镍掺杂花状纳米碳片的制备及其在超级电容器中的应用
2011-11-10易观贵谢春林莫珊珊贺文启率刘应亮袁定胜黄浪欢谭绍早
易观贵 肖 勇 谢春林 莫珊珊 贺文启 赵 率刘应亮 袁定胜 黄浪欢 谭绍早
(暨南大学化学系,纳米化学研究所,广州 510632)
镍掺杂花状纳米碳片的制备及其在超级电容器中的应用
易观贵 肖 勇 谢春林 莫珊珊 贺文启 赵 率刘应亮*袁定胜 黄浪欢 谭绍早
(暨南大学化学系,纳米化学研究所,广州 510632)
本文通过溶剂热法“一锅”制备了镍掺杂的花状纳米碳片(Ni/FCNAs)。借助X射线衍射仪(XRD)、扫描电子显微镜(SEM)和透射电子显微镜(TEM)对该复合材料的表面形貌和结构进行了分析。循环伏安和恒流充/放电测试结果表明,Ni/FCNAs具有较大的比电容值且电化学稳定性良好。在电流密度为0.1 A·g-1时,Ni/FCNAs电极的比电容可达176 F·g-1。本文同时也提出了Ni/FCNAs可能的形成机理。
镍掺杂;纳米碳片;溶剂热;超级电容器
Electrochemical double layer capacitor(EDLC)has attracted considerable attention because of its wide potential applications in electric vehicles and other high power applications due to its high power density and long cycle life[1-4].Various materials have been tried for developing supercapacitors[5-10],but the application is still limited due to the low energy density.Carbon materials can be promising candidates for supercapacitor applications because of their chemical stability,low-cost,fineconductivity andkinds of existing forms[11].Novel carbon materials,such as carbon nanofibers[12],nanotubes[13],nanospheres[14-16]and nanoflakes[17]have successfully applied for EDLC.Recently,Yuan et al.[18]reported the synthesis of flower-like mesoporouscarbon from metal-organic coordination polymers with high specific surface area and high specific capacitance.Flower-like microconstruction is composed of a number of 1 or 2-dimensoinal subunits with one end aggregating together[19].The microstructure gives rise to a relatively high specific surface area and evenly distributed mesoporosity,which are favorable for their application as electrode material in supercapacitors.
In recent years,several works have been reported on Ni-doped carbon materials applied as an electrode material for supercapacitors[20-21].Their results show that the doped nickel particles play an important role in improving electrochemical reaction of the Ni-doped carbon materials.Therefore,the Ni-doped carbon materials exhibit an excellent capacitive behavior compared with the pure carbon materials.
In this paper,we report an easy route for the largescale production ofNi-doped flower-like carbon nanosheet aggregations(Ni/FCNAs)via a one-pot solvothermal method followed by a subsequent calcination process at 900 ℃ in nitrogen atmosphere.The electrochemical capacitive properties for this material have been characterized via cyclic voltammetry and galvanostatic charge/discharge experiments.The results show that the Ni/FCNAs have good electrochemical performance.To the best of our knowledge,there has been no reporton Ni-doped flower-like carbon nanosheet aggregations as electrode materials for supercapacitors.
1 Experimental
1.1 Synthesis of flower-like products
All the chemical reagents were analytically pure and used without further purification.In a typical procedure,0.3 g of Nickelacetate tetrahydrate was introduced to 30 mL of a mixed H2O and ethylene glycol(EG)solution (1 ∶1,V/V).About 0.8 mL of furfural was added under stirring until a reddish-brown solution was obtained.The solution was then transferred into a 40 mL Teflon-lined stainless-steel autoclave that was sealed and heated at 180℃for 24 h before cooled to room temperature.The resulting solid material was collected by centrifugation,washed with deionized water and ethanol,and finally dried at 80℃for 4 h in vacuum.The as-obtained precursor sample was heated to 900℃ at a rate of 2℃·min-1and kept at the final temperature for 3 h in flowing N2.The as-synthesized black wool-like raw product was referred as Ni/FCNAs.For comparison, Nickel-free flower-like carbon nanosheet aggregations was prepared following the similar procedure,the difference was that the precursor was boiled in nitric acid before calcined at 900℃,and the nickel-free material was denoted as FCNAs.
1.2 Characterization of flower-like products
The structure of as-prepared product was analyzed by a MSAL-XD2 X-ray diffractometer(Cu Kα,36 kV,20 mA, λ =0.154 060 nm).The morphologies were observed via on a Philips SEM-XL30S scanning electron microscope and a JEOL TEM-2010 transmission electron microscope using an accelerating voltage of 200 kV.The specific surface area was measured via the Micromeritics TriStar 3000 analyzer.
1.3 Electrochemical performance
The working electrode was prepared by pressing the mixture of active materials、carbon black and 5%-PTFE (75∶15∶10,w/w)onto a foam nickel electrode under 35 MPa.Cyclic voltammetry and galvanostatic charge/discharge measurements were conducted on CHI 660B electrochemical workstation.All experiments were carried out at room temperature in a standard three-compartment cell containing a nickel~foil~electrode as the current collector and an Hg/HgO(6.0 mol·L-1KOH)as a reference electrode and the abovementioned as working electrode.Different sweep rates were employed in cyclic voltammetry within the range of-0.9 to 0.1 V.Cycling stability of Ni/FCNAs was evaluated by repeated cyclic voltammetry at a rate of 20 mV·s-1.
2 Results and discussion
2.1 Structural characterization
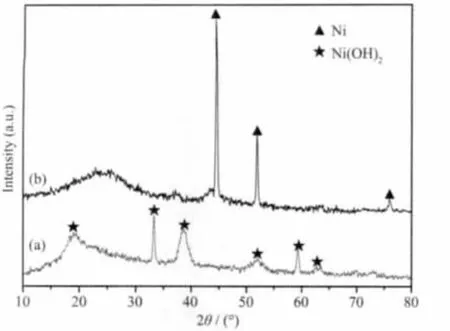
Fig.1 XRD patterns of the precursor(a)and Ni/FCNAs(b)

Fig.1 shows the X-ray diffraction(XRD)patterns of the the precursor and as-synthesized products.The peaks in Fig.1a at about 19°,33°,39°,52°,59°,63°can be indexed to diffraction planes ofβ-Ni(OH)2,respectively.Fig.1b at about 45°,52°and 76°can be assigned to the(111),(200)and (220)planes of the cubic structure of Ni.The broad low intensity peaks were produced by disordered carbon.According to the following Scherrers equation:where D is the average diameter of the crystals in nm,λ is the X-ray wavelength (λ=0.154 060 nm),k is the shape factor (k=0.89),and θ is the Bragg angle in degrees and β=B-b.Here B is the full-width at half maximum (FWHM)and b represents the instrumental line broadening (assuming null instrumental in the paper).The reflecting peaks at 2θ=45°,52°and 76°are chosen to calculate the average diameter,the average size of Ni particles is about 18 nm.
Typical SEM image of the precursor is shown in Fig.2a.According to the observation of SEM image,flower-like microstructures account for the majority of the precursor,which consists of nanosheets.Fig.2b shows the SEM image of Ni/FCNAs,the flower-like morphology can be maintained during the subsequent calcination step.The nanosheets are just a few nanometers in thickness.Fig.2c shows a SEM image of the product FCNAs.The flower-like morphology is only slightly distorted even though the precursor was boiled in nitric acid before calcined.
Fig.3a shows a TEM image of Ni/FCNAs.The product is composed of nanosheets that root deeply inside the flower-like aggregation.The dispersion and size of Ni nanoparticles on the carbon materials are further characterized by the high resolution TEM(HRTEM)analysis.Fig.3b shows the typical HTEM image of the Ni/FCNAs.The dark spots with diameters ranging from 10 nm to 25 nm are Ni nanoparticles.Abetter dispersion of Ni nanoparticles on carbon support with narrower size distribution is found.The average size of the particles is estimated to be 18 nm by counting more than 100 Ni particles from the HTEM image,which is consistent with the XRD results.

Fig.2 SEM images of the precursor(a),Ni/FCNAs(b)and FCNAs(c)
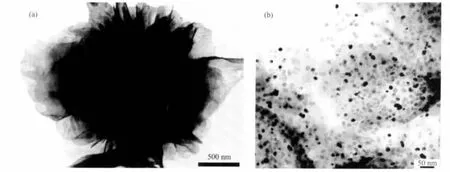
Fig.3 TEM image(a)and HTEM image(b)of Ni/FCNAs
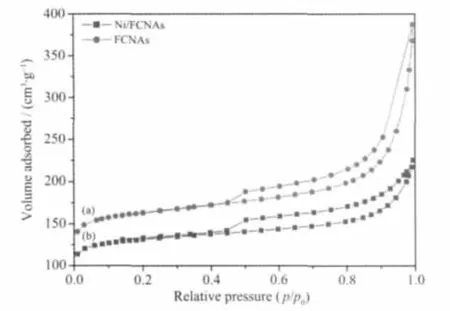
Fig.4 Adsorption-desorption isotherms of FCNAs(a)and Ni/FCNAs(b)
The nitrogen adsorption-desorption isotherms of Ni/FCNAs and FCNAs are shown in Fig.4.The isotherm of typeⅣwith a sharp capillary condensation step is observed and a hysteresis loop near relative pressure of~0.5 in the desorption branch indicates the presence of mesopores.BET specific area of Ni/FCNAs and FCNAs are measured to be 455 m2·g-1and 562 m2·g-1,respectively.
2.2 Growth mechanism of Ni/FCNAs
Based on the experimental results,the procedure for the formation of Ni/FCNAs has been proposed in Scheme 1.The procedure can be divided into two steps:(1)Formation of Ni(OH)2nanoplatelets;and (2)the growth of flower-like product.The possible formation mechanism can be explained as following.During the formation of Ni(OH)2nanoplatelets in solvothermal condition,the related reaction equationscan be described as follows[22].

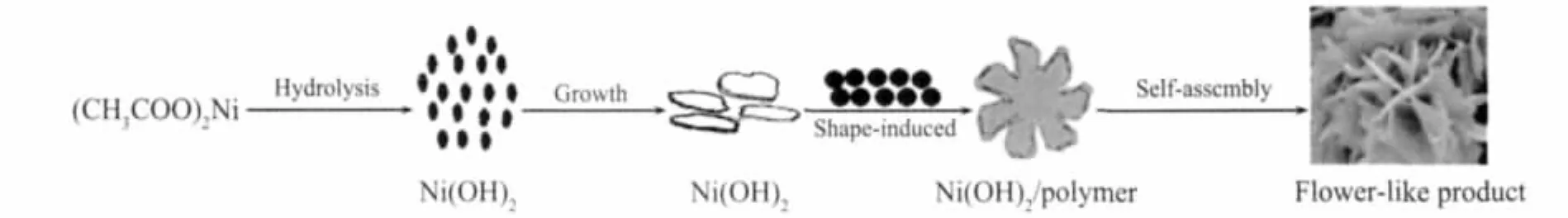
Scheme 1 Possible mechanism for the formation of Ni/FCNAs
In the primary stage of the reaction,ethylene glycol (EG)molecules react with Ni2+ions to form coordinated complexes (EG-Ni2+)(Eq.(2)),and the hydrolysis of the acetate group provides OH-ions(Eq.(3)).Ni(OH)2nuclei are formed instantaneously in solution via the reaction between Ni2+cations and OH-anions,which are released through complexationdissociation equilibrium and hydrolysis equilibrium(Eq.(4)).The ions are released slowly through the above-mentioned chemical regulation process,wherefore itisbeneficialto form nanoplatelets.Synchronously,furfural polymerizes to form colloidal carbon spheres and strongly adsorbs on the surface of the Ni(OH)2nanoplatelets.As a result,large Ni(OH)2/polymer nanoplatelets are produced.The nanoplatelets can aggregate into the flower-like morphology through self-assembly process by the driving forces,such as electrostatic and dipolar fields associated with the aggregate,hydrophobic interactions,hydrogen bonds,crystal-face attraction,and van der Waals forces[23].During the subsequent carbonization process,flowerlike morphology can be maintained and nickel ions are reduced to metal nickel,consequently this leads to the formation of Ni/FCNAs.
2.3 Electrochemical tests
For researching the electrochemical performance of the as-synthesized products,we have employed two electrochemical methods involving cyclic voltammetry and galvanostatic charge/discharge.The voltammograms of Ni/FCNAs and FCNAs under different sweep rates are shown in Fig.5a and b,respectively.It is well known that an ideal capacitance behavior of a carbon material electrode is expressed in the form of a rectangular shape on the voltammetry characteristics.As shown in Fig.5a,cyclic voltammogram (CV)of Ni/FCNAs presents a near rectangular shapes with slightly distorted,which is charac teristic of electrochemical capacitor.Note that the slopes of current variation near the vertex potentials are almost verticalatCV,illustrating the excellent behaviour of Ni/FCNAs as electrode materials with very small equivalent series resistance.However,in Fig.5b the slopes of current variation vs potentials are obvious to indicate that forms IR drops in the FCNAs material electrodes.
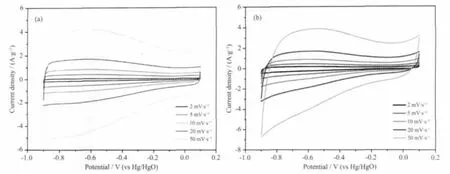
Fig.5 CVs of Ni/FCNAs and FCNAs in 6 mol·L-1KOH at room temperature

The specific capacitances (C)of electrodes is calculated according to the following equation from the measured CVs:where i,w and△v are the sample current,the weight of active materials and the voltage window,respectively.The data calculated from CVs of Ni/FCNAs and FCNAs is summarized in Table 1.A maximum C of 165 F·g-1is obtained for Ni/FCNAs at the sweep rate of 2 mV·s-1.Although the surface area of sample FCNAs is larger than that of the Ni/FCNAs materials,the specific capacitance is only 79 F·g-1at 2 mV·s-1,less than half of that for Ni/FCNAs.Total electroactive sites on the surface of carbon materials are strongly affected not only by the specific surface area for electrolyte accessibility but also by the electrical conductivity for electron transfer.Probably,the higher capacitance of Ni/FCNAs may be due to that the doped metallic nickel considerably may alleviate the electron-conducting resistance and facilitate the electrochemical reaction.The introduction of Ni species imparts a surface polarity to the carbon surface,thus enhancing dipole affinity towards OH-to cause capacitance increase[24].In addition,the surface polarity can improve the wettability of carbon micropores,thus increasing the surface area accessible to electrolyte or hydrated molecules for double-layer formation, thereby increasing the capacitive behavior of the Ni/FCNAs.
The capacitances per surface area (CSA)were also calculated.It was found that the value of Ni/FCNAs is larger than usually reported for the double-layer capacitance(0.1~0.2 F·m-2)[25].
The galvanostatic charge/discharge measurements are performed with various current densities in order to further investigate the performances of as-synthesized products.The specific capacitance (C)of the sampleswas calculated according to the following equation:which i,t,w and Δv are the constant current and discharge time and the weight of active materials and the voltage window,respectively.Fig.6a shows the potential-time curves of Ni/FCNAs as well as FCNAs at the constant current density of 0.1 A·g-1.The charge/discharge curves ofNi/FCNAs are linear and symmetrical,and the IR drop is not obvious,which shows good capacitance,highly reversible charge/discharge efficiency.In particular,discharging time of the FCNAs electrode unloaded Ni content was 1 322 s,while that of the Ni/FCNAs electrodes increased 450 s.
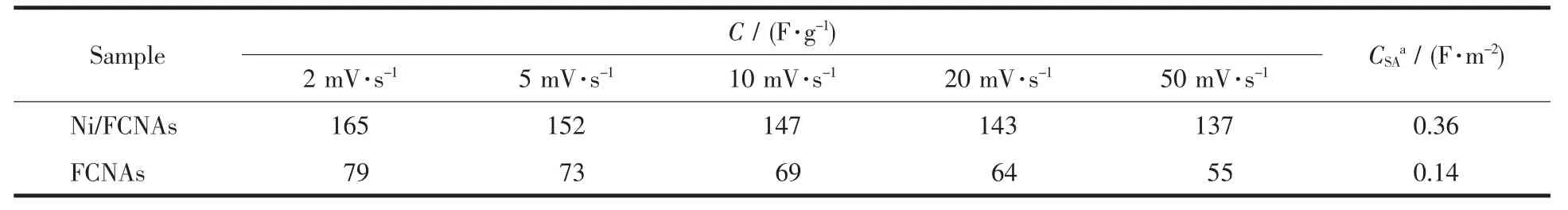
Table 1 Specic gravimetric capacitances(C)and specific capacitances per surface area(CSA)of Ni/FCNAs as well as FCNAs

Fig.6b is galvanostatic charge/discharge curves of Ni/FCNAs at current densities of 0.1,0.25,0.5 and 1 A·g-1,respectively.The specific capacitance of Ni/FCNAs is 176 F·g-1with the current density of 0.1 A·g-1.The data agrees well with from CV tests.The results show that over two times of capacitance enhancement can be achieved through carbon without Ni and carbon with an appreciable amountNi (the specific capacitance of FCNAs is 86 F·g-1in 0.1 A·g-1).With the increase of current densities from 0.1 A·g-1to 2 A·g-1,the capacitances remained 78%and 71%for Ni/FCNAs and FCNAs(Fig.7a),respectively.
The cyclic voltammetry experiments were performed at a sweep rate of 20 mV·s-1for 1000 cycles in order to investigate the cycling stability of Ni/FCNAs(Fig.7b)The specific capacitance of Ni/FCNAs is to a 163 F·g-1for the first cycle and the 160 F·g-1after 1000 cycles.Over 98% of the originalspecific capacitance remained for Ni/FCNAs.
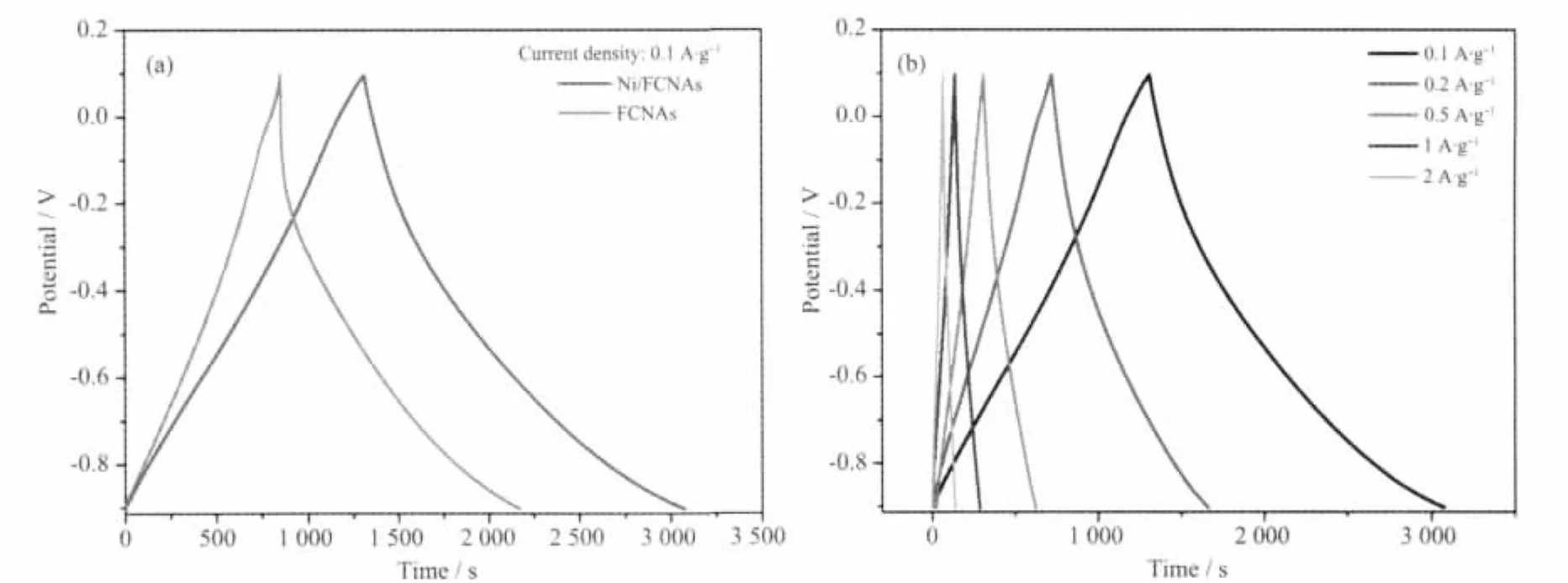
Fig.6 Galvanostatic charge/discharge curves of Ni/FCNAs and FCNAs with current 0.1 A·g-1(a)and of Ni/FCNAs(b)with currents of 0.1,0.2,0.5,1 and 2 A·g-1
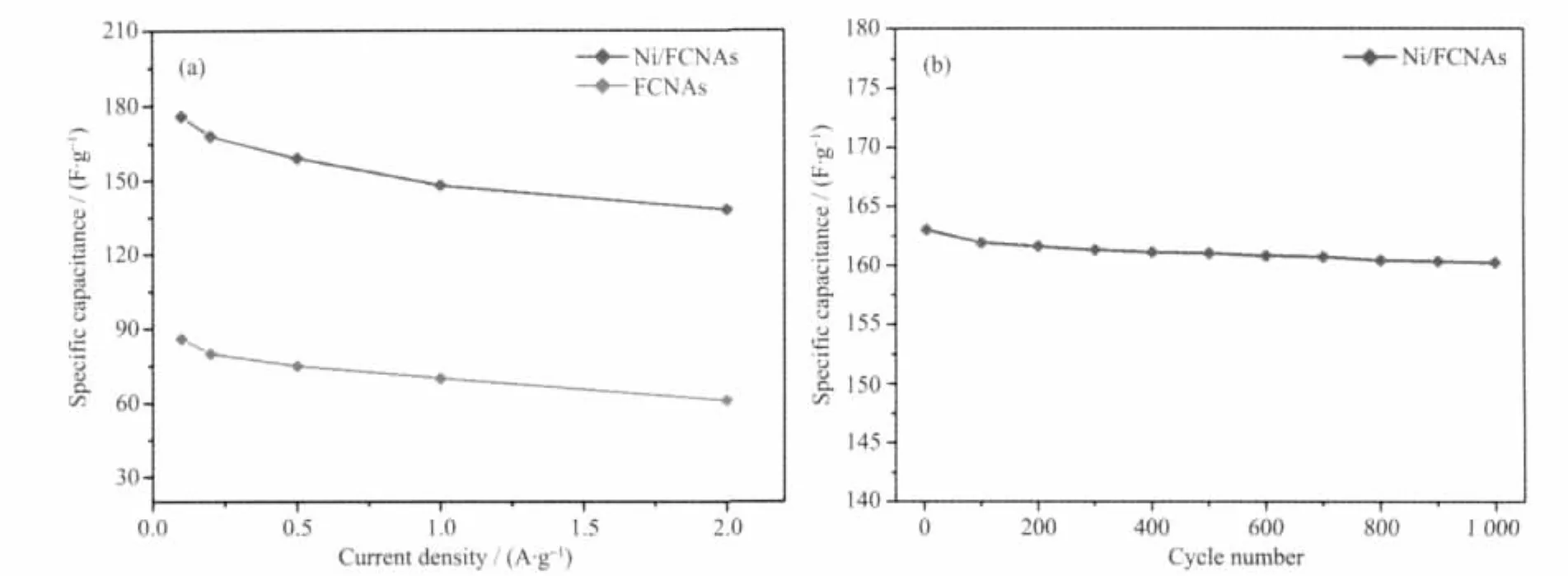
Fig.7 Dependence of specific capacitances on the different current density for Ni/FCNAs and FCNAs electrodes(a)and cycle life for Ni/FCNAs measured at 20 mV·s-1(b)
3 Conclusions
Ni-doped flower-like carbon nanosheet aggregations was successfully prepared via a one-pot solvothermal method followed by a subsequent calcination process at 900℃in nitrogen atmosphere.The asproduced Ni/FCNAs possess a specific surface area of 493.6 m2·g-1after the precursors were calcined at 900℃.The experimental results show that the specific capacitance of the Ni/FCNAs is176 F·g-1at the current density of 0.1 A·g-1and the retained capacitance is up to 160 F·g-1at 20 mV·s-1after 1 000 cycles,which will make Ni/FCNAs the potential material for supercapacitors.
[1]Lee J,Yoon S,Hyeon T,et al.Chem.Commun.,1999,21:2177-2178
[2]Moriguchi I,Koga Y,Matsukura R,et al.Chem.Commun.,2002,24:1844-1854
[3]Lee J,Yoon S,Oh S M,et al.Adv.Mater.,2000,12:359-362
[4]MI Hong-Yu(米红宇),ZHANG Xiao-Gang(张校刚),WANG Xin-Lei(王兴磊),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(1):159-163
[5]Liu F J.J.Power Sources,2008,182:383-388
[6]SHEN Lai-Fa(申来法),ZHANG Xiao-Gang(张校刚),YUAN Chang-Zhou(原长洲),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(9):1601-1606
[7]WANG Xiao-Fen(王晓峰),WANG Da-Zhi(王大志),LIANG Ji(梁吉).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2003,19(2):137-141
[8]Zhao D D,Xu M W,Zhou W J,et al.Electrochim.Acta,2008,53:2699-2705
[9]QIAO Song(乔松),SUN Gang-Wei(孙刚伟),ZHANG Jian-Hua(张建华),et al.Carbon Techniques(Tansu Jishu),2010,29(1):14-19
[10]DU Xuan(杜嬛),WANG Cheng-Yang(王成扬),CHEN Ming-Ming(陈明鸣),et al.J.Inorg.Mater.(Wuji Cailiao Xuebaoi),2010,23(6):1193-1198
[11]Frackowiak E.Phys.Chem.Chem.Phys.,2007,9:1774-1785
[12]Kim C,Park S H,Lee W J,et al.Electrochim.Acta,2004,50:877-881
[13]Wang L,Li C,Zhou Q,et al.Physica B,2007,398:18-22
[14]Li F,Zou Q Q,Xia Y Y.J.Power Sources,2008,177:546-552
[15]Li H P,Zhao N Q,He C N,et al.J.Alloys Compd.,2008,465:387
[16]Yuan D S,Chen J X,Zeng J H,et al.Electrochem.Commun.,2008,10:1067-1070
[17]Yuan D S,Chen J X,Hu X C,et al.Int.J.Electrochem.Sci.,2008,3:1268-1276
[18]Yuan D S,Chen J X,Tan S X,et al.Electrochem.Commun.,2009,11:1191-1194
[19]ShenJM,Feng YT.J.Phys.Chem.C,2008,112:13114-13120
[20]Li J,Liu E H,Li W,et al.J.Alloys Compd.,2009,478:371-374
[21]Wu M S,Lin K H.J.Phys.Chem.C,2010,114:6190-6196
[22]Wang L,Zhao Y,Lai Q Y,et al.J.Alloys Compd.,2010,495:82-87
[23]Xu L P,Ding Y S,Chen C H,et al.Chem.Mater.,2008,20:308-316
[24]Tai Y L,Teng H.Carbon,2004,42:2335-2342
[25]Rojo J M,Fuertes A B,Pico F.J.Power Sources,2004,133:329-336
Synthesis of Ni-doped Flower-Like Carbon Nanosheet Aggregations for Supercapacitors
YI Guan-GuiXIAO Yong XIE Chun-Lin MO Shan-Shan HE Wen-QiZHAO Shuai LIU Ying-Liang*YUAN Ding-Sheng HUANG Lang-Huan TAN Shao-Zao
(Department of Chemistry and Institute of Nanochemistry,Jinan University,Guangzhou 510632,China)
Ni-doped flower-like carbon nanosheet aggregations (Ni/FCNAs)were synthesized via a one-pot solvothermal method followed by a subsequent calcination process at 900℃in nitrogen atmosphere.The surface morphologies and structures of composites were examined by scanning electron microscope (SEM),transmission electron microscope (TEM)and X-ray diffraction (XRD).Cyclic voltammetry and galvanostatic charge/discharge experiments were adopted to investigate their electrochemical behaviors.The results show that the doped nickel particles could enhance both specific capacitance and electrochemical stability.The Ni/FCNAs show large specific capacitance up to 176 F·g-1with the current density of 0.1 A·g-1.The possible formation mechanism of Ni/FCNAs has also been proposed on the basis of experimental results.
Ni-doped;carbon nanosheet;solvothermal;supercapacitors
TB333;TB383
A
1001-4861(2011)04-0764-07
2010-08-24。收修改稿日期:2010-11-09。
国家-广东联合基金(No.U0734005);国家自然科学青年基金(No.20906037);中央高校基本科研业务费专项资金(No.21610102)资助项目。*
。 E-mail:tliuyl@jnu.edu.cn;会员登记号:S060017521P。
