黄芪提取物调节IL-6/STAT3信号通路治疗马兜铃酸Ⅰ诱导肝肾损伤小鼠模型的效果观察
2023-04-29朱哿瑞皮亚妮王静黄恺彭渊陈高峰刘成海陶艳艳
朱哿瑞 皮亚妮 王静 黄恺 彭渊 陈高峰 刘成海 陶艳艳
摘要:目的探討黄芪提取物(AR)改善马兜铃酸Ⅰ(AAⅠ)致小鼠急性肝、肾损伤效果及其调控IL-6/STAT3信号通路的作用机制。方法健康雄性C57BL/6小鼠38只,采用简单随机分组法分为正常组(n=8)、模型组(n=10)、AR组(n=10)和N-乙酰半胱氨酸(NAC)组(n=10)。模型组小鼠以20 mg/kg AAⅠ 腹腔注射,1次/d,持续5 d。正常组小鼠腹腔注射相同容积羧甲基纤维素钠。AR组、NAC组20 mg/kg AAⅠ 腹腔注射,1次/d,持续3 d;第4天分别按AR 75 mg/kg、NAC 150 mg/kg小鼠体质量剂量灌胃,1次/d,持续8 d。NAC为阳性对照药。给药造模结束后,处死小鼠并收集血清及肝、肾组织。试剂盒检测血清ALT、AST、肌酐(SCr)、尿素氮(BUN)水平;HE染色观察肝、肾组织病理;荧光PCR及免疫组化分析肝、肾组织中p-STAT3表达量;酶联免疫吸附实验检测肝、肾组织IL-6、IL-1β及TNF-α表达水平。计量资料多组间比较采用单因素方差分析,进一步两组间比较采用SNK-q检验。结果与正常组小鼠相比,模型组小鼠肾体比上升(P<0.05);与模型组相比,AR组ALT、AST、SCr和BUN水平显著降低(F值分别为49.29、31.31、58.89、85.88,P值均<0.01);HE染色结果表明,AR可有效减轻AAⅠ 导致的肝、肾组织结构破坏和炎性细胞浸润;荧光PCR及免疫组化染色结果表明,AR可减少肝、肾组织p-STAT3表达;酶联免疫吸附检测发现,AR可下调IL-6、IL-1β及TNF-α表达。NAC与AR效应相似,两者间无明显差异。结论AR对AAⅠ 所致急性肝、肾损伤有保护作用,其部分作用机制可能与抑制IL-6/STAT3信号通路激活,减轻炎症反应有关。
关键词:马兜铃酸; 化学性与药物性肝损伤; 急性肾损伤; STAT3转录因子; 黄芪
基金项目:上海市科委科技支撑项目(19401901500); 上海市中医药三年行动计划(ZY-〔2018-2020〕-CCCX-5001)
Efficacy of Astragali Radix extract in treatment of a mouse model of aristolochic acid Ⅰ-induced liver and renal injury by regulating the IL-6/STAT3 signaling pathway
ZHU Gerui PI Yani WANG Jing HUANG Kai PENG Yuan CHEN Gaofeng LIU Chenghai TAO Yanyan(1. Institute of Liver Diseases, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; 2. Institute of TCM International Standardization, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; 3. Shanghai Key Laboratory of Traditional Chinese Clinical Medicine, Shanghai 201203, China; 4. Key Laboratory of Liver and Kidney Diseases, Ministry of Education, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China)
Corresponding author:TAO Yanyan, taoyanyan1023@126.com (ORCID: 0000-0002-8962-3137)
Abstract:ObjectiveTo investigate the mechanism of action of Astragali Radix (AR) extract in improving aristolochic acid Ⅰ (AAⅠ)-induced acute liver and renal injury in mice by regulating the IL-6/STAT3 signaling pathway. MethodsA total of 38 healthy male C57BL/6 mice were randomly divided into normal group with 8 mice, model group with 10 mice, AR group with 10 mice, and N-Acetyl-L-cysteine (NAC) group with 10 mice. The model group mice were intraperitoneally injected with 20 mg/kg AAⅠ once a day for 5 days. Normal mice were intraperitoneally injected with the same volume of Carboxymethyl cellulose sodium. AR group and NAC group received intraperitoneal injection of 20 mg/kg AAⅠ once a day for 3 days; On the 4th day, mice were gavaged with AR 75 mg/kg and NAC 150 mg/kg body mass doses, once a day, for 8 days. NAC was used as a positive control drug. After the end of administration and modeling, the mice were sacrificed to collect serum samples and liver and renal tissue samples. The kit was used to measure the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (SCr), and blood urea nitrogen (BUN); HE staining was used to observe liver and renal histopathology; quantitative real-time PCR and immunohistochemistry were used to measure the expression level of p-STAT3 in the liver and renal tissue; ELISA was used to measure the expression levels of interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) in the liver and renal tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the SNK-q test was used for further comparison between two groups. ResultsCompared with the normal group, the model group had a significant increase in kidney-to-body ratio (P<0.05). Compared with the model group, the AR group had significant reductions in the levels of ALT, AST, SCr, and BUN (F=49.29, 31.31, 58.89, and 85.88, all P<0.01). HE staining showed that AR could effectively alleviate AAⅠ-induced structural damage and inflammatory cell infiltration in the liver and renal tissue; quantitative real-time PCR and immunohistochemistry showed that AR could reduce the expression of p-STAT3 in the liver and renal tissues; ELISA showed that AR could downregulate the expression of IL-6, IL-1β, and TNF-α. NAC and AR had a similar effect with no significant differences. ConclusionAR exerts a protective effect against AAⅠ-induced acute liver and renal injury, possibly by inhibiting the activation of the IL-6/STAT3 signaling pathway and alleviating inflammatory response.
Key words:Aristolochic Acid; Chemical and Drug Induced Liver Injury; Acute Kidney Injury; STAT3 Transcription Factor;Astmgali Radix
Research funding:Science and Technology Innovation Action Plan of the Shanghai Municipal Science and Technology Commission (19401901500); Three-Year Action Plan of for the Development of TCM in Shanghai (ZY-〔2018-2020〕-CCCX- 5001)
马兜铃酸主要存在于马兜铃属和细辛属植物中[1],如马兜铃、天仙藤、广防已等。研究[2]证明,马兜铃酸具有免疫激活、抗炎、肾毒性、致癌性和致突变等作用。笔者团队前期研究[3-4]发现,马兜铃酸Ⅰ(aristolochic acid Ⅰ, AAⅠ)可导致急性肝、肾损伤,其毒理机制可能与氧化应激、炎症反应、糖代谢等途径相关。黄芪是中医临床常用药亦是补气圣药,《神农本草经》记载:“黄芪,味甘,性微温,归肺、脾、肝、肾经”,可补益元气而脱毒,治一切气衰血虚之症[5]。黄芪的药理作用包括保护心、脑、肾等组织,改善肝脏损伤,提高免疫功能等方面,广泛应用于临床[6]。本研究以AAⅠ诱导小鼠急性肝、肾损伤,并观察黄芪提取物(astragali radix extract, AR)的调控作用及其机制。
1材料与方法
1.1实验动物健康雄性C57BL/6小鼠,8周龄,SPF级,共38只,体质量(25±1)g,购于北京维通利华生物有限公司[实验动物生产许可证号: SCXK(京) 2016-0006]。所有小鼠均饲养在上海中医药大学实验动物中心[实验动物使用许可证号:SYXK(沪) 2020-0009],自由饮水,环境温度22 ℃,湿度55 %。
1.2药物与试剂AAⅠ(批号:3503),购自上海诗丹德标准技术服务有限公司,纯度>98.5%。4 ℃冰箱存放备用。AR委托上海现代制药股份有限公司代加工制成,具体方法如下:黄芪1 000 g,以70%乙醇10 L提取2次,每次回流1 h。提取液组合、过滤、50 ℃减压浓缩、冻干,并采用中药指纹图谱进行质量控制,其主要活性物质为黄芪总皂苷,其中毛蕊异黄酮苷含量为0.306 mg/g、黄芪甲苷含量为0.874 mg/g。N-乙酰半胱氨酸(N-Acetyl-L-cystein,NAC)颗粒(商品名:富露施,0.1 g/包,海南赞绑制药有限公司,批号:1001542)。羧甲基纤维素钠(批号:F20051103),购自国药集团化学试剂有限公司。
Trizol Reagent(批号:F919KB3054)、总RNA提取试剂盒(批号:B518811),购自上海生工生物工程股份有限公司;反转录试剂盒(批号:QP016)、扩增试剂盒(批号:1725122)均购自日本TAKARA公司。苏木素-伊红(HE)染液(货号:D006-1-1),购自南京建成公司。免疫组化抗体:磷酸化STAT3(phospho-signal transducer and activator of transcription 3, p-STAT3)抗体(批号:#9145)購自美国CST公司;SABC免疫组化染色试剂盒(货号:SA1022-兔IgG)、DAB显色试剂盒(货号:AR1022)均购自武汉博士德公司。
1.3主要仪器IX70荧光倒置显微镜(日本Olympus公司);微孔板分光光度计(美国Bio-Tek公司);ABI Viia7实时荧光定量PCR仪(美国Life Technonogy公司);ASP300全自动组织脱水机、EG1140石蜡包埋机、RM2235 石蜡切片机均由德国莱卡公司提供;HI1210 水浴摊片机、HI1220 烘片机均由荷兰飞利浦公司提供。
1.4研究方法
1.4.1分组与造模、给药小鼠适应性饲养1周,按体质量采用简单随机分组法分为4组:正常组(n=8)、模型组(n=10)、AR组(n=10)和NAC组(n=10)。 模型组小鼠以20 mg/kg AAⅠ 腹腔注射,1次/d,持续5 d[7-8]。正常组小鼠腹腔注射相同容积羧甲基纤维素钠。AR组、NAC组以20 mg/kg AAⅠ 腹腔注射,1次/d,持续3 d;第4天分别按AR 75 mg/kg[9]、NAC 150 mg/kg[10-12]小鼠体质量剂量灌胃,1次/d,持续8 d。NAC作为阳性对照药使用。
1.4.2检测指标与方法
1.4.2.1肝、肾脏器指数测定小鼠以3%戊巴比妥钠麻醉后,摘取肝脏及双肾,肉眼观察大体形态,称重,除以小鼠体质量,计算肝体比(%)、肾体比(‰)。肝体比=肝脏质量(g)/体质量(100 g)×100%。肾体比=肾脏质量(g)/体质量(100 g)×1 000 ‰。
1.4.2.2血清肝、肾功能测定小鼠麻醉后,用1 mL注射器从下腔静脉抽取新鲜血液,以4 ℃,3 000 r/min,离心15 min,吸取上层血清送至上海中医药大学附属曙光医院检验科检测血清ALT、AST活性及肌酐(SCr)、尿素氮(BUN)水平。
1.4.2.3肝、肾组织HE染色小鼠肝、肾组织经4%中性甲醛缓冲液固定,自动脱水机脱水,石蜡包埋,4 μm切片,HE染色,光学显微镜下200倍观察并拍照。
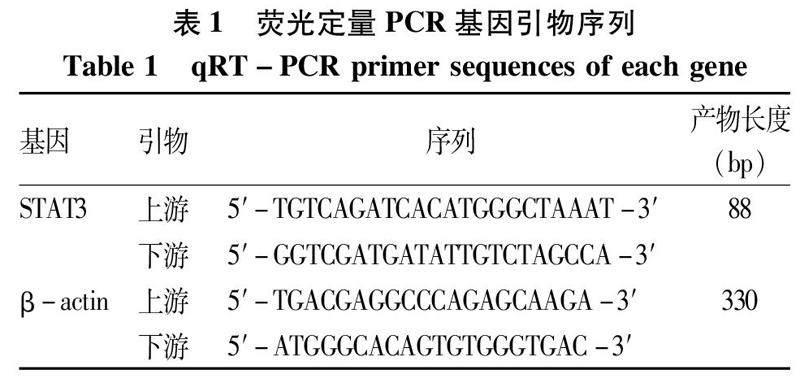
1.4.2.4肝、肾组织STAT3的基因表达取全部小鼠肝、肾组织各50 mg,加入500 μL Trizol试剂及100 μL氯仿抽提总RNA,应用 Prime ScriptTM RT试剂盒进行逆转录制备cDNA。以β-actin为内参进行荧光定量PCR扩增以检测各组肝、肾组织中mRNA的表达水平。反应条件:95 ℃、2 min预变性;95 ℃变性15 s,60 ℃退火1 min,45个循环。各组均设3个复孔,采用2-△△CT法计算各目的基因的相对表达量。扩增引物由上海生工生物工程有限公司合成,引物序列见表1。
1.4.2.5肝、肾组织STAT3免疫组化肝、肾组织切片脱蜡至水,经过抗原修复(微波修复法)后冷却至室温。滴加封闭液,加稀释的一抗(p-STAT3,稀释比例1∶100),4 ℃冰箱过夜。二抗37 ℃孵育,DAB显色。苏木素复染,二甲苯透明,中性树胶封片,光学显微镜下200倍观察并拍照。
1.4.2.6肝、肾组织IL-6、IL-1β及TNF-α水平检测取全部小鼠肝、肾组织各50 mg,加入0.5 mL生理盐水,匀浆机获得组织匀浆液后,4 ℃、15 840×g离心10 min,收集组织上清,ELISA法测定IL-6、IL-1β及TNF-α水平,具体操作步骤遵试剂盒说明书。
1.5统计学方法采用SPSS 24.0统计软件进行数据分析。计量资料以x±s 表示,多组间比较采用单因素方差分析,进一步两组间比较采用SNK-q检验。P<0.05为差异有统计学意义。
2结果
2.1各组小鼠一般情况正常组小鼠反应灵敏且动作迅速,被毛滑润有光泽,饮食正常。模型组小鼠倦怠,动作迟缓,被毛粗乱无光泽,食欲明显下降,AAⅠ 染毒第4天模型组死亡2只,占比20%。AR组及NAC组小鼠精神萎靡,皮毛無光泽,食欲下降;AAⅠ 染毒第4天NAC组死亡1只,占比10%。
2.2AR对AAⅠ 模型小鼠体质量和脏器指数的影响
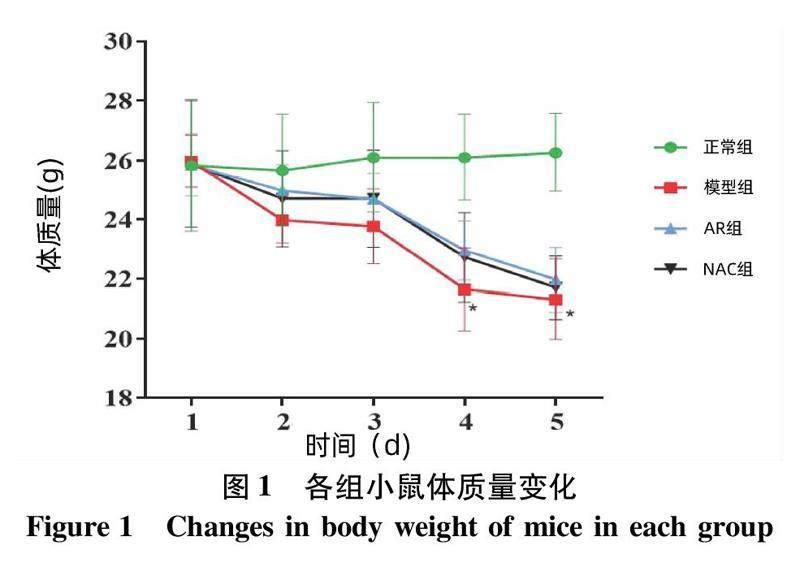
各组小鼠活动、饮水饮食均不受限,体质量变化如图1所示。结果显示,与正常组相比,模型组体质量下降明显(P<0.05);且小鼠肝脏及肾脏外观苍白。与模型组相比,AR组小鼠肝脏、肾脏外观未见明显苍白;NAC组肝脏外观正常未见明显苍白,肾脏肉眼可见苍白改变(图2)。
与正常组小鼠相比,模型组小鼠肝体比有下降趋势,但差异无统计学意义(P>0.05),肾体比显著升高(P<0.01)。与模型组小鼠相比,AR组小鼠肝体比有升高趋势,但差异无统计学意义(P>0.05),肾体比下降(P<0.01);NAC组小鼠肝体比升高,肾体比略有下降,但差异均无统计学意义(P值均>0.05)(表2)。
2.3AR对AAⅠ 模型小鼠肝、肾功能的影响血清肝功能提示,与正常组相比,模型组小鼠血清ALT、AST活性明显升高(P值均<0.01);与模型组相比,AR组ALT、AST及NAC组AST活性均下降(P值均<0.05)。血清肾功能提示,与正常组相比,模型组小鼠血清SCr、BUN含量明显升高(P值均<0.01);与模型组相比,AR组SCr、BUN含量及NAC组BUN含量明显下降(P值均<0.01)(表3)。
2.4AR对AAⅠ模型小鼠肝、肾组织形态学改变的影响HE染色结果显示,正常肝组织小叶结构完整、肝细胞排列整齐;模型组肝组织主要表现为灶状坏死、部分坏死、轻度汇管区炎症。正常肾组织结构正常,肾小球、肾小管、肾间质均未见到明显改变;模型组肾小球水肿、肾小球固缩,肾小管上皮细胞可见变性、坏死、细胞脱落。与模型组比较,AR组及NAC组可见肝细胞的坏死和变性程度有不同程度的减轻,肾小管上皮细胞坏死和变性程度也有明显减轻,其中AR组改善较NAC组明显(图3)。
2.5AR对AAⅠ 模型小鼠肝、肾组织STAT3基因表达的影响与正常组相比,模型组肝组织STAT3 mRNA表达上升(P<0.01);与模型组相比,AR组及NAC组肝组织STAT3表达量均下降(P值均<0.01)。
与正常组相比,模型组肾组织STAT3 mRNA表达上升(P<0.01),与模型组相比,AR组及NAC组肾组织STAT3表达量均下降(P值均<0.01)(表4)。
2.6各组小鼠肝、肾组织p-STAT3免疫组化染色结果免疫组化染色显示(图4),正常组小鼠肝、肾组织偶见p-STAT3阳性表达,主要表达部位在细胞核,在胞浆也可见阳性表达。AAⅠ 染毒后,模型组小鼠肝组织p-STAT3数量明显增多(P<0.01),主要分布在肝细胞核及胞浆;与模型组相比,AR组及NAC组肝组织p-STAT3表达均显著减少(P值均<0.01)。模型组小鼠肾组织p-STAT3数量也明显增多(P<0.01),主要分布在近曲小管上皮细胞及胞浆,肾小球及远区小管部也可见阳性表达;与模型组相比,AR组及NAC组肾组织p-STAT3也明显减少(P值均<0.01)(表5)。
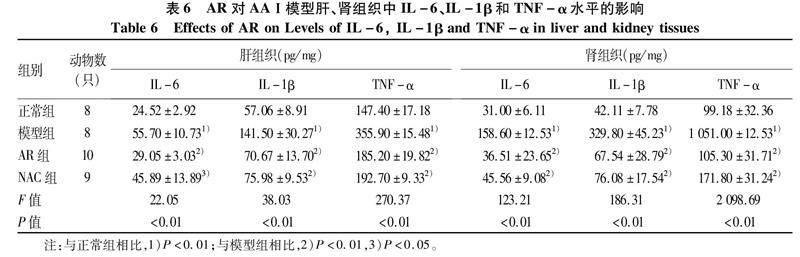
2.7AR对AAⅠ模型小鼠肝、肾组织IL-6、IL-1β和TNF-α水平的影响与正常组比较,模型组小鼠肝组织中IL-6、IL-1β和TNF-α均明显升高(P值均<0.01);与模型组比较,AR组及NAC组IL-6、IL-1β和TNF-α水平明显下降(P值均<0.05),AR与NAC效应相似。与正常组比较,模型组小鼠肾组织中IL-6、IL-1β和TNF-α均可见升高(P值均<0.01),且TNF-α升高约10倍;与模型组比较,AR组及NAC组IL-6、IL-1β和TNF-α可见明显下降(P值均<0.01),两药物组间无明显差异(表6)。
3讨论
《素问·阴阳应象大论》曰“肾生骨髓,髓生肝”,明代李中梓《医宗必读》提出“乙癸同源,肝肾同源”理论,中医理论揭示了肝、肾在物质方面相生相养,生理功能上相互为用、互相制约的密切关系,故在病理上也必然相互影响[13]。笔者团队前期研究中发现,AAⅠ 可导致急性肝、肾损伤,主要与炎症反应等有关。本研究结果提示,肝、肾组织中IL-6、IL-1β、TNF-α较正常组明显升高,这与相关研究[14]结果一致。IL-6、IL-1β、TNF-α等促炎因子激活JAK-STAT3信号通路,磷酸化的STAT3入核后介导炎症反应,导致肝、肾损伤[15-18]。因此,抑制炎症反应可能是缓解AAⅠ 致急性肝、肾毒性的良策。本研究以AAⅠ 诱导的急性肝、肾损伤模型,相比于正常组,模型组小鼠血清 ALT、AST 活性及 SCr、BUN 水平显著增加,肝组织可见肝小叶结构紊乱、灶状坏死和轻度汇管区炎症,肾组织可观察到肾小球水肿、肾小球固缩以及肾小管上皮细胞坏死、脱落,呈现明显的肝、肾毒性。
黄芪是中医临证最常用的药材,具有 “十药八芪”的说法,素有“补药之长”的美誉[19]。现代药理学研究[20-22]证明,黄芪具有增强机体免疫、抗氧化、抗炎等多种药理作用,能保护肝脏、肾脏、心脑血管等多种脏器。NAC是L-半胱氨酸加上乙酰基形成的,最初归为祛痰药类中的黏液溶解药,新研究表明NAC具有清除自由基和抗氧化作用,有助于保护线粒体功能、抑制炎症,改善肝功能[23-24]和肾功能[25-26],广泛应用于临床。
本研究以AR干预AAⅠ 诱导的急性肝、肾损伤,结果显示,AR组小鼠未见死亡;ALT、AST、SCr和BUN较模型组下降明显;病理结果提示,肝、肾组织炎性细胞浸润、结构破坏较模型组减轻,p-STAT3在肝、肾组织的表达也较模型组减少;ELISA结果显示肝、肾组织IL-6、IL-1β及TNF-α等炎性因子的表达降低,AR组保护肝、肾损伤的效用与NAC疗效相当。且AR对AAⅠ 诱导的小鼠急性肝、肾损伤的作用机制可能与其抑制肝、肾组织中 p-STAT3表达、下调促炎因子IL-6、IL-1β及TNF-α表达水平,减轻AAⅠ 诱导炎症反应有关。
伦理学声明:本研究方案于2021年8月经由上海中医药大学实验动物伦理委员会审批,批号:PZSHUTCM
210926011,符合实验室动物管理与使用准则。利益冲突声明:本文不存在任何利益冲突。作者贡献声明:朱哿瑞负责动物实验,资料分析,撰写论文;皮亚妮协助动物实验和数据整理;王静、黄恺、彭渊、陈高峰负责修改论文;刘成海指导课题设计,修改论文;陶艳艳负责拟定课题设计和写作思路,指导撰写文章并最后定稿。
参考文献:
[1]JI HJ, LI JY, WU SF, et al. Two new aristolochic acid analogues from the roots of Aristolochia contorta with significant cytotoxic activity[J]. Molecules, 2020, 26(1): 44. DOI: 10.3390/molecules26010044.
[2]HAN JY, XIAN Z, ZHANG YS, et al. Systematic overview of aristolochic acids: Nephrotoxicity, carcinogenicity, and underlying mechanisms[J]. Front Pharmacol, 2019, 10: 648. DOI: 10.3389/fphar.2019.00648.
[3]ZHU GR, WANG J, HUANG K, et al. A transcriptomic analysis of acute hepatotoxicity induced by aristolochic acid Ⅰ in mice[J]. J Clin Hepatol, 2021, 37(10): 2389-2394. DOI: 10.3969/j.issn.1001-5256.2021.10.026.朱哿瑞, 王靜, 黄恺, 等. 马兜铃酸 Ⅰ 致小鼠急性肝毒性的转录组学分析[J]. 临床肝胆病杂志, 2021, 37(10): 2389-2394. DOI: 10.3969/j.issn.1001-5256.2021.10.026.
[4]WANG F, WANG J, HUANG K, et al. Study on mechanism of aristolochic acid I induced acute kidney injury[J]. Nat Prod Res Dev, 2022, 34(5): 848-855. DOI: 10.16333/j.1001-6880.2022.5.014.王帆, 王静, 黄恺, 等. 马兜铃酸I致急性肾损伤的分子机制研究[J]. 天然产物研究与开发, 2022, 34(5): 848-855. DOI: 10.16333/j.1001-6880.2022.5.014.
[5]CAO YX, LI K, QIN XM, et al. Comparative study on different areas of Astragali Radix based on oligosaccharides characteristic map and immunological activity evaluation of partial acid hydrolyzed[J]. Chin Tradit Herb Drugs, 2020, 51(21): 5598-5606. DOI: 10.7501/j.issn.0253-2670.2020.21.025.曹宇欣, 李科, 秦雪梅, 等. 基于部分酸水解寡糖特征图谱及免疫活性评价的不同产地黄芪的品质比较[J]. 中草药, 2020, 51(21): 5598-5606. DOI: 10.7501/j.issn.0253-2670.2020.21.025.
[6]HU NN, ZHANG XJ. Research progress on chemical constituents and pharmacological effects of Astragalus membranaceus[J]. Inf Tradit Chin Med, 2021, 38(1): 76-82. DOI: 10.19656/j.cnki.1002-2406.210118.胡妮娜, 张晓娟. 黄芪的化学成分及药理作用研究进展[J]. 中医药信息, 2021, 38(1): 76-82. DOI: 10.19656/j.cnki.1002-2406.210118.
[7]CUI Y, LI HS, SONG NN, et al. Metabonomics reveals that aristolochic acid Ⅰ affects β-oxidation of fatty acids, glucose metabolism and the TCA cycle in the mice liver[J]. Chin J Pharmacovigil, 2019, 16(8): 449-466. DOI: 10.19803/j.1672-8629.2019.08.001.崔媛, 李海山, 宋乃寧, 等. 马兜铃酸Ⅰ影响小鼠肝脏脂肪酸β氧化和糖代谢以及TCA循环的代谢组学研究[J]. 中国药物警戒, 2019, 16(8): 449-466. DOI: 10.19803/j.1672-8629.2019.08.001.
[8]LIU X, XIAO Y, GAO HC, et al. Metabonomic study of aristolochic acid I-induced acute renal toxicity urine at female and male C57BL/6J mice based on 1H NMR[J]. Chem J Chin Univ, 2010, 31(5): 927-932.刘霞, 肖瑛, 高红昌, 等. 基于1H NMR代谢组学方法分析马兜铃酸I诱导的雌雄小鼠急性肾毒性[J]. 高等学校化学学报, 2010, 31(5): 927-932.
[9]PENG Y, ZHU GR, MA YY, et al. Network pharmacology-based prediction and pharmacological validation of effects of Astragali Radix on acetaminophen-induced liver injury[J]. Front Med, 2022, 9: 697644. DOI: 10.3389/fmed.2022.697644.
[10]LUO JH, YANG YB. Effect of N-acetylcysteine on oxidative stress in acute kidney injury induced by cisplatin[J]. Chin J Exp Tradit Med Formulae, 2012, 18(19): 170-175. DOI: 10.13422/j.cnki.syfjx.2012.19.052.罗景慧, 杨迎暴. N-乙酰半胱氨酸对顺铂诱导急性肾损伤后肾脏组织氧化应激水平的影响[J]. 中国实验方剂学杂志, 2012, 18(19): 170-175. DOI: 10.13422/j.cnki.syfjx.2012.19.052.
[11]WANG J, ZHAO S, REN BH. Protective effect and mechanism of N-acetylcysteine on cisplatin-induced nephrotoxicity[J]. Chin J Immunol, 2020, 36(4): 390-394. DOI: 10.3969/j.issn.1000-484X.2020.04.002.王健, 赵硕, 任博环. N-乙酰半胱氨酸对顺铂导致肾毒性的保护作用及作用机制分析[J]. 中国免疫学杂志, 2020, 36(4): 390-394. DOI: 10.3969/j.issn.1000-484X.2020.04.002.
[12]WANG D, QI J, PAN XQ, et al. The antagonistic effect and mechanism of N-acetylcysteine on acrylamide-induced hepatic and renaltoxicity[J]. Chin J Ind Hyg Occup Dis, 2016, 34(1): 13-17.王敦, 齐健, 潘校琦, 等. N-乙酰半胱氨酸拮抗丙烯酰胺的肝肾毒性及机制[J]. 中华劳动卫生职业病杂志, 2016, 34(1): 13-17.
[13]WU N, GAO X, YE ZH, et al. Inheritance and innovation of theory of homogeny of liver and kidney for LI Hanmin[J]. Chin Arch Tradit Chin Med, 2018, 36(3): 619-622. DOI: 10.13193/j.issn.1673-7717.2018.03.025.吴娜, 高翔, 叶之华, 等. 李瀚旻教授对“肝肾同源”理论的继承创新[J]. 中华中医药学刊, 2018, 36(3): 619-622. DOI: 10.13193/j.issn.1673-7717.2018.03.025.
[14]ANGER EE, YU F, LI J. Aristolochic acid-induced nephrotoxicity: Molecular mechanisms and potential protective approaches[J]. Int J Mol Sci, 2020, 21(3): 1157. DOI: 10.3390/ijms21031157.
[15]DAI ZC, WANG XH, PENG RX, et al. Induction of IL-6Rα by ATF3 enhances IL-6 mediated sorafenib and regorafenib resistance in hepatocellular carcinoma[J]. Cancer Lett, 2022, 524: 161-171. DOI: 10.1016/j.canlet.2021.10.024.
[16]CLEMENS MM, KENNON-MCGILL S, VAZQUEZ JH, et al. Exogenous phosphatidic acid reduces acetaminophen-induced liver injury in mice by activating hepatic interleukin-6 signaling through inter-organ crosstalk[J]. Acta Pharm Sin B, 2021, 11(12): 3836-3846. DOI: 10.1016/j.apsb.2021.08.024.
[17]CHEN W, YUAN H, CAO WM, et al. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation[J]. Theranostics, 2019, 9(14): 3980-3991. DOI: 10.7150/thno.32352.
[18]ZHENG C, HUANG L, LUO W, et al. Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice[J]. Cell Death Dis, 2019, 10(11): 848. DOI: 10.1038/s41419-019-2085-0.
[19]SINCLAIR S. Chinese herbs: A clinical review of Astragalus, Ligusticum, and Schizandrae[J]. Altern Med Rev, 1998, 3(5): 338-344.
[20]JIANG H, GU SL, ZHANG YT, et al. Research progress on chemical constituents and pharmacological effects of Astragalus membranaceus[J]. J Anhui Univ Chin Med, 2020, 39(5): 93-96. DOI: 10.3969/j.issn.2095-7246.2020.05.022.姜辉, 顾胜龙, 张玉婷, 等. 黄芪化学成分和药理作用研究进展[J]. 安徽中医药大学学报, 2020, 39(5): 93-96. DOI: 10.3969/j.issn.2095-7246.2020.05.022.
[21]LI Y, MA W. Analysis on the medication rule of Chinese medicine in the treatment of interstitial pneumonia based on data mining[J]. Chin Med Herald, 2023, 20(14): 146-150. DOI: 10.20047/j.issn1673-7210.2023.14.31.李杨, 马伟. 基于数据挖掘的含有黄芪的中成药配伍规律研究[J]. 中国医药导报, 2023, 20(14): 146-150. DOI: 10.20047/j.issn1673-7210.2023.14.31.
[22]HUANG LJ, DENG XL, QIN JF, et al. Effects of Huangqi injection assisted PHGF therapy on liver function and serum sST2 and IL-33 in patients with viral hepatitis[J]. Clin Misdiagn Misther, 2022, 35(4): 21-25, 30.黄丽静, 邓喜亮, 覃金凤, 等. 黄芪注射液辅助PHGF治疗对病毒性肝炎患者肝功能及血清sST2、IL-33的影响[J]. 临床误诊误治, 2022, 35(4): 21-25, 30.
[23]WANG SM, GENG JB, WANG M, et al. Therapeutic effect of acetylcysteine on drug-induced liver injury[J]. Chin Hepatol, 2017, 22(1): 32-34. DOI: 10.14000/j.cnki.issn.1008-1704.2017.01.011.王寿明, 耿家宝, 王敏, 等. 乙酰半胱氨酸治疗药物性肝损伤疗效观察[J]. 肝脏, 2017, 22(1): 32-34. DOI: 10.14000/j.cnki.issn.1008-1704.2017.01.011.
[24]AI G, WANG M, ZHU JL, et al. Preliminary study on the clinical efficacy of N-acetylcysteine in the treatment of patients with chronic icteric hepatitis B[J]. J Pract Hepatol, 2020, 23(3): 336-339. DOI: 10.3969/j.issn.1672-5069.2020.03.009.艾國, 王鸣, 朱纪玲, 等. 应用N-乙酰半胱氨酸治疗慢性乙型肝炎重度患者临床疗效初步研究[J]. 实用肝脏病杂志, 2020, 23(3): 336-339. DOI: 10.3969/j.issn.1672-5069.2020.03.009.
[25]MAGNER K, ILIN JV, CLARK EG, et al. Meta-analytic techniques to assess the association between N-acetylcysteine and acute kidney injury after contrast administration: A systematic review and meta-analysis[J]. JAMA Netw Open, 2022, 5(7): e2220671. DOI: 10.1001/jamanetworkopen.2022.20671.
[26]LI QW, LIAO JZ, CHEN WJ, et al. NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway[J]. Free Radic Biol Med, 2022, 187: 158-170. DOI: 10.1016/j.freeradbiomed.2022.05.024.
收稿日期:2022-11-23;录用日期:2023-01-18
本文编辑:朱晶
引证本文:ZHU GR, PI YN, WANG J, et al. Efficacy of Astragali Radix extract in treatment of a mouse model of aristolochic acid Ⅰ-induced liver and renal injury by regulating the IL-6/STAT3 signaling pathway[J]. J Clin Hepatol, 2023, 39(8): 1903-1910.