Characterization of Coal Tar Pitch and Paving Pitch by UV,EA and NMR
2011-04-09XIAOYanhuaFENGRuijieCAOSumeiLIUXiangyongGAOTingPANZhiquan
XIAO Yan-hua,FENG Rui-jie,CAO Su-mei,LIU Xiang-yong,GAO Ting,PAN Zhi-quan
(1.School of Chemical Engineering & Pharmacy,Wuhan Institute of Technology,Key Laboratory for Green Chemical Process of Ministry of Education, Hubei Key Laboratory of Novel Reactor & Green Chemical Technology,Wuhan 430073,China; 2.Wuhan Pingmei Wugang Unite Coking Chemical Corp.Ltd,Wuhan Iron & Steel Group Co.,Wuhan 430081, China)
At the present rate,availability production of coal is expected to last over 215 years,in contrast with the reserves of petroleum and gas,whose life expectancy has been estimated to be 40 and 60 years respectively[1].15 million tons of CTP,which is the main byproduct generated in the coal carbonization process,are produced every year.CTPs are the liquid products made from the coal in the process of dry distillation and gasification,and are complex combinations of rocyclic oxygen,polycyclic aromatic hydrocarbons(PAH's),nitrogen compounds and sulphur[2-4].CTP is an increasingly important commodity with high value such as binders for copolymers,electrodes for aluminum processing and steel manufacture,super capacitors for fuel cells,and in the production of carbon materials[5-6].In the world,demand for CTP has been outstripping supply in recent years.
As the saying goes “want to be rich,build the way”,transportation plays a very important role in the development of the Chinese economy.Now the demand to the quality and the quantity of PP was increasing day and day in order to construct more and more highway.For example in China,from“12th Five-Year” Plan to the 2015,35 thousand kilometers highway will be constructed.Especially in this financial crisis,the Chinese government plans to invest much in construction of infrastructural facilities,among them including building freeway[7-9].Now the mainly PP is the petroleum asphaltenes.As presented above,it is very important to enlarge the application of CTP into PP as an alternative resource to supply the insufficiency of petroleum asphalt.Before using the CTP as PP,differences between them must be clear so the methods can be chosen to change some characterization of CTP to satisfy the need of PP.
CTP has its advantages:good moist and adhesion,fighting against the erosion of oil,having an extremely high coefficient of friction.On the counterpart,it has some disadvantages,for example its thermal sensitivity is inferior so it is easy to be soft in summer and easy to be brittle rupture in winter,easy to degrade,possess poor ductility.These weaknesses should be got over before it would be used as PP.At all,freeway should be in a good condition in different seasons for a long time.So the characterization of CTP was studied to discuss the difference from that of PP before it was applied in paving road.
At present,many researches were focused on the components to identify the chemicals by standard analytical techniques such as gas chromatography(GC),size-exclusion chromatography(SEC),matrix-assisted laser desorption/ionization mass spectrometry(MALDI-MS) and other analytical methods.Though these researches,parts of the chemicals were known,but more complex hydrocarbons have led to debate and to a level of uncertainty in Refs.[10-20].No single method is unambiguously capable of indicating molecular mass distributions or chemical structural features in complex fuel-derived mixtures[11].At the same time,the CTP was used as an entirety.It is constructed by complex mixtures,so the study on the integral nature occupies a more important position than chemicals’ study in this respect.In this study,CTP and PP were identified as a whole through detection of their physical nature,UV-absorption(UV-A),elemental analyses(EA) and nuclear magnetic resonance(NMR) were used to explain the diversities of characterization between CTP and PP,and fractionation allows the less abundant high mass fractions to be observed more clearly.At this point,the research is different from the other report.
1 Material and methods
1.1 Instruments
UV was obtained on a Lambda 35 spectrometer,the solvents were methanol.NMR spectra analyses was recorded on a Bruker Advance 400 spectrometer with TMS as internal standard,the solvents were chloroform.Elemental analyses were obtained on a Vario Micro cube(Elementar Co.,Germany).Needle penetration was tested on JNLY-97A(Wuxi Jinniu Equipment Co.,Ltd).Ductility was detected on JN1005A of extensometer(Wuxi Jinniu Equipment Co.,Ltd).Emulsification was obtained on a high shearing machine FT800(FLUKO Equipment Shanghai Co.,Ltd.).All solvents were purchased with analytical reagent from Wuhan Lieran Technology Co.,Ltd.
1.2 Pitch material
The CTP was collected in November 2008 from Wuhan Pingmei Wugang Unite Coking Chemical Corp.Ltd.of Wuhan Iron & Steel Group Corp.(WISCO),Hubei Province of China.The CTP,whose medium temperature asphalt(softening point 65—90 ℃),was collected by engineer Su-mei Cao at Wuhan Pingmei Wugang Unite Coking Chemical Corp.Ltd. of WISCO.A voucher specimen(WG-37) was deposited at Wuhan Institute of Technology.WG-70 PP was supported by Engineer Su-mei Cao.
1.3 The physical nature of CTP and PP
In order to get the macroscopical physical properties,several physical natures were detected through different tools(shown in Table 1).
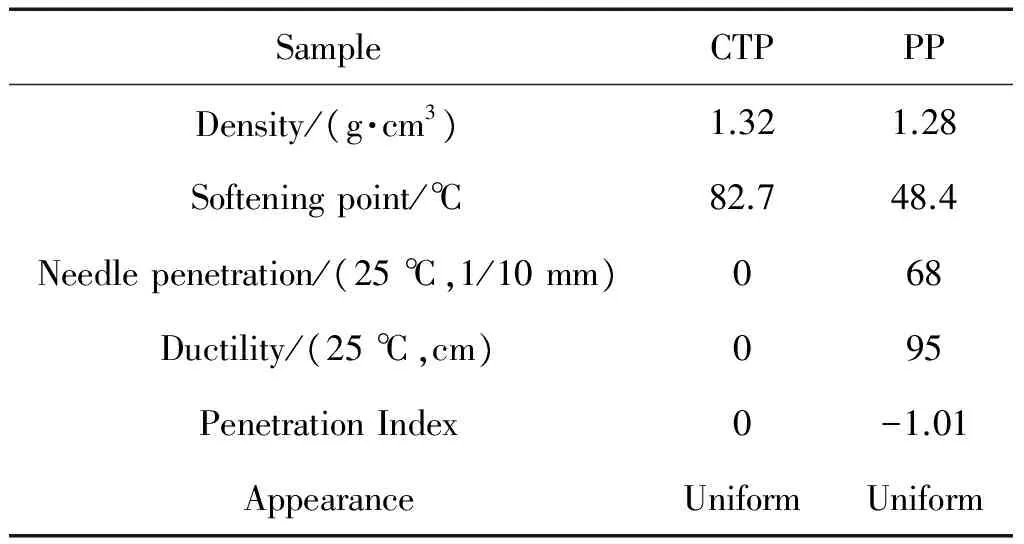
Table 1 Properties of CTP and PP
It was shown in Table 1 the properties of CTP and PP were very different.The density and appearance of CTP were almost the same as that of PP.The higher softening point of CTP than that of PP could make it difficult to be used in building freeway at relative low temperature.At the same time,the lower needle penetration and ductility of CTP than that of PP showed CTP was hot short.The road surface was easy cracking and raveling when CTP was used in building freeway.
1.4 Solvent fractionation
The pitches consist of the solid residue obtained after distillation of the tars from the pyrolysis of coal in a coke oven.The present sample was a “medium temperature” pitch,containing some light components(anthracene oil fraction),such as phenanthrene,and has been extensively investigated[21-23].In this paper,the pitch was fractionated by solvent-solubility in Soxhlet apparatus.Three fractions were produced:the pitchn-heptane soluble(PHS) fraction,the pitch toluene soluble-heptane insoluble(PTS) fraction,and the pitch ethanol soluble(PES) fraction,with each fraction making up different percent of the whole.The characterization of the asphaltenes was aided by separating each into three sub-fractions using Soxhlet extraction.These fractions fell into three groups: lighter oil,low-and high-aromaticity groups.The fractions of pitch materials follow the method procedures described below.
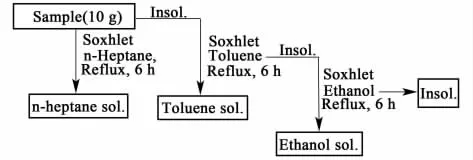
Fig.1 Separation process of pitch
The separation method of asphaltenes is described in Figure 1.The medium temperature CTP in the process of dry distillation product pitch(10 g) was pulverized and mixed with zeolite,enwrapped in non-woven fabrics,then extracted withn-heptane(600 mL) for 6 h in Soxhlet apparatus,followed by changing round bottom flask to one of same volume with toluene(600 mL).The residue was extracted sequencely with toluene and ethanol at the same condition.Then the solutions were evaporated under reduced pressure to give three fractions.
2 Results and discussion
2.1 Weight and ratio of different solvent fractions
Solvent-solubility separation of the CTP(10 g) involvedn-heptane,toluene and ethanol was carried out step by step in a Soxhlet apparatus,different from the separation method reported in Ref.[21].n-Heptane,toluene and ethanol were evaporated under reduced pressure to give 3.76 g(PHS-1),3.33 g(PTS-1) and 0.17 g(PES-1) residue, respectively.Portions of the CTP were isolated as PHS-1,PTS-1 and PES-1(38%,33% and 1.7%,respectively,by weight).The ethanol dissolvable residue occupied 27.4%. With the same method,WG-70 PP(PP) was separated by n-heptane,toluene and methanol in a Soxhlet apparatus continuously.Correspondingly,the three portions have 4.62 g(PHS-2),4.90 g(PTS-2),0.48 g(PES-2),a further 11% was observed to be soluble in heptane; the heptane-insoluble fraction was 50.0% of the crude.Samples PHS-2,PTS-2,and PES-2 were 46.2%,49.0%,and 4.8%,respectively.No chemical was determined to be ethanol insoluble.
2.2 Results of UV
It is known that the polyaromatic cores of the asphaltenes are responsible for the absorbance in the UV-vis region.Clearly,the UV-absorption(UV-A) detector wasn’t sensitive to purely aliphatic material,although PP was known to contain material with a more aromatic character than lighter fractions.UV-A detectors was operated at low sample concentrations to avoid self-absorption.The shift between CTP and PP was small, showing that the aromatic systems were similar in the CTP and PP.
There are some differences still between CTP fractions that we have used and PP fractions that we have examined. The UV-vis(230—450 nm) absorption spectra of CTP and PP were shown in Table 2.The spectra of the two types of asphaltenes have been arbitrarily scaled to have similar absorbance in the long-wave length region(i>450 nm).Such scaling also leads to a crossing of the two spectra(i.e.,a coincidence in absorbance) at 290 nm.
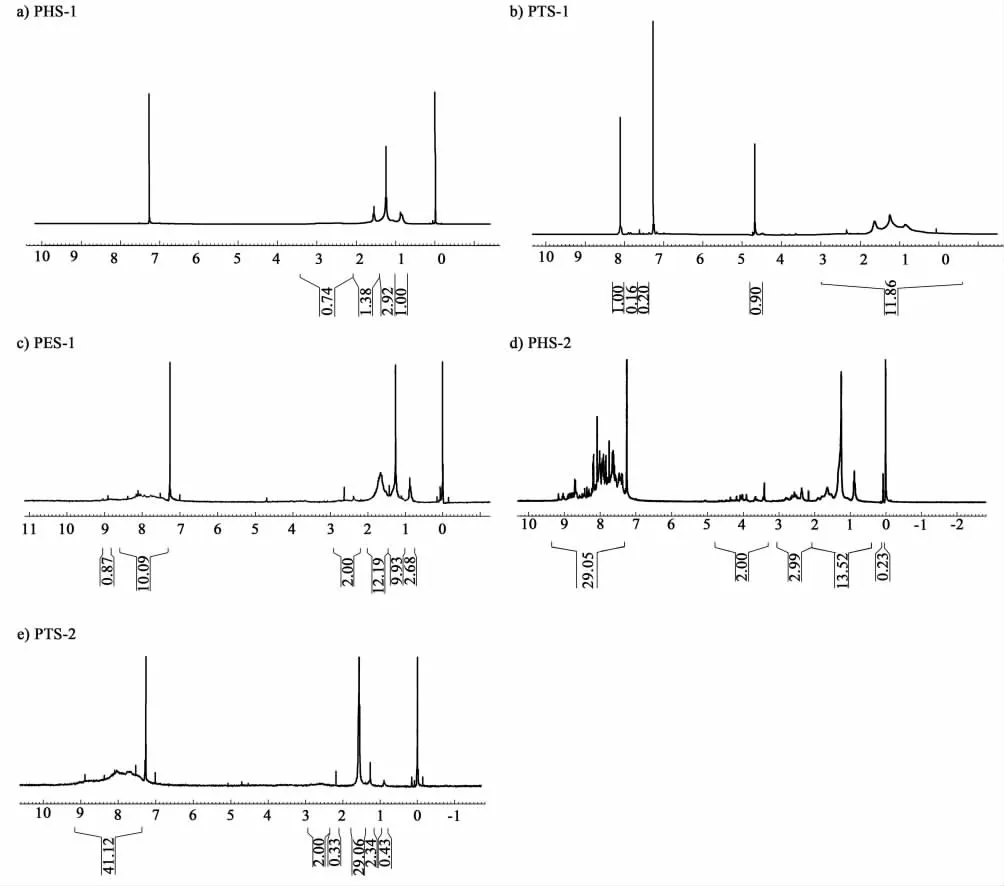
2.3 1 H-NMR results
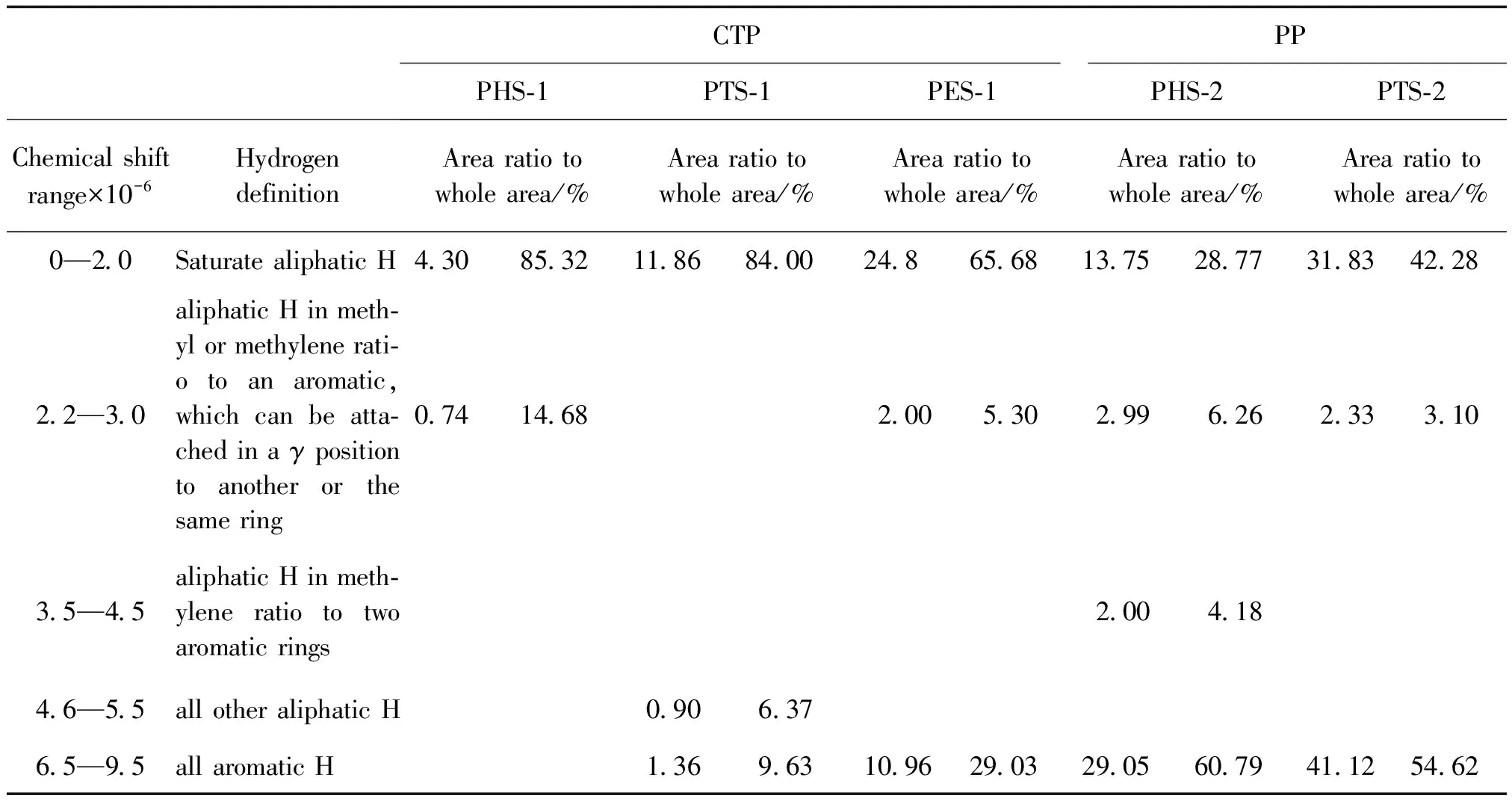
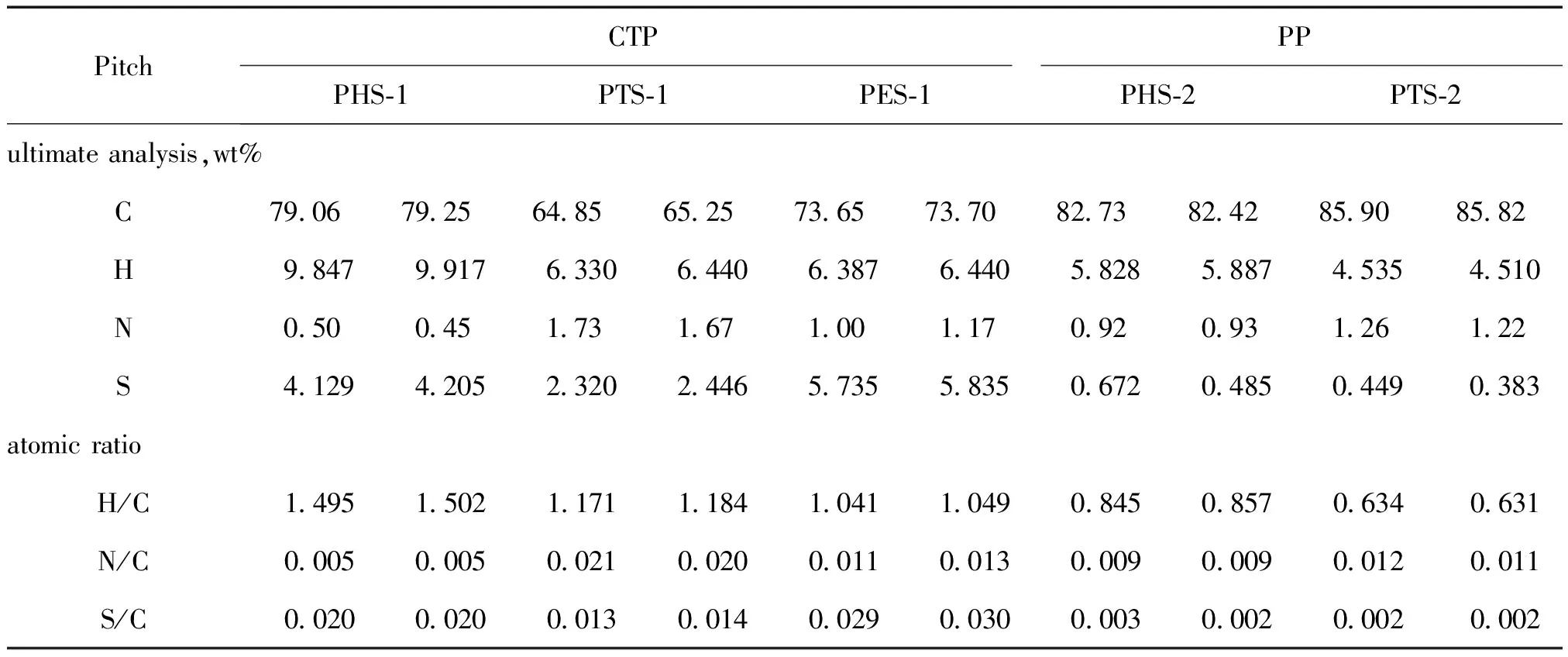
Firstly compare the different parts of the same sample in Table 3 and combining with different proportion of PHS-1(3.76 g,38%),PTS-1(3.33 g,33%),PES-1(0.17 g,1.7%),PHS-2(4.62 g,46%),PTS-2 (4.90 g,49%),PES-2(0.48 g,4.8%)(Shown in Figure 2).The portion of aromatic rings was found to steadily increase in the following order:PHS Table 2 UV-A of several pitch samples Table 3 Chemical shifts classifications and assignments of1 H-NMR of several pitch samples using CDCl3 as the solvent Comparing the counterparts of CTP and PP in Table 4,it was found that in PHS-1 and PHS-2 the A of aromatic H was 0 and 28.1.From this result,it suggested there were aromatic rings in the light part of PP,but in the CTP there was no aromatic ring or couldn’t be detected because the amount was too small.As well as the PTS-1 and PTS-2,it suggested that continental type structures in PTS-2 were greater than those in PTS-1.It was found that for PHS-1 and PHS-2,the1H NMR results suggested there were more portions of saturate aliphatic H in CTP than that of PP,and the experimental value of 4.69×10-6in PHS-2 showed there are double bond existed in aliphatic styles,in this point PP is very different from CTP. The results of EA for different parts of the same sample are listed in table 5. The atomic ratio of H/C was 1.50,1.17,and 1.04 for the PHS-1,PTS-1 and PES-1 in CTP and was 0.85 and 0.63 in PP for PHS-2 and PTS-2. From these data,the atomic ratio of H/C was found to steadily increase in the following order:PHS>PTS>PES whether in CTP or in PP.If we com-pared the counterparts of CTP and PP,it was found the atomic ratio of H/C was increased in the following order:PHS-1>PHS-2 and PTS-1>PTS-2.The atomic ratio of H/C was the most important parameter in decision the aromatized situation in CTP or PP.Taken together,the EA data imply that the aromatized structures in the PP is greater than that in the CTP.The conclusion was the same as the interpretations from the detection results of1H NMR. Fig.2 1H-NMR spectra Chemicalshiftrange/×10-6HydrogendefinitionCTPPPPHS⁃1PTS⁃1PES⁃1PHS⁃2PTS⁃20—20SaturatealiphaticH3242771113320722—30aliphaticHinmethylormethyleneratiotoanaromat⁃ic,whichcanbeattachedinaγpositiontoanotherorthesamering56009291535—45aliphaticHinmethyleneratiototwoaromaticrings1946—55allotheraliphaticH2165—95allaromaticH3205281268 Table 5 EA of asphaltene sub-fractions There are significant differences between the CTP fractions that we have used and PP that we have examined. First,the relative proportions of high-mass material in the PP samples were considerably smaller. The ethanol-insoluble fraction of the CTP was 27.4%,whereas the ethanol-insoluble fractions of the PP residues was almost zero.Second,the data showed aromatic degree of PP was higher than that of CTP.These two results seemed to contradict to each other.We considered in CTP chemical were existed in two extreme ways,either aliphatic chemicals or high polycondensed arrangements,but in PP the situation was different.The molecules in PP are highly aromatized than that of CTP in smaller size on average.A large portion of saturate aliphatic in CTP but a relative large proportion of polycyclic aromatic structures resulted that CTP is easy to be crisp in cold winter and is easy to be soft in summer.The following research will focus on how to change the chemical construction into the relative similar structures with PP in order that a large amount of CTP produced annual could be used as PP,such as adding some light oil tar or polyer.Among the two solutions,the latter attracted much attention because many polyers can play the role as hydrogen donor to realize hydrogen accepting modification of CTP,such as rubber,plastic and resin,etc.From the consideration of environmental protection,now our researchers pay much attention to plastics(known as white pollution). [1]SCHOBERT H H,SONG C.Chemicals and materials from coals in the 21st century[J].Fuel,2002,81:15-32 [2]BLANCO C G,CANGA J S,DOMNGUEZ A,etal.Flame ionization detection relative response factors of some polycyclic aromatic compounds:Determination of the main components of the coal tar pitch volatile fraction[J].Journal of Chromatography,1992,607:295-302 [3]GUILLEN M D,IGLESIAS M J,DOMNGUEZ A,etal.Polynuclear aromatic hydrocarbon retention indices on SE-54 stationary phase of the volatile components of a coal tar pitch:Relationships between chromatographic retention and thermal reactivity[J].Journal of Chromatography,1992,591:287-295 [4]NOEL L,AUTHIER-MARTIN M,PATRY G,etal.Quantitative analysis of PAH in pitch by HPLC with fluorescence detector[J].Polycyclic Aromatic Compounds,1996,9:373-380 [5]WANG Y,LI H.Review of the usage of coal tar pitch[J].Coal chemical industry,2000,94:13-34 [6]TURNER N R.Recent trends in binder pitches for reduction anodes[J].Jom-Journal of the Minerals Metals & Materials Society,1993,45:39-42 [7]WANG X.Who will benefit from the five trillion investments on traffic and transportation[J].Modern Enterprise Education,2008,11:40-42 [8]HOU X.The things of reform and opening-up among 30 years[J].China Traffic Construction Engineering Consultants,2008,10:68-71 [9]DING W.Application and development tendency of testing technology to road[J].China Highway,2007,10:69-73 [10]KARACA F,MORGAN T J,GEORGE A,etal.Molecular mass ranges of coal tar pitch fractions by mass spectrometry and size-exclusion chromatography[J].Rapid Communications in Mass Spectrometry,2009,23:2 087-2 098 [11]MORGAN T J,GEORGE A,DAVIS D B,etal.On the limitations of UV-fluorescence spectroscopy in the detection of high-mass hydrocarbon molecules[J].Energy & Fuels,2005,19:164-165 [12]HEROD A A,KANDIYOTI R,BARTLE K D.Characterization of heavy hydrocarbons by chromatographic and mass spectrometric methods:An overview[J].Energy & Fuels,2007,21:2 176-2 203 [13]BADRE S,GONCALVES C C,NORINAGE K,etal.Molecular size and weight of asphaltene and asphaltene solubility fractions from coals,crude oils and bitumen[J].Fuel,2006,85:1-11 [14]MULLINS O C.Rebuttal to comment by professors Herod,Kandiyoti,and Bartle on “Molecular size and weight of asphaltene and asphaltene solubility fractions from coals,crude oils and bitumen[J].Fuel,2007,86:309 [15]TANAKA R,SATO S,TAKANOHASHI T,etal.Analysis of the molecular weight distribution of petroleum asphaltenes using laser desorption-mass spectrometry[J].Energy & Fuels,2004,18:1 405-1 413 [16]ACEVEDO S,GUTIERREZ L B,NEGRIN G,etal.Molecular weight of petroleum asphaltenes:A comparison between mass spectrometry and vapor pressure osmometry[J].Energy & Fuels,2005,19:1 548-1 560 [17]BECKER C,QIAN K N,RUSSELL D H.Molecular weight distributions of asphaltenes and deasphaltened oils studied bylaser desorption ionization and ion mobility mass spectrometry[J].Analytical Chemistry,2008,80:8 592-8 597 [18]MORGAN T J,GEORGE A,DAVIS D B,etal.Optimization of 1H and 13C NMR methods for structural characterization of acetone and pyridine soluble/insoluble fractions of a coal tar pitch[J].Energy & Fuels,2008,22:1 824-1 835 [19]SEKI H, KUMATA F.Structural change of petroleum asphaltenes and resins by hydrodemetallization[J].Energy & Fuels,2000,14:980-985 [20]ISLAS C A,SUELVES I,MILLAN M,etal.Matching average masses of pitch fractions of narrow polydispersity,derived from matrix-assisted laser desorption ionisation time-of-flight mass spectrometry with the polystyrene calibration of SEC[J].Journal of Separation Science,2003,26:1 422-1 428 [21]HEROD A A,MILLAN M,MORGAN T J,etal.Positive-ion electrospray ionisation mass spectrometry of acetone- and acetonitrile-soluble fractions of coal-derived liquids[J].European Journal of Mass Spectrometry,2005,11:429-442 [22]LAZAROIAN M J,HEROD A A,KANDIYOTI R.Comparison of the fractionation of a coal tar pitch by solvent solubility and by planar chromatography[J].Fuel,1999,78:795-801 [23]HEROD A A,LAZARO M J,SUELVES I,etal.Size exclusion chromatography of soots and coal-derived materials with 1-methyl-2-pyrrolidinone as eluent:Observations on high molecular mass material[J].Energy & Fuels,2000,14:1 009-1 020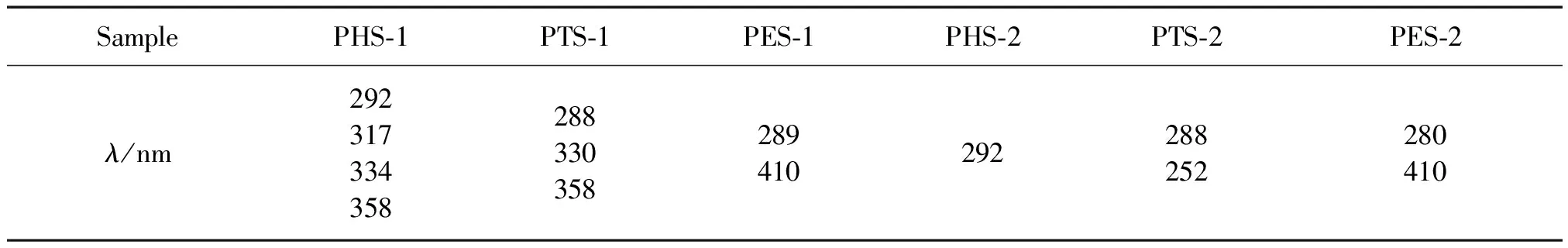
2.4 Elemental analysis
3 Conclusion
