间的血象变化Fig.3Hemogram change during progression and treatment
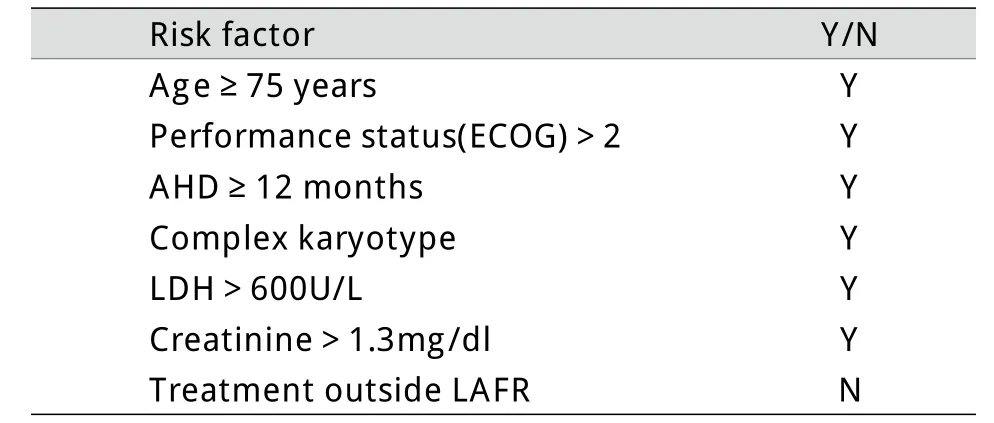
表2 本例生存相关危险因素Tab.2Risk factors related to the predicting survival of the case[13]
老年AML的治疗至今仍无标准方案,小剂量阿糖胞苷和最佳支持治疗均不能延长患者的总生存[3,14],长期生存(5年)率仅5%~15%[3,15-16],因此,老年AML仍被归为难治性白血病类型。故有必要针对老年AML开展临床试验研究,探索高效低毒的治疗方法,有效提高缓解率,延长生存率,改善患者生活质量。
地西他滨是一种DNA甲基化转移酶抑制剂,具有去甲基化作用,可重新激活由于DNA过度甲基化而失活的基因,从而诱导肿瘤细胞分化、凋亡[17-18]。多项临床试验表明,小剂量地西他滨对老年MDS/AML具有一定疗效,能够提高生存质量,改善血液学指标,延缓MDS向AML的转化,延长无进展生存和总生存期[19-21]。综合文献报道,地西他滨治疗60岁以上老年AML的单次剂量在15~45mg不等,一个疗程的总剂量在100~200mg,治疗总反应率25%~64%,中位生存期5.5~12.8个月,治疗相关死亡率13%~25%[15,22-23](表3)。但对于由MDS转化而来的80岁以上高龄AML患者,当前报道的地西他滨剂量由于血液学毒性大而难以适用,缓解后的维持治疗也不能单纯依靠地西他滨。本研究应用超低剂量地西他滨联合自体CIK细胞输注方案治疗MDS转化的高龄AML,效果良好。本例诊断AML时87岁,合并右肺肺泡癌,病程中出现高白细胞血症,预期生存仅1个月[13],给予超低剂量地西他滨联合自体CIK细胞治疗8个周期,患者生存期达22个月,显著高于文献报道。对于本方案的疗效机制,我们推测可能与地西他滨和CIK细胞的协同作用有关。地西他滨在高剂量时主要显示细胞毒作用,而在低剂量时则表现为对肿瘤相关基因的表观调控作用[24-26],例如能使失活的抑癌基因重新表达,使肿瘤细胞表达新的癌抗原,从而增强CIK细胞对肿瘤细胞的识别和杀伤;反过来,CIK细胞具有潜在的增强体内其他免疫效应细胞功能的作用[7,27-29],这也会提高患者的免疫力,进而降低地西他滨治疗后骨髓抑制期发生感染的风险。
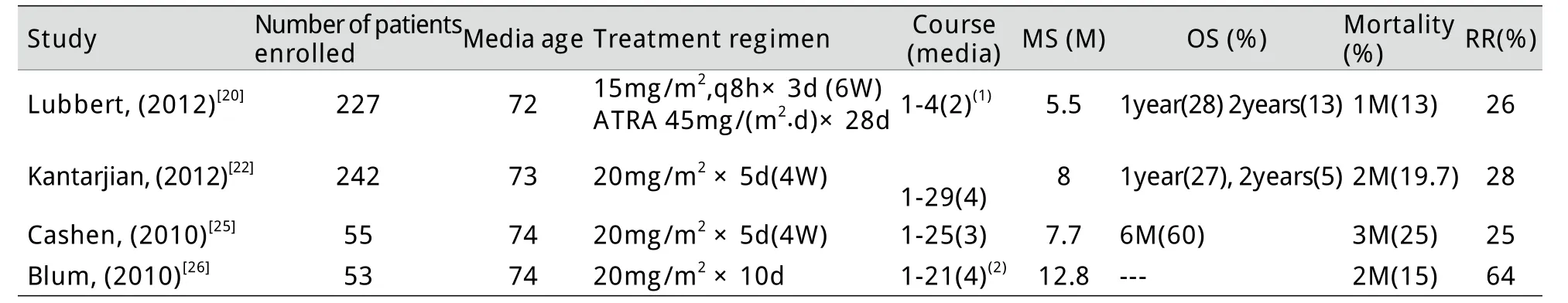
表3 地西他滨治疗老年AML用药方案及疗效总结Tab.3Dosage regimen and efficacy of decitabine in treatment of AML in elderly patients (≥60) with AML
总之,对于老年MDS转化的AML,超低剂量地西他滨联合CIK细胞输注是一种可供选择的安全有效的治疗方法,未来尚需积累更多病例加以验证。
[1]Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients[J]. J Clin Oncol, 2007, 25(14): 1908-1915.
[2]Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediateor high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group[J]. J Clin Oncol, 2011, 29(15): 1987-1996.
[3]Lübbert M, Rüter BH, Claus R, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy[J].Haematologica, 2012, 97(3): 393-401.
[4]Yang B, Lu XC, Yu RL, et al. Repeated transfusions of autologous cytokine-induced killer cells for treatment of haematological malignancies in elderly patients: a pilot clinical trial[J]. Hematol Oncol, 2012, 30(3): 115-122.
[5]Liu Y, Bao EN, Yang B, et al. Clinical study of autologous cytokine induced killer cell infusion treating for elderly patients,with myelodysplastic syndrome[J]. J Exp Hematol, 2011, 19(3):787-792.[刘洋, 包尔宁, 杨波, 等. 自体CIK细胞输注治疗老年骨髓增生异常综合征的临床研究[J]. 中国实验血液学杂志, 2011, 19(3): 787-792.]
[6]Wang Y, Bo J, Dai HR, et al. CIK cells from recurrent or refractory AML patients can be efficiently expanded in vitro and used for reduction of leukemic blasts in vivo[J]. Exp Hematol,2013, 41(3): 241-252.
[7]Lu XC, Yang B, Zhu HL, et al. Cytokine-induced killer plus IL-2for elderly patients with a hematological malignancy[J]. Med J Chin PLA, 2010, 35(10): 1270-1272. [卢学春, 杨波, 朱宏丽, 等. 自体细胞因子诱导的杀伤细胞联合IL-2治疗老年人血液系统恶性肿瘤的临床经验探讨[J]. 解放军医学杂志,2010, 35(10): 1270-1272.]
[8]Pievani A, Borleri G, Pende D, et al. Dual-functional capability of CD3+CD56+CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity[J]. Blood, 2011,118(12): 3301-3310.
[9]Pedersen-Bjergaard J, Ersb ll J, S rensen HM, et al. Risk of acute nonlymphocytic leukemia and preleukemia in patients treated with cyclophosphamide for non-Hodgkin's lymphomas.Comparison with results obtained in patients treated for Hodgkin's disease and ovarian carcinoma with other alkylating agents[J]. Ann Intern Med, 1985, 103(2): 195-200.
[10]Pagano L, Mele L, Casorelli I, et al. Acute lymphoblastic leukemia in the elderly. A twelve-year retrospective, single center study[J]. Haematologica, 2000, 85(12): 1327-1329.
[11]Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study[J]. Blood, 1997, 89(9): 3323-3329.
[12]Li TT, Sun XD, Dong Z, et al. Comparison of molecular biology,immunological characteristics and clinical efficacy in patients with acute myelogenous leukemia with or without FLT3-ITD gene mutation[J]. Med J Chin PLA, 2013, 38(6): 501-505.[李婷婷, 孙雪冬, 董征, 等. FLT3-ITD阳性与阴性急性髓细胞白血病的实验室特征及预后比较[J]. 解放军医学杂志, 2013, 38(6): 501-505.]
[13]Kantarjian H, O'brien S, Cortes J, et al. Results of intensive chemotherapy in 998patients age 65years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome:predictive prognostic models for outcome[J]. Cancer, 2006,106(5): 1090-1098.
[14]Miller KB, Kim K, Morrison FS, et al. The evaluation of low-dose cytarabine in the treatment of myelodysplastic syndromes: a phase-III intergroup study[J]. Ann Hematol, 1992, 65(4): 162-168.
[15]Büchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia:a study by the German Acute Myeloid Leukemia Cooperative Group[J]. J Clin Oncol, 2009, 27(1): 61-69.
[16]Ziogas DC, Voulgarelis M, Zintzaras E. A network meta-analysis of randomized controlled trials of induction treatments in acute myeloid leukemia in the elderly[J]. Clin Ther, 2011, 33(3): 254-279.
[17]Ma YY, Zhou SJ, Chen FY, et al. Induction effects of decitabine in combination with valproic acid sodium on apoptosis of myeloma cells and its underlying mechanism[J]. Med J Chin PLA, 2013,38(10): 837-841. [马泳泳, 周淑娟, 陈枫煜, 等. 俞康地西他滨联合丙戊酸钠诱导的骨髓瘤细胞凋亡及其机制研究[J]. 解放军医学杂志, 2013, 38(10): 837-841.]
[18]Lv HR, Deng Q, Li YM. Effect of Decitabine combined with atra on proliferation and differentiation of hl-60cells[J]. Tianjin Med J, 2013, 41(2): 154-157. [吕海容, 邓琦, 李玉明. 地西他滨联合全反式维甲酸对HL-60细胞增殖及分化的影响[J].天津医药, 2013, 41(2): 154-157.]
[19]Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter,randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia[J]. J Clin Oncol, 2012,30(21): 2670-2677.
[20]Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study[J]. Cancer, 2006, 106(8): 1794-1803.
[21]Bordoni RE, Feinberg BA, Gilmore JW, et al. Hematologic outcomes of myelodysplastic syndromes treatment with hypomethylating agents in community practice[J]. Clin Lymphoma Myeloma Leuk, 2011, 11(4): 350-354.
[22]Cashen AF, Schiller GJ, O'Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia[J]. J Clin Oncol, 2010, 28(4): 556-561.
[23]Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine[J]. Proc Natl Acad Sci USA, 2010, 107(16): 7473-7478.
[24]Wang LX, Mei ZY, Zhou JH, et al. Low dose decitabine treatment induces CD80expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses[J]. PLoS One, 2013,8(5): e62924.
[25]Turcan S, Fabius AW, Borodovsky A, et al. Efficient induction of differentiation and growth inhibition in IDH1mutant glioma cells by the DNMT Inhibitor Decitabine[J]. Oncotarget, 2013,4(10): 1729-1736.
[26]Tsai HC, Li H, Van Neste L, Cai Y, et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells[J]. Cancer Cell, 2012,21(3): 430-446.
[27]Weng DS, Zhou J, Zhou QM, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas[J]. J Immunother, 2008, 31(1): 63-71.
[28]Shi M, Zhang B, Tang ZR,et al. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma[J]. World J Gastroenterol,2004, 10(8): 1146-1151.
[29]M rten A, Ziske C, Sch ttker B, et al. Interactions between dendritic cells and cytokine-induced killer cells lead to an activation of both populations[J]. J Immunother, 2001, 24(6): 502-510.
