新型1,3,4-噁二唑衍生物的合成*
2012-05-09严云南陈江韩陈智勇潘文龙宋化灿
严云南,陈江韩,陈智勇,潘文龙,宋化灿
(1.中山大学化学与化学工程学院,广东 广州 510275;2.赣南医学院药学院,江西 赣州 341000;3.中国广州分析测试中心,广东 广州 510070)
1 引 言
咪唑衍生物广泛用于医药和农药[1-3]、光电材料[4-12]、不对称有机合成催化剂配体[13]、离子液体[14]、阴离子传感器[15]等领域。本课题组多年从事具有杂环结构的发光材料的设计合成和发光性能的研究[16-18],曾报道过一系列含有咪唑结构单元的大共轭体系的2,5-二芳基-1,3,4-噁二唑的合成[19]。但是该类化合物的溶解性能不佳,为改善其溶解性,从而改善在染料等方面的应用性能,增加烷基的长度,合成了系列含N-正癸基咪唑结构的2,5-二芳基-1,3,4-噁二唑。合成路线和目标产物的结构见图1。
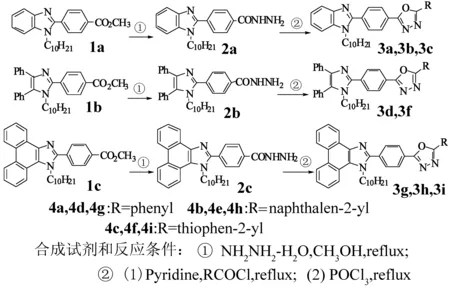
图1 1,3,4-噁二唑衍生物的合成路线
2 实验部分
2.1 仪器和试剂
温度计未校准,X4 熔点仪,Bruker AVANCE-300 NMR 核磁共振仪,SHIMADZU LCMS-2010A 质谱仪,Elementar Analysensysteme GmbH Vario EL元素分析仪。其余试剂均为分析纯,未经处理直接使用。
2.2 4-(2-(1-正癸基咪唑基))苯甲酸甲酯1a,1b,1c的合成
化合物1a,1b,1c参照文献[19]合成,其结构表征如下。
4-(2-(1-正癸基苯并咪唑基))苯甲酸甲酯(1a): 淡黄色,黏稠液体,产率75%。1H NMR (DMSO-d6)δ: 0.87 (t,J= 6.6 Hz,3H,CH3),1.20~1.30 (m,14H,CH2),1.77~1.83 (m,2H,CH2),3.96 (s,3H,OCH3),4.23 (t,J= 7.5 Hz,2H,NCH2),7.27~7.35 (m,2H,ArH),7.38~7.44 (m,1H,ArH),7.78~7.83 (m,3H,ArH),8.19 (d,J= 8.7 Hz,2H,ArH)。ESI-MS (m/z): 393 [M + H]+。
4-(2-(1-正癸基-4,5-二苯基咪唑基))苯甲酸甲酯(1b): 无色固体。产率81%,熔点81~82 ℃。1H NMR (DMSO-d6,δ: 0.83 (t,J= 6.9 Hz,3H,CH3),0.86~0.94 (m,6H,CH2),0.96~1.05 (m,2H,CH2),1.07~1.16 (m,4H,CH2),1.18~1.33 (m,4H,CH2),3.89 (s,3H,OCH3),3.94 (t,J= 7.2 Hz,2H,NCH2),7.09~7.22 (m,3H,ArH),7.38~7.48 (m,4H,ArH),7.50~7.57 (m,3H,ArH),7.91 (d,J= 8.1 Hz,2H,ArH),8.10 (d,J=8.4 Hz,2H,ArH)。ESI-MS (m/z): 495 [M + H]+。
4-(2-(1-正癸基菲并[9,10-d]咪唑基))苯甲酸甲酯(1c): 浅黄色固体,产率76%,熔点72~73 ℃。1H NMR (CDCl3)δ: 0.86 (t,J= 6.9 Hz,3H,CH3),1.15~1.34 (m,14H,CH2),1.87~2.00 (m,2H,CH2),3.98 (s,3H,OCH3),4.60 (t,J= 7.2 Hz,2H,NCH2),7.58~7.71 (m,4H,ArH),7.84 (d,J= 8.1 Hz,2H,ArH),8.19-8.25 (m,3H,ArH),8.69 (d,J= 8.1 Hz,1H,ArH),8.77 (d,J= 7.8 Hz,1H,ArH),8.84 (d,J= 7.8 Hz,1H,ArH)。 ESI-MS (m/z): 493 [M + H]+。
2.3 4-(2-(1-癸基咪唑基))苯甲酰肼2a,2b,2c的合成
化合物2a,2b,2c参照文献[19]合成,其结构表征如下。
4-(2-(1-正癸基苯并咪唑基))苯甲酰肼(2a): 无色固体,产率70%,熔点95~96℃。1H NMR (CDCl3)δ: 0.86 (t,J= 6.6 Hz,3H,CH3),1.12~1.29 (m,14H,CH2),1.70~1.84 (m,2H,CH2),4.18 (t,J= 6.9 Hz,2H,NCH2),4.72 (s,2H,NH2),7.29~7.35 (m,2H,ArH),7.38~7.44 (m,1H,ArH),7.63~7.70 (m,2H,ArH),7.83~7.88 (m,3H,ArH),8.42 (s,1H,NH)。ESI-MS (m/z): 393 [M + H]+。
4-(2-(1-正癸基-4,5-二苯基咪唑))苯甲酰肼(2b): 无色固体,产率82%,熔点111~112 ℃。1H NMR (DMSO-d6)δ: 0.83 (t,J= 6.9 Hz,3H,CH3),0.88~0.96 (m,6H,CH2),0.99~1.07 (m,2H,CH2),1.09~1.17 (m,4H,CH2),1.19~1.29 (m,4H,CH2),3.92 (t,J= 7.2 Hz,2H,NCH2),4.54 (s,2H,NH2),7.09~7.21 (m,3H,ArH),7.39~7.48 (m,4H,ArH),7.50~7.57 (m,3H,ArH),7.81 (d,J= 8.4 Hz,2H,ArH),7.98 (d,J= 8.1 Hz,2H,ArH),9.89 (s,1H,CONH)。ESI-MS (m/z): 495 [M + H]+。
4-(2-(1-正癸基菲并[9,10-d]咪唑-2))苯甲酰肼(2c): 无色针状晶体,产率78%,熔点197~198 ℃。1H NMR (CDCl3)δ: 0.87 (t,J= 6.6Hz,3H,CH3),1.11~1.32 (m,14H,CH2),1.88~1.99 (m,2H,CH2),4.20 (s,2H,NH2),4.59 (t,J= 7.5 Hz,2H,NCH2),7.61~7.74 (m,4H,ArH),7.81 (d,J= 8.4Hz,2H,ArH),7.94 (d,J= 8.4 Hz,2H,ArH),8.26 (d,J= 7.5 Hz,1H,ArH),8.72 (d,J= 7.8 Hz,1H,ArH),8.79 (dd,J1= 1.5 Hz,J2= 9.3 Hz,1H,ArH),8.86 (d,J= 7.8 Hz,1H,ArH)。 ESI-MS (m/z): 493 [M + H]+。
2.4 2,5-二芳基-1,3,4-噁二唑衍生物3a,3b,3c,3d,3e,3f,3g,3h,3i的合成
参照文献[19]完成合成,粗产物经柱层析后得到纯的目标物3a,3b,3c,3d,3e,3f,3g,3h,3i。
2-(4-(2(1-正癸基苯并咪唑基))苯基)-5-苯基-1,3,4-噁二唑(3a): 无色固体,产率45%,熔点78~80 ℃。1H NMR (CDCl3)δ: 0.85 (t,J= 6.6 Hz,3H,CH3),1.14~1.23 (m,14H,CH2),1.79~1.87 (m,2H,CH2),4.27 (t,J= 7.5 Hz,2H,NCH2),7.29~7.36 (m,2H,ArH),7.40~7.44 (m,1H,ArH),7.50~7.58 (m,3H,ArH),7.80~7.86 (m,1H,ArH),7.92 (d,J= 8.7 Hz,2H,ArH),8.14~8.17 (m,2H,ArH),8.31 (d,J= 8.7 Hz,2H,ArH)。13C NMR (CDCl3)δ: 14.0,22.5,26.6,28.9,29.1,29.3,29.3,29.7,31.7,44.8,110.0,119.9,122.4,122.9,123.4,124.6,126.7,126.9,128.8,129.7,131.6,133.6,135.5,142.8,151.8,163.6,164.5。元素分析(w/%): C31H34N4O·H2O,计算值: C,74.97; H,7.31; N,11.28;实测值: C,74.69; H,7.33; N 11.23。 ESI-MS (m/z): 479 [M + H]+。
2-(4-(2-(1-正癸基苯并咪唑基))苯基)-5-(2-萘基)-1,3,4-噁二唑(3b): 无色固体,产率47%,熔点102~104 ℃。1H NMR (CDCl3)δ: 0.85 (t,J= 6.6 Hz,3H,CH3),1.14~1.32 (m,14H,CH2),1.80~1.87 (m,2H,CH2),4.28 (t,J= 7.5 Hz,2H,NCH2),7.29~7.36 (m,2H,ArH),7.54~7.61 (m,3H,ArH),7.83~7.92 (m,3H,ArH),7.95~8.00 (m,3H,ArH),8.36 (d,J= 8.1 Hz,2H,ArH),8.65 (s,1H,ArH)。13C NMR (CDCl3)δ: 13.9,22.4,26.4,28.8,29.0,29.1,29.2,29.5,31.6,44.6,109.9,119.6,120.4,122.3,122.6,122.7,124.3,126.6,126.8,127.4,127.5,128.3,128.4,128.5,129.4,132.2,133.1,134.1,135.3,142.4,151.5,163.4,164.3。元素分析(w/%): C35H36N4O·0.21H2O,计算值: C,78.95; H,6.89; N,10.52;实测值: C,78.42; H,6.28; N 9.76。ESI-MS (m/z): 529 [M + H]+。
2-(4-(2-(1-正癸基苯并咪唑基))苯基)-5-(2-噻吩基)-1,3,4-噁二唑(3c): 黄色固体,产率40%,熔点94~95 ℃。1H NMR (CDCl3)δ: 0.85 (t,J= 6.6 Hz,3H,CH3),1.15~1.24 (m,14H,CH2),1.78~1.85 (m,2H,CH2),4.26 (t,J= 7.5Hz,2H,NCH2),7.18~7.21 (m,1H,ArH),7.28~7.36 (m,2H,ArH),7.41~7.44 (m,1H,ArH),7.59 (d,J= 4.8 Hz,1H,ArH),7.81~7.87 (m,2H,ArH),7.91 (d,J= 8.1Hz,2H,ArH),8.28 (d,J= 8.1 Hz,2H,ArH)。13C NMR (CDCl3)δ: 14.0,22.6,26.7,28.9,29.2,29.3,29.4,29.8,31.8,44.9,110.0,119.9,122.4,122.9,124.4,126.9,128.1,129.7,129.8,130.2,133.7,135.6,142.9,151.9,160.9,163.2。元素分析(w/%): C29H32N4OS,计算值: C,71.87; H,6.66; N,11.56;实测值: C,71.60;H,6.50; N 11.52。 ESI-MS (m/z): 485 [M + H]+。
2-(4-(2-(1-正癸基-4,5-二苯基咪唑基))苯基)-5-苯基-1,3,4-噁二唑(3d): 无色固体,产率39%,熔点96~98 ℃。1H NMR (DMSO-d6)δ: 0.75 (t,J= 6.9 Hz,3H,CH3),0.81~1.17 (m,14H,CH2),1.22~1.36 (m,2H,CH2),3.98 (t,J= 6.9 Hz,2H,NCH2),7.10~7.23 (m,3H,ArH),7.41~7.49 (m,4H,ArH),7.51~7.58 (m,3H,ArH),7.60~7.67 (m,3H,ArH),8.02 (d,J= 8.4 Hz,2H,ArH),8.12~8.19 (m,2H,ArH),8.29 (d,J= 8.4 Hz,2H,ArH)。13C NMR (CDCl3)δ: 14.1,22.6,26.1,28.5,29.0,29.1,29.3,30.3,31.8,44.9,123.6,126.2,126.6,126.7,126.9,127.9,128.6,128.9,129.3,130.4,130.8,131.0,131.6,134.2,134.5,138.1,146.0,163.9,164.4。 ESI-MS (m/z): 581 [M + H]+。
2-(4-(2-(1-正癸基-4,5-二苯基咪唑基))苯基)-5-(2-萘基)-1,3,4-噁二唑(3e): 无色固体,产率43%,熔点133~134 ℃。1H NMR (DMSO-d6)δ: 0.73 (t,J= 6.6 Hz,3H,CH3),0.83~1.20 (m,14H,CH2),1.24~1.36 (m,2H,CH2),3.98 (t,J= 7.2 Hz,2H,NCH2),7.10~7.23 (m,3H,ArH),7.42~7.50 (m,4H,ArH),7.52~7.59 (m,3H,ArH),7.62~7.70 (m,2H,ArH),8.04 (d,J= 8.4 Hz,3H,ArH),8.14~8.23 (m,3H,ArH),8.34 (d,J= 8.4 Hz,2H,ArH),8.80 (s,1H,ArH)。13C NMR (CDCl3)δ: 14.2,22.7,26.2,28.7,29.2,29.3,29.4,30.5,31.9,45.0,120.9,123.1,123.8,126.3,126.7,127.1,127.2,127.9,128.0,128.7,128.9,129.4,130.4,130.9,131.1,132.7,134.2,134.6,138.2,146.1,164.1,164.8。 ESI-MS (m/z): 631 [M + H]+。
2-(4-(2-(1-正癸基-4,5-二苯基咪唑基))苯基)-5-(2-噻吩基)-1,3,4-噁二唑(3f): 无色固体,产率40%,熔点106~108 ℃。1H NMR (DMSO-d6)δ: 0.76 (t,J= 6.6 Hz,3H,CH3),0.83~1.22 (m,10H,CH2),1.22~1.36 (m,2H,CH2),3.97 (t,J= 7.2 Hz,2H,NCH2),7.09~7.23 (m,3H,ArH),7.32~7.35 (m,1H,ArH),7.40~7.50 (m,4H,ArH),7.51~7.59 (m,3H,ArH),7.96~8.01 (m,4H,ArH),8.24 (d,J= 8.4 Hz,2H,ArH)。13C NMR (CDCl3)δ: 14.1,22.6,26.1,28.6,29.1,29.2,29.3,30.4,31.8,44.9,123.4,124.9,126.2,126.6,126.9,127.9,128.0,128.6,128.9,129.3,129.7,130.1,130.4,130.8,131.0,134.2,134.6,138.2,146.0,160.8,163.4。 ESI-MS (m/z): 587 [M + H]+。
2-(4-(2-(1-正癸基菲并[9,10-d]咪唑基))苯基)-5-苯基-1,3,4-噁二唑(3g): 浅黄色固体,产率36%,熔点160~162 ℃。1H NMR (CDCl3)δ: 0.82 (t,J= 6.6 Hz,3H,CH3),1.07~1.30 (m,14H,CH2),1.86~2.03 (m,2H,CH2),4.69 (t,J= 6.9 Hz,2H,NCH2),7.49~7.74 (m,7H,ArH),8.00 (d,J= 7.5 Hz,2H,ArH),8.10~8.20 (m,2H,ArH),8.27 (d,J= 7.5 Hz,1H,ArH),8.32 (d,J= 7.5 Hz,2H,ArH),8.67 (d,J= 7.8 Hz,1H,ArH),8.81 (t,J= 7.2 Hz,2H,ArH)。13C NMR (CDCl3)δ: 14.1,22.6,26.2,28.8,29.2,29.3,29.4,30.1,31.8,47.3,120.8,122.7,122.8,123.6,124.3,124.7,125.1,125.8,126.2,126.8,126.9,127.3,128.1,128.9,129.2,130.6,131.7,150.4,163.8,164.6。ESI-MS(m/z): 579 [M + H]+。
2-(4-(2-(1-正癸基菲并[9,10-d]咪唑基))苯基)-5-(2-萘基)-1,3,4-噁二唑(3h): 黄色固体,产率30%,熔点151~153 ℃。1H NMR (CDCl3)δ: 0.83 (t,J= 6.6 Hz,3H,CH3),1.17~1.30 (m,14H,CH2),1.87~2.06 (m,2H,CH2),4.73 (t,J= 7.2 Hz,2H,NCH2),7.56~7.74 (m,6H,ArH),7.87~7.94 (m,1H,ArH),7.98~8.05 (m,4H,ArH),8.23 (d,J=7.8,1H,ArH),8.30 (d,J= 7.5 Hz,1H,ArH),8.39 (d,J= 8.4 Hz,2H,ArH),8.61~8.71 (m,2H,ArH),8.83 (d,J= 8.1 Hz,1H,ArH),8.87 (d,J= 8.4 Hz,1H,ArH)。13C NMR (CDCl3)δ: 14.1,22.6,26.2,28.8,29.2,29.3,29.4,30.1,31.8,47.4,120.7,120.8,122.7,122.8,122.9,124.2,124.7,125.2,125.9,126.1,126.8,126.9,127.2,127.3,127.7,127.8,128.1,128.6,128.8,129.2,130.7,132.6,134.5,150.2,163.6,164.7。 ESI-MS (m/z): 629 [M + H]+。
2-(4-(2-(1-正癸基菲并[9,10-d]咪唑基)苯基)-5-(2-噻吩基)-1,3,4-噁二唑(3i): 黄色固体,产率32%,熔点118~119 ℃。1H NMR (CDCl3)δ: 0.79 (t,J= 6.6 Hz,3H,CH3),1.02~1.07 (m,14H,CH2),1.83 (m,2H,CH2),4.95 (t,J= 7.2 Hz,2H,NCH2),7.20 (t,J= 7.8 Hz,1H,ArH),7.39 (m,1H,ArH),7.47 (t,J= 7.8 Hz,1H,ArH),7.59~7.75 (m,4H,ArH),8.10 (d,J= 7.8 Hz,2H,ArH),8.23 (d,J= 8.1 Hz,1H,ArH),8.31 (t,J= 8.4 Hz,3H,ArH),8.50 (d,J= 7.8 Hz,1H,ArH),8.91 (d,J=8.1 Hz,1H,ArH)。13C NMR (CDCl3)δ: 13.9,22.4,25.8,28.4,29.0,29.1,29.2,29.3,31.6,48.5,120.7,120.8,121.6,122.4,124.1,124.3,124.7,124.9,126.6,126.8,127.4,127.5,128.0,128.1,128.3,129.4,130.2,130.8,131.6,147.0,160.8,162.0。 ESI-MS (m/z): 585 [M + H]+。
3 结果与讨论
众所周知,咪唑衍生物的溶解性往往较差,尤其是具有大共轭体系的咪唑化合物,如本文中的4-(2-咪唑基)苯甲酸甲酯衍生物,其溶解性更差,往往不溶于一般的有机溶剂,使其在合成应用方面受到极大的限制。因此,本合成研究的首要工作是实现4-(2-咪唑基)苯甲酸甲酯衍生物的癸基化,也是本合成工作的关键所在。为得到目标物,采用了前期报道的w=50% K2CO3-丁酮-TBAB体系[19]。该体系对4-(2-咪唑基)苯甲酸甲酯衍生物的长链烷基化具有显著的优势,产率为75%以上,溶剂可循环使用,产物的提纯较为简便,适合批量生产。
参考文献:
[1]PESQUET A,DAIECH A, VAN H.General and versatile entry to 4,5-fused polycyclic imidazolones systems.Use of the tandem transposition/π-cyclization of N-acyliminium species [J].Journal of Organic Chemistry,2006,71(14): 5303-5311.
[2]SHI W,QIAN X,ZHANG R,et al.Synthesis and quantitative structure-activity relationships of new 2,5-disubstituted-1,3,4-oxadiazoles [J].Journal of Agricultural and Food Chemistry,2001,49: 124-130.
[3]CHEN H,LI Z,HAN Y.Synthesis and fungicidal activity against rhizoctonia solani of 2-alkyl (alkylthio) -5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles) [J].Journal of Agricultural and Food Chemistry,2000,48: 5312-5315.
[4]BELLINA F,CAUTERUCCIO S,ROSSI R.Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles [J].Tetrahedron,2007,63: 4571-4624.
[5]PARK S,KWON O,KIM S,et al.Imidazole-based excited-state intramolecular proton-transfer materials: synthesis and amplified spontaneous emission from a large single crystal [J].Journal of the American Chemical Society,2005,127: 10070-10074.
[6]SUN Y F,CUI Y P.The synthesis,structure and spectroscopic properties of novel oxazolone-,pyrazolone- and pyrazoline-containing heterocycle chromophores [J].Dyes and Pigments,2009,81: 27-34.
[7]LI H L,KANG S S,XING Z T,et al.The synthesis,optical properties and x-ray crystal structure of novel 1,3,4-oxadiazole derivatives carrying a thiophene unit [J].Dyes and Pigments,2009,80: 163-167.
[8]YEH K M,LEE C C,CHEN Y.Host copolymers containing pendant carbazole and oxadiazole groups: synthesis,characterization and optoelectronic applications for efficient green phosphorescent OLEDs [J].Journal of Polymer Science(Part A): Polymer Chemistry,2008,46(15): 5180-5193.
[9]FROEHLICH J D,YOUNG R,NAKAMURA T,et al.Synthesis of Multi-Functional POSS Emitters for OLED Applications [J].Chemistry of Materials,2007,19(20): 4991-4997.
[10]AHN J H,WANG C S,PEREPICHKA I F,et al.Blue organic light emitting devices with improved color purity and efficiency through blending of poly(9,9-dioctyl-2,7-fluorene) with an electron transporting material [J].Journal of Materials Chemistry,2007,17(29): 2996-3001.
[11]LEE J H,TSAI H H,LEUNG M K,et al.Phosphorescent organic light-emitting device with an ambipolar oxadiazole host [J].Applied Physics Letters,2007,90(24): 243501-243503.
[12]LEUNG M K,YANG C C,LEE J H,et al.The Unusual electrochemical and photophysical behavior of 2,2'-bis(1,3,4-oxadiazol-2-yl)biphenyls,effective electron transport hosts for phosphorescent organic light emitting diodes[J].Organic Letters,2007,9(2): 235-238.
[13]ZHOU Y,WANG W H,DOU W,et al.Synthesis of a new C2-symmetric chiral catalyst and its application in the catalytic asymmetric borane reduction of prochiral ketones [J].Chirality,2008,20(2): 110-114.
[14]PETER W,WILHELM K.Ionic liquids-new "solutions" for transition metal catalysis [J].Angewandte Chemie,International Edition,2000,39(21): 3772-3789.
[15]ZHAO Q,LIU S J,SHI M,et al.Tuning photophysical and electrochemical properties of cationic iridium(Ⅲ) complex salts with imidazolyl substituents by proton and anions [J].Organometallics,2007,26: 5922-5930.
[16]PAN W L,SONG J G,YU W J,et al.One-step synthesis of 2-benzimidazolyl alkene under microwave irradiation[J].Acta Scientiarum Naturalium Universitis Sunyatseni,2006,45(6): 58-61.
[17]PAN W L,TAN H B,CHEN Y,et al.The synthesis and preliminary optical study of 1-alkyl-2,4,5-triphenyl- imidazole derivatives [J].Dyes and Pigments,2008,76: 17-23.
[18]YAN Y N,LIN D Y,PAN W L,et al.Synthesis and optical behaviors of 2-(9-phenanthrenyl)-,2-(9-anthryl)-,and 2-(1-pyrenyl)-1-alkylimidazole homologues [J].Spectrochimica Acta: Part A,2009,74: 233-242.
[19]YAN Y N,PAN W L,SONG H C.Synthesis and preliminary optical study of novel 1,3,4-oxadiazole derivatives containing imidazole unit [J].Dyes and Pigments,2010,86: 249-258.
