柔性环己烷六酸和刚性草酸配体桥连的稀土配位聚合物的水热合成与结构研究
2011-11-10苏婷婷
王 静 徐 敏 苏婷婷
(广州大学化学化工学院,广州 510006)
柔性环己烷六酸和刚性草酸配体桥连的稀土配位聚合物的水热合成与结构研究
王 静*徐 敏 苏婷婷
(广州大学化学化工学院,广州 510006)
草酸铽与水合环己烷六酸(H6LⅠ·H2O)(顺式椅式构型LⅠ:a,e,a,e,a,e)在水热条件下反应生成一种新颖的三维稀土配位聚合物 [Tb4(LⅡ)(ox)3(H2O)8](LⅡ为反式椅式构型:e,e,e,e,e,e;ox为草酸根),通过元素分析和红外光谱对这个配位聚合物进行了表征。X射线单晶衍射分析表明该配合物属于三斜晶系,P1空间群,晶胞参数为:a=0.60203(4)nm,b=1.08278(8)nm,c=1.29446(9)nm,α=67.9080(10)°,β=82.1090(10)°,γ=83.8870(10)°,V=0.77307(9)nm3,Z=2。 在这个配合物的形成中,顺式构型的 H6LⅠ配体发生构型转变形成LⅡ配体,LⅡ配体采取μ8-桥连模式将Tb离子连接成一个具有孔洞的二维(Tb-LⅡ)配位层。由μ2-和μ4-桥连模式构成的一维(Tb-ox)链将二维(Tb-LⅡ)层连接成一个具有孔道的三维配位框架,ox配体和水分子通过配位作用和氢键作用填充在孔道中。
稀土配位聚合物;环己烷六酸;草酸;水热合成
The crystal engineering of lanthanide complexes has attracted considerable attention over the past decade,not only due to their fascinating structural diversity and the intriguing topological networks[1-2],but also due to the potential application in medicine,magnetism,bioinorganic chemistry and luminescence[3-4].As is well known,lanthanide ions have their high and variable coordination numbers and flexible coordination environments,which can provide unique impetus for discovery of unusual network topologies[5-6].Consequently,a variety of lanthanide coordination polymers with interesting architectures and topologies have been synthesized successfully[7-8].
In order to construct multicarboxylate coordination frameworks,two main kinds of organic ligands are extensively studied,one is rigid ligands such as benzenepolycarboxylates and pyridinepolycarboxylates[9-11],the other is flexible ligands,such as cyclohexanepolycarboxylate[12-13].Recently, cyclohexane-1,2,3,4,5,6-hexacarboxylic acid (H6L,L stands for the ligand with different conformations)with versatile flexible conformations has been proved by us to act as excellent building blocks with charge and multi-connecting ability in the construction of functional coordination polymers[14-18].And in our recent work,we have investigated the coordination chemistry of the cyclohexanehexacarboxylic ligand and trapped its four conformations in different coordination polymers by carefully controlling the reaction conditions[14-19].As our continuing investigation on this interesting metal-H6L system,we employed the lanthanide Tbto react with the H6LⅠ(cis-chair conformation LⅠ:a,e,a,e,a,e)ligand to investigate the ligand flexible conformations.Herein,we report a novel three-dimensional lanthanide coordination polymer[Tb4(LⅡ)(ox)3(H2O)8](trans-chair conformation LⅡ:e,e,e,e,e,e,ox:oxalate)bridged by flexible cyclohexanehexacarboxylate and rigid oxalate ligands.The conformation of the cyclohexanehexacarboxylate ligand transformed from LIto LⅡ(Scheme 1).
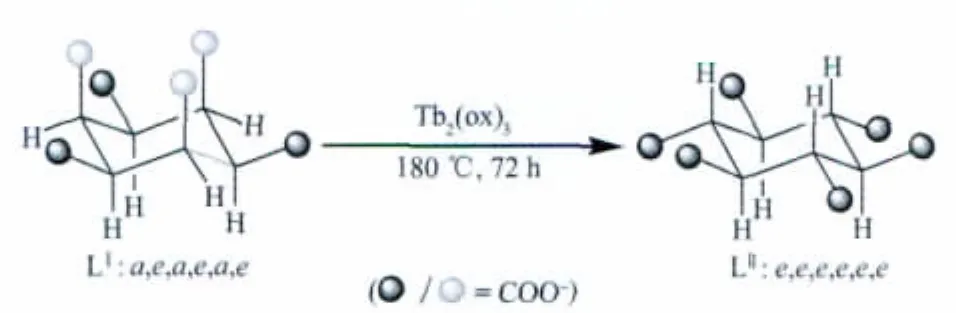
Scheme 1 Summary of hydrothermal condition and ligand conformation transformation in the preparation of the title complex
1 Experimental section
1.1 Materials and physical measurements
The starting material cyclohexanehexacarboxylic acid hydrate(H6LⅠ·H2O)employed was commercially available and used as received withoutfurther purification.The C and H microanalyses were carried out with an Elementar Vario-EL CHNS elemental analyzer.The FTIR spectra were recorded from KBr tablets in the range 4000~400 cm-1on a Bio-Rad FTS-7 spectrometer.
1.2 Hydrothermal synthesis
A mixture of H6LⅠ·H2O (0.087 g,0.25 mmol)and Tb2(ox)3·H2O(0.345 g,0.50 mmol)in H2O(15 mL)were placed to a 25 mL Teflon reactor and heated in an oven to 180 ℃ for 72 h.After being cooled at a rate of ca.5℃·h-1,the colorless crystals of the title complex in single phase (in ca.15% yield based on H6LⅠ)were obtained,isolated by filtration and washed with water.Elemental analysis calcd.for C9H11O16Tb2(%):C 15.60,H 1.60;found(%):C 15.49,H 1.73.IR(KBr,4000~400 cm-1):3429(s),2370(m),1686(s),1608(vs),1384(vs),1320(m),1262(w),1126(w),1041(w),799(w),619(w),521(w).
1.3 Crystal structure determination
Data collectionsofthe title complex were performed on a Bruker Smart Apex CCD diffractometer with Mo Kα radiation(λ=0.071 073 nm)at 293(2)K.The raw data frames were integrated with SAINT+,and the corrections were applied for Lorentz and polarization effects.Absorption correction was applied by using the multiscan program SADABS[20].The structure was solved by direct methods,and all non-hydrogen atoms were refined anisotropically by least-squares on F2using the SHELXTL program[21].Hydrogen atoms on organic ligands were generated by the riding mode(CH=0.093 nm).Crystal data as well as details of data collections and refinements for the complexare summarized in Table 1.Selected bond distances and bond angles are listed in Table 2.
CCDC:790926.
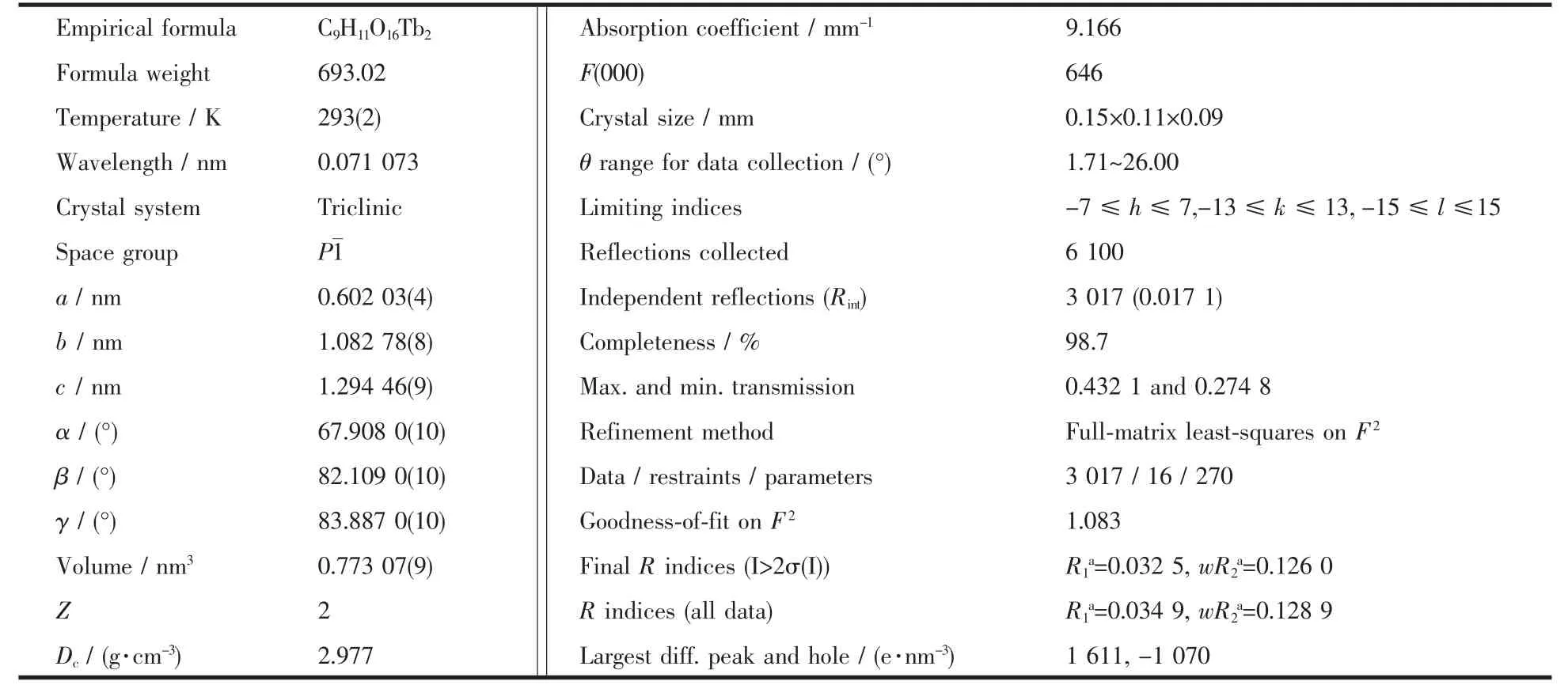
Table 1 Crystal data and structure parameters for the title complex


Table 2 Bond lengths(nm)and angles(°)for the title complex
2 Results and discussion
2.1 Synthesis
Hydrothermal synthesis is being widely used as a synthetic technique,not only for its advantageous preparation of highly stable metal-ligand frameworks,but also for its special reaction condition for the interesting reactions.It is well known that lanthanide ions are easily precipitated by oxalate,but under the condition of hydrothermal synthesis the preparation of new lanthanide frameworks containing both oxalate and other ligands is possible[21-22].In the formation of the title complex,it is of interest to note that the complex contains both rigid ox and the flexible cyclohexanepolycarboxylate ligands,which was rarely observed before for lanthanide frameworks.
From our previous studies on cyclohexanehexacarboxylic acid(H6L),we observed that the size and versatile coordination environments of the metal ions,different alkali metal ions,the presence of auxiliary ligands and different reaction temperatures may play an important role in controlling the conformation of carboxylate groups on the cyclohexane ring[14-19].Considering the characters of lanthanide ions,we employed Tbto react with the H6L,attempting to trap the L ligand conformations (Scheme 1).As a result,the LIIin situ transformed from the starting form LIcan be observed in the final crystal complex,proving that the Tbwith the presence of the ox ligand can also trap the LⅡconformation of the cyclohexanehexacarboxylate ligand.
2.2 Structure of[Tb4(LⅡ)(ox)3(H2O)8]
X-ray diffraction crystal structure analysis reveals that the title complex is a three-dimensional framework crystallizing in P1 space group.The asymmetric unit contains two crystallographically independent Tbatoms,one LⅡligand transformed from the H6LⅠlying on a special position,two ox ligands,one of which lies on a special position,and four coordinated water molecules(Fig.1a).Tb1 adopts nine-coordinated geometry,with eight oxygen atoms from the LⅡand ox ligands as well as a coordinated water molecule(Tb-O 0.2274(5)~0.2528(8)nm)(Table 2),presenting a geometry close to that of a monocapped square antiprism.Differently,Tb2 atom is eight-coordinated with five oxygen atoms from the LⅡand ox ligands as well as three water molecules(Tb-O 0.229 8(6)~0.244 7(5)nm)(Table 2),giving a distorted bicapped trigonal prism geometry.The LⅡligand transformed from H6LⅠadopts μ8-bridging mode through its six e-carboxylate groups(Fig.1b),while the two kinds of ox ligands adopting μ2-and μ4-bridging modes,respectively.

Fig.1 (a)ORTEP drawing of coordination environment of the Tb atoms(with thermal ellipsoids at 50%);(b)Coordination mode of the LⅡligand
The μ8-bridging LⅡligands connect the Tb atoms to form a two-dimensional porous(Tb-LⅡ)layer extended through the ab plane(Fig.2a).The thickness of the layer is about 0.8 nm viewed along the b axis(Fig.2b).There are two types of pores in the layers along the c axis (Fig.2a),distorted quadrangularporeswith dimensions 0.55 nm ×0.70 nm and elliptic pores with dimensions 0.62 nm×1.25 nm,respectively.Meanwhile,there are one-dimensional channels with dimensions 0.65 nm×1.00 nm in the layers along the b axis(Fig.2b).Meanwhile,the μ2-and μ4-bridging ox ligands connect the Tb atom to form a one-dimensional(Tb-ox)chain.As showed in Fig.3a,the μ4-bridging ox ligands link the Tb1 atoms to make up the main(Tb-ox)chain,and the μ2-bridging ox ligands link the Tb1 and Tb2 atoms to act as the decorations of the (Tb-ox)chain.Furthermore,the two-dimensional porous(Tb-LⅡ)layers are linked by the one-dimensional(Tb-ox)chains to generate a three-dimensional coordination framework with channels(Fig.3b,c).
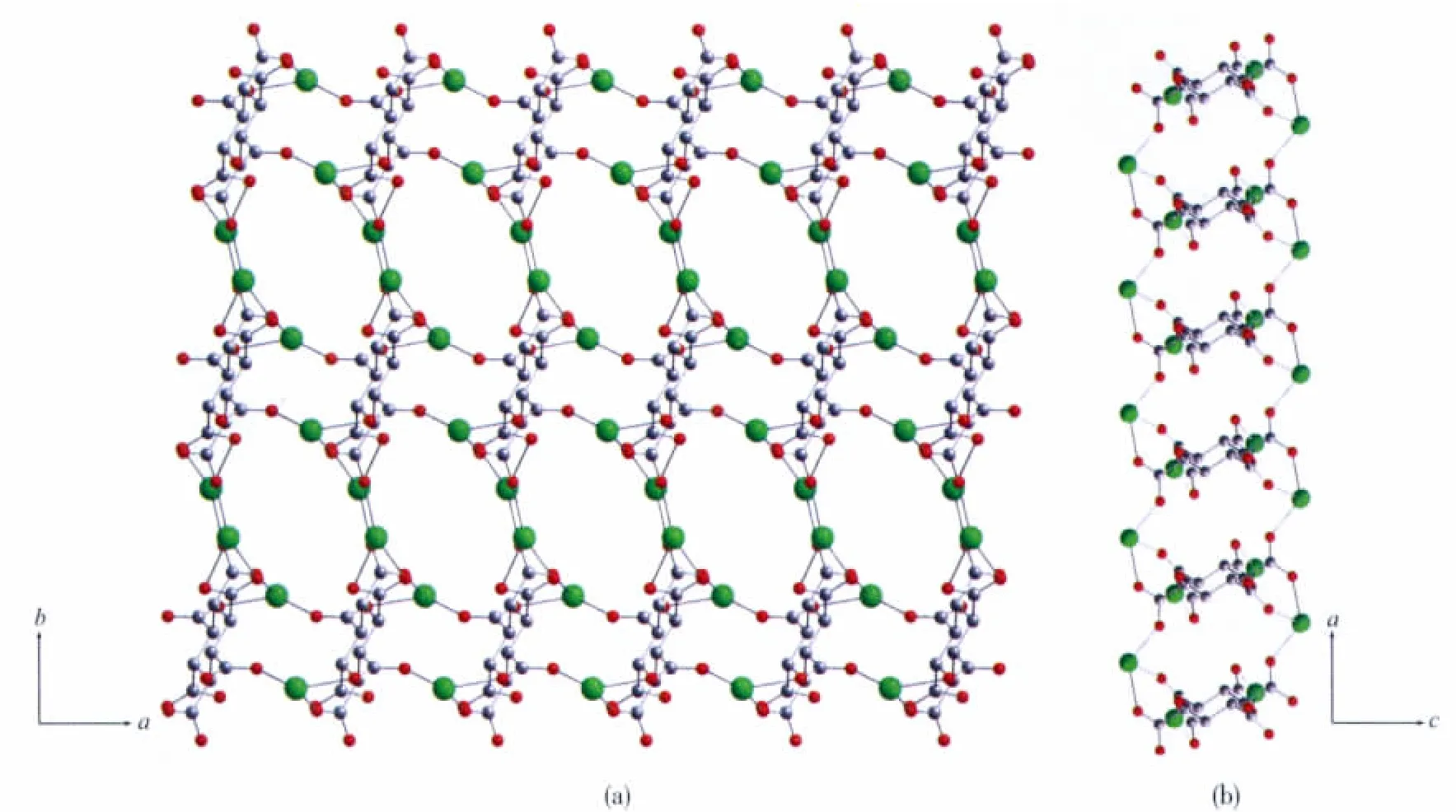
Fig.2(a)Perspective views of the two-dimensional porous(Tb-LⅡ)layer bridged by the LⅡ ligand viewed along the c axis;(b)Along b axis
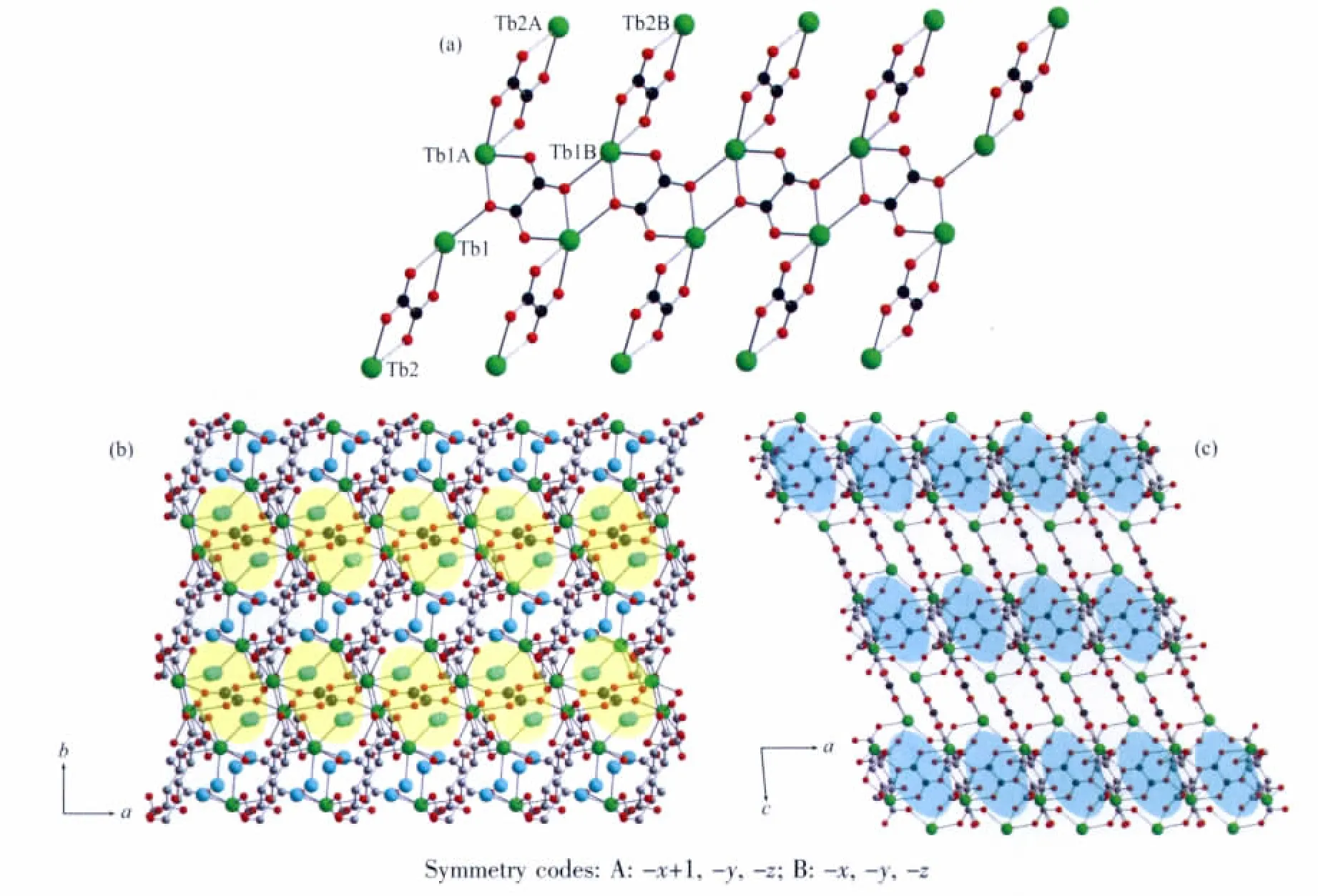
Fig.3 (a)Perspective views of the one-dimensional(Tb-ox)chain;(b)Three-dimensional framework bridged by LⅡand ox ligands with elliptic channels occupied by ox lgiand and water molecules viewed along the c axis;(c)Along b axis(yellow and blue ellipse shapes stand for the elliptic channels,and big blue balls in(b)stand for the water molecules)
It should be noted that the two kinds of elliptic channels in the framework are occupied by the μ4-bridging ox ligands and coordinated water molecules,while the quadrangular are filled with coordinated water molecules(Fig.3b).Moreover,there are rich hydrogen interactions between the water molecules and the carboxylate groups of the LIIand ox ligands (O…O 0.2674(8)~0.2785(8)nm,∠O-H…O122(5)°~169(6)°)(Table 3),which further strengthen the three-dimensional framework.

Table 3 Hydrogen bond lengths and bond angles for the title complex
[1]Bradshaw D,Claridge J B,Cussen E J,et al.Acc.Chem.Res.,2005,38:273-282
[2]Ye B H,Tong M L,Chen X M.Coord.Chem.Rev.,2005,249:545-565
[3]Zhao B,Chen X Y,Cheng P,et al.J.Am.Chem.Soc.,2004,126:15394-15395
[4]Bunzli J C G,Piguet C.Chem.Soc.Rev.,2005,34:1048-1077
[5]Zhang M B,Zhang J,Zheng S T,et al.Angew.Chem.,Int.Ed.,2005,44:1385-1388
[6]Cheng J W,Zhang J,Zheng S T,et al.Angew.Chem.,Int.Ed.,2006,45:73-76
[7]Shi W,Chen X,Zhao Y,et al.Chem.Eur.J.,2005,11:5031-5039
[8]Sun Y Q,Zhang J,Yang G Y.Chem.Commun.,2006:1947-1949
[9]Chui S S Y,Lo S M F,Charmant J P H,et al.Science,1999,283:1148-1150
[10]Humphrey S M,Wood P T.J.Am.Chem.Soc.,2004,126:13236-13237
[11]PENG Meng-Xia(彭梦侠),CHEN Zi-Yun(陈梓云).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(6):1055-1061
[12]Bi W,Cao R,Sun D,et al.Chem.Commun.,2004:2104-2105
[13]Kitagawa S,Uemura K.Chem.Soc.Rev.,2005,34:109-119
[14]Wang J,Hu S,Tong M L.Eur.J.Inorg.Chem.,2006:2069-2077
[15]Wang J,Zheng L L,Li C J,et al.Cryst.Growth Des.,2006,6:357-359
[16]Wang J,Zhang Y H,Tong M L.Chem.Commun.,2006:3166-3168
[17]Wang J,Lin Z J,Ou Y C.Chem.Eur.J.,2008,24:7218-7235
[18]Wang J,Ou Y C,Shen Y,et al.Cryst.Growth Des.,2009,9:2442-2450
[19]WANG Jing(王静),LIU Zhao-Qing(刘兆清).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(11):2077-2082
[20]Sheldrick G M.SADABS 2.05,University of Göttingen,2000.
[21]SHELXTL 6.10,Bruker Analytical Instrumentation,Madison,Wisconsin,USA,2000.
[22]Song J L,Mao J G.Chem.-Eur.J.,2005,11:1417-1424
[23]Zhu W H,Wang Z M,Gao S.Inorg.Chem.,2007,46:1337-1342
Hydrothermal Synthesis and Crystal Structure of a Novel Lanthanide Coordination Polymer Bridged by Flexible Cyclohexane-1,2,3,4,5,6-hexacarboxylate and Rigid Oxalate Ligands
WANG Jing*XU Min SU Ting-Ting
(School of chemistry and chemical engineering,Guangzhou University,Guangzhou 510006,China)
Reaction of Tb2(ox)3·6H2O andcyclohexane-1,2,3,4,5,6-hexacarboxylicacid hydrate(H6LⅠ·H2O)(cischair conformation LⅠ:a,e,a,e,a,e)resulted in formation of a novel three-dimensional lanthanide coordination polymer[Tb4(LⅡ)(ox)3(H2O)8](trans-chair conformation LⅡ:e,e,e,e,e,e;ox:oxalate)under hydrothermal condition,which was characterized by elemental analysis and IR.X-ray diffraction crystal structure analysis shows that the complex crystallizes in triclinic system,space group P1 with a=0.602 03(4)nm,b=1.082 78(8)nm,c=1.294 46(9)nm,α=67.9080(10)°,β=82.1090(10)°,γ=83.8870(10)°,V=0.77307(9)nm3,Z=2.In the formation of the complex,the cis-chair H6LⅠligand transformed to the trans-chair LⅡligand,which adopts μ8-bridging mode connecting the Tb atoms to form two-dimensional(Tb-LⅡ)layers with channels.The one-dimensional(Tb-ox)chains bridged by μ2-and μ4-ox ligands link the(Tb-LⅡ)layers to generate a three-dimensional framework with channels,which were filled with ox ligands and water molecules through coordination and hydrogen interactions.CCDC:790926.
lanthanide coordination polymer;cyclohexanehexacarboxylate;oxalate;hydrothermal synthesis
O614.341
A
1001-4861(2011)04-0737-06
2010-09-06。收修改稿日期:2010-11-13。
国家自然科学青年基金(No.20901018)和广东省自然科学基金(No.9451009101003177)资助项目。*
。 E-mail:wangjgzhu@yahoo.com.cn
