长链非编码小核仁RNA宿主基因(lncRNA SNHG)在肝细胞癌发生发展中的作用
2023-04-29李雨韩郭北辰周凤韩涛
李雨韩 郭北辰 周凤 韩涛
摘要:肝细胞癌 (HCC) 是原发性肝癌中最常见的类型,也是癌症相关死亡的主要原因之一。尽管目前对HCC的研究逐渐深入,但其潜在的分子机制仍不清楚。近年来研究发现,长链非编码RNA (lncRNA) 在HCC的发生和发展中起着重要作用,而长链非编码小核仁RNA宿主基因 (lncRNA SNHG) 在HCC中异常表达并在肿瘤细胞的增殖、侵袭、转移、血管生成、干细胞特性及耐药性等方面发挥调节作用。本文综述了lncRNA SNHG基因家族在HCC中的发生机制及预后价值,以期为该领域的基础和临床研究提供新思路。关键词:RNA, 长链非编码; 癌, 肝细胞; 作用机制; 预后基金项目:天津市自然科学基金 (19JCZDJC36700)
Research advances in long non-coding small nucleolar RNA host gene in hepatocellular carcinoma
LI Yuhan GUO Beichen ZHOU Feng HAN Tao(1. Department of Gastroenterology and Hepatology, Clinical Medical College of Tianjin Medical University, Tianjin 300121, China; 2. Department of Gastroenterology and Hepatology, Tianjin Union Medical Center, Nankai University, Tianjin 300121, China)
Corresponding author:HAN Tao, hantaomd@126.com (ORCID:0000-0003-4216-6968)
Abstract:Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and is one of the leading causes of cancer-related deaths. Despite the in-depth research on HCC at present, the underlying molecular mechanisms are still unclear. Recent studies have revealed that long non-coding RNAs (lncRNAs) play an important role in the development and progression of HCC, and long non-coding RNA small nucleolar RNA host genes (lncRNA SNHGs) show abnormal expression in HCC and play regulatory roles in the proliferation, invasion, metastasis, angiogenesis, stemness, and drug resistance of tumor cells. This article reviews the mechanism and prognostic value of the lncRNA SNHG family in HCC, so as to provide new ideas for basic and clinical research in this field.
Key words:RNA, Long Noncodings; Carcinoma, Hepatocellular; Mechanism; Prognosis
Research funding:The Natural Science Foundation of Tianjin (19JCZDJC36700)
肝细胞癌 (HCC) 是全球第五大恶性肿瘤,约占原发性肝癌的90%,并具有病死率高,易复发、易转移等特点, 临床预后极差, 其治疗方案取决于疾病分期[1-2]。然而,由于HCC早期临床症状和体征多不明显,多数患者确诊时已属中晚期,失去了根治性治疗的机会。HCC发生包括癌基因的激活、抑癌基因失活等多种分子改变[3]。但其具体的作用机制尚不清楚,因此深入了解HCC的分子機制对于寻求新的诊断和治疗策略,提高患者生存率及改善预后有着重要价值。
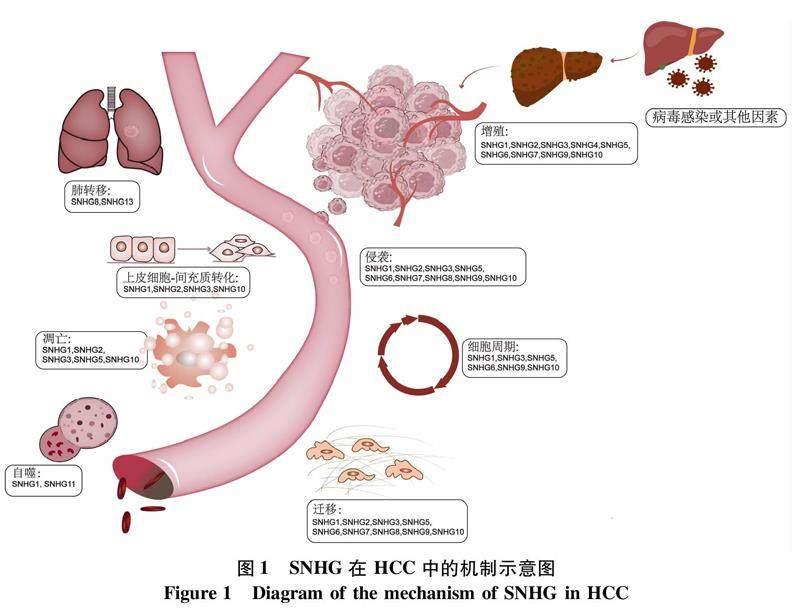
长链非编码RNA (long non-coding RNA, lncRNA)是一类长度 > 200个核苷酸的片段,通常缺乏编码蛋白质的能力。lncRNA 在调控基因表达和正常生理过程等多方面都发挥着重要作用,其表达和功能失调是包括癌症在内多种疾病发生和发展的关键[4]。小核仁RNA (small nucleolar RNA, snoRNA) 是一类主要存在于核仁中的非编码RNA,超过一半的snoRNA是从宿主基因转录而来,其中小核仁RNA宿主基因 (small nucleolar RNA host gene, SNHG) 占了将近4/5。目前SNHG家族包括22个成员(SNHG1~SNHG22),大多数成员已被证明可在癌症进展中发挥重要作用[5]。SNHG作为一种lncRNA可通过多种分子调节机制参与肿瘤发生:在细胞核中,SNHG通过甲基化酶影响DNA甲基化或与转录因子相互作用调控基因转录;在细胞质中,可以通过充当微小RNA (microRNA, miRNA) 海绵来影响mRNA的稳定性或其翻译[6]。SNHG在HCC中发挥的作用多样且复杂(图1),本文对SNHG家族分子在HCC中已知的作用机制及预后价值进行综述。1SNHG在HCC发生发展中的作用
1.1SNHG调节HCC细胞的生长和转移研究发现,一些SNHG可影响HCC细胞体内外生长和转移能力。SNHG1,位于染色体11q12.3,有研究[7]发现SNHG1上调可以抑制细胞质中的miR-140-5p的表达,进而上调细胞周期依赖性激酶4的表达,促进细胞生长、细胞周期进展,上皮-间充质转化 (epithelial-mesenchymal transition, EMT) 也参与这一过程,促进肿瘤转移。GAS5,位于1q25,也被称为SNHG2,是几种癌症的潜在肿瘤抑制因子,如乳腺癌、前列腺癌、肺癌。研究[8]表明,在HCC中,GAS5可抑制HCC细胞的增殖、迁移和侵袭。已有研究[9]报道SNHG3可能是HCC的致癌因子,miR-139-5p是SNHG3的靶点,BMI1是miR-139-5p的直接靶点mRNA,敲除SNHG3或BMI1,以及过表达miR-139-5p可以抑制HCC细胞的增殖、迁移和侵袭。Yang等[10]发现在HCCLM3和MHCC97H细胞中,敲除SNHG7可以明显抑制细胞的增殖、迁移和侵袭,其机制可能是SNHG7通过调节miR-122-5p和核糖体蛋白L4的稳定性来促进HCC细胞的生长和转移。SNHG8表达水平在HCC中显著上调,Dong等[11]研究发现SHNG8可能通过miR-149促进HCC细胞侵袭和肺部转移,是影响HCC患者肿瘤复发的独立预后因素。研究[12]发现,SNHG9在HCC组织中上调,通过招募DNA甲基化酶 (DNA methyltransferase, DNMT) 包括 DNMT1、DNMT3A和DNMT3B,增加谷胱甘肽转移酶P1 (glutathione-S-transferase Pi 1, GSTP1) 启动子甲基化,从而促进HCC细胞的增殖、迁移和侵袭。SNHG10位于14q32.13,其表达水平在多种癌症中存在差异。Lan等[13]发现SNHG10与其同源基因SCARNA13形成正反馈环,促进HCC的转移,SOX9是SCARNA13的一个下游蛋白,可以对癌细胞的细胞周期和EMT产生巨大影响,高表达的SNHG10通过调控SOX9,促进HCC细胞恶性表型、细胞增殖、侵袭和迁移。SNHG12可直接与miR-199a/b-5p相互作用影响混合谱系激酶3的表达,进而通过NF-κB通路促进HCC的发生和转移[14]。此外,SNHG12还可通过海绵化miR-516-5p,激活Wnt/β-catenin信号通路以诱导EMT[15]。Xu等[16]在收集的55对HCC组织及相应的癌旁组织中用RT-qPCR检测SNHG14的表达,与癌旁组织相比,HCC组织中SNHG14的表达显著升高,同时与部分关键的致癌基因(IGF1R、KLF5、E2F3、MTDH)存在正相关关系,通过海绵化miR-217促进HCC细胞的增殖。Li等[17]研究发现SNHG16的下调可以抑制肿瘤细胞的转移和EMT的进展,通过吸附let-7b-5p促进G2/M细胞周期转换和EMT进展。有研究表明,SNHG17的表达在HCC中明显上调,Ma等[18]认为,SNHG17的高表达可以与miR-3180-3p相互作用,促进HCC细胞的增殖、侵袭和迁移。Tu等[19]提出,HBV可通过SNHG20/PTEN信号通路促进HCC细胞增殖。
1.2SNHG调节HCC血管生成HCC是高度血管化的肿瘤,其特征是肿瘤内血管丰富,血管生成在肿瘤的生长转移和侵袭中扮演重要角色[20]。研究[21]表明,SNHG14过度表达诱导人脐静脉内皮细胞的毛细血管结构分支增多,其机制可能是SNHG14/细胞质多聚腺苷酸结合蛋白1通过PTEN信号通路在体内/外促进血管生成。此外,SNHG17的表达在公共数据库和收集的 HCC 组织及 HCC 细胞系中均显著上调,其表达升高与瘤体较大、分化较差、血管浸润有明显的相关性[22]。
1.3SNHG调节HCC免疫中的作用SNHG3可上调PD-1的表达水平,并进一步通过调节抗沉默功能1B(anti-silencing function 1B, ASF1B)蛋白抑制HCC的免疫反应,从而激活肿瘤免疫耐受和免疫逃逸能力[23]。自然杀伤(NK) 细胞被认为是抵抗癌症的第一道防线,是固有免疫应答的组成部分, 并且负责癌细胞的快速识别和消除, Fang等[24]研究发现, 在HCC中,GAS5在NK细胞中表达下调;而GAS5高表达通过调节miR-544/Runt相关转录因子3增强NK细胞的杀伤力,促进IFN-γ的分泌,进而抑制肿瘤生长和转移。
1.4SNHG调节肿瘤干细胞(cancer stem cell,CSC)特性CSC是肿瘤内的细胞群,具有自我更新、逃避药物作用和重建异源性肿瘤的能力,有研究[25]表明,CSC可能是肿瘤转移、疾病复发和最终患者死亡的原因。Li等[26]证实SNHG5在肝脏 CSC 特性中的关键作用,SNHG5改变(敲低或上调)显著影响肝脏 CSC 的增殖和自我更新能力,SNHG5下调后,CSC标志物和干细胞因子的表达下降。
2SNHG在HCC中的临床价值
2.1SNHG与HCC预后的关系HCC的预后相关指标与SNHG表达密切相关。Gao等[27]研究发现SNHG1和AFP的组合诊断比单独的AFP具有更高的诊断准确性。SNHG15的升高与HCC患者的总生存期呈正相关,并可作为HCC患者的独立预后指标[28]。SNHG3与HCC的不良预后显著相关[29]。Zhu等[30]研究发现高表达SNHG4的患者总生存期明显短于低表达患者。SNHG7的表达与HCC的进展呈正相关,与HCC患者的总生存期和无进展生存期呈负相关, SNHG7的高表达与肿瘤数量、淋巴结转移和血管侵袭有关,但与性别、年龄、AFP水平、HBsAg水平和肿瘤大小无关[10]。Ye等[12]研究表明,SNHG9在HCC中的高表达与较短的无病生存期有关。Kaplan-Meier分析显示,SNHG10、SNHG11高表达的患者,总生存期有缩短趋势[13,31]。Lan等[14]研究显示,SNHG12在HCC中的表达水平与肿瘤大小、血管浸润和TNM分期呈正相关。DANCR的上调与患者较短的生存期相关;同时,Kaplan-Meier分析显示,术后DANCR表达水平较高的患者復发率较高,生存率较低[32-33]。此外,SNHG14、SNHG17的高表达与血管浸润、TNM 分期和总生存期有关[16,22]。研究[8]表明,GAS5表达水平越高,HCC患者的生存期越长,GAS5是HCC患者的独立预后因素。Liu等[34]研究表明,与相应的癌旁组织相比,SNHG18在HCC组织中表达水平明显降低,并且与肿瘤大小和血清AFP水平相关。
2.2SNHG和HCC耐藥性的关系部分晚期HCC患者可以通过药物治疗获益[35]。然而,多数患者最终会产生耐药性,导致不良预后。因此,了解HCC耐药的分子机制十分重要。研究[36]表明,SNHG1通过上调SLC3A2激活HCC细胞的AKT通路来增加HCC患者对索拉非尼耐药性。GAS5可以调节HCC细胞对化疗的反应,即GAS5的敲除可以增加HCC细胞对多柔比星的耐药性;GAS5的过表达通过调节miR-222削弱HCC细胞对顺铂的耐药性[37-38]。此外,SNHG3高表达可以增加HCC细胞对索拉非尼的耐药性[39]。敲除DANCR可以改善HCC对索拉非尼的治疗反应,其机制可能是通过增加PSMD10的表达和激活IL-6/ STAT3信号通路[33]。此外,敲除SNHG16也可以增加肿瘤细胞对顺铂和索拉非尼的敏感性[17,40]。
3小结
HCC是一种高度恶性肿瘤,预后较差,目前的治疗方案疗效有限。近年研究证实,lncRNA 可作为癌基因或抑癌基因在各种癌症中发挥作用,SNHG 是新近发现在许多恶性肿瘤中异常表达的 lncRNA,其表达水平与临床病理特征如TNM 分期、淋巴转移、肿瘤大小和总生存期较短等有关。在HCC中,SNHG可作用于相关miRNA, 解除miRNA对靶基因的抑制作用;此外,还可以通过调控P53、Notch或Wnt/β-catenin等信号通路调节细胞增殖、细胞周期、侵袭、转移、凋亡和自噬等过程进一步影响HCC患者的预后。此外,SNHG家族的一些成员可影响HCC细胞的化疗反应,可能是克服癌症耐药性的潜在靶标。
虽然已有研究证明SNHG在HCC发生发展中的重要性,但相关研究仍处于早期阶段,仍有许多问题亟待解决,比如:(1) SNHG 家族的其他成员 (例如SNHG19~SNHG21) 尚未在HCC中进行研究,需要通过生物信息学和基础实验进一步研究验证;(2) 目前对于HCC患者血浆或血清等体液中 SNHGS 的表达水平知之甚少,为今后的临床应用带来局限。因此,未来仍需要更深入的研究来揭示SNHG在肝癌中的作用。
利益冲突声明:本文不存在任何利益冲突。作者贡献声明:李雨韩撰写论文;郭北辰、周凤负责查阅及分析文献;韩涛负责指导文章撰写。
参考文献:
[1]WEI L, WANG X, LV L, et al. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma[J]. Mol Cancer, 2019, 18(1): 147. DOI: 10.1186/s12943-019-1086-z.
[2]LI Y, WANG X, CHEN S, et al. Long non-coding RNA small nucleolar RNA host genes: functions and mechanisms in hepatocellular carcinoma[J]. Mol Biol Rep, 2022, 49(3): 2455-2464. DOI: 10.1007/s11033-021-07018-0.
[3]JIANG Y, HAN QJ, ZHANG J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy[J]. World J Gastroenterol, 2019, 25(25): 3151-3167. DOI: 10.3748/wjg.v25.i25.3151.
[4]WANG PS, WANG Z, YANG C. Dysregulations of long non-coding RNAs - The emerging “lnc” in environmental carcinogenesis[J]. Semin Cancer Biol, 2021, 76: 163-172. DOI: 10.1016/j.semcancer.2021.03.029.
[5]SAEINASAB M, ATLASI Y, M MATIN M. Functional role of lncRNAs in gastrointestinal malignancies: the peculiar case of small nucleolar RNA host gene family[J]. FEBS J, 2022. DOI: 10.1111/febs.16668. [Online ahead of print]
[6]LI J, YU H, YAO J, et al. Integrative analysis and experimental validation indicated that SNHG17 is a prognostic marker in prostate cancer and a modulator of the tumor microenvironment via a competitive endogenous RNA regulatory network[J]. Oxid Med Cell Longev, 2022, 2022: 1747604. DOI: 10.1155/2022/1747604.
[7]LI B, LI A, YOU Z, et al. Epigenetic silencing of CDKN1A and CDKN2B by SNHG1 promotes the cell cycle, migration and epithelial-mesenchymal transition progression of hepatocellular carcinoma[J]. Cell Death Dis, 2020, 11(10): 823. DOI: 10.1038/s41419-020-03031-6.
[8]ZHANG WY, ZHAN HL, LI MK, et al. Long noncoding RNA Gas5 induces cell apoptosis and inhibits tumor growth via activating the CHOP-dependent endoplasmic reticulum stress pathway in human hepatoblastoma HepG2 cells[J]. J Cell Biochem, 2022, 123(2): 231-247. DOI: 10.1002/jcb.30159.
[9]WU J, LIU L, JIN H, et al. LncSNHG3/miR-139-5p/BMI1 axis regulates proliferation, migration, and invasion in hepatocellular carcinoma[J]. Onco Targets Ther, 2019, 12: 6623-6638. DOI: 10.2147/OTT.S196630.
[10]YANG X, SUN L, WANG L, et al. LncRNA SNHG7 accelerates the proliferation, migration and invasion of hepatocellular carcinoma cells via regulating miR-122-5p and RPL4[J]. Biomed Pharmacother, 2019, 118: 109386. DOI: 10.1016/j.biopha.2019.109386.
[11]DONG J, TENG F, GUO W, et al. lncRNA SNHG8 promotes the tumorigenesis and metastasis by sponging miR-149-5p and predicts tumor recurrence in hepatocellular carcinoma[J]. Cell Physiol Biochem, 2018, 51(5): 2262-2274. DOI: 10.1159/000495871.
[12]YE S, NI Y. lncRNA SNHG9 promotes cell proliferation, migration, and invasion in human hepatocellular carcinoma cells by increasing GSTP1 methylation, as revealed by CRISPR-dCas9[J]. Front Mol Biosci, 2021, 8: 649976. DOI: 10.3389/fmolb.2021.649976.
[13]LAN T, YUAN K, YAN X, et al. LncRNA SNHG10 facilitates hepatocarcinogenesis and metastasis by modulating its homolog SCARNA13 via a positive feedback loop[J]. Cancer Res, 2019, 79(13): 3220-3234. DOI: 10.1158/0008-5472.CAN-18-4044.
[14]LAN T, MA W, HONG Z, et al. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2017, 36(1): 11. DOI: 10.1186/s13046-016-0486-9.
[15]CHEN PP, ZHANG ZS, WU JC, et al. LncRNA SNHG12 promotes proliferation and epithelial mesenchymal transition in hepatocellular carcinoma through targeting HEG1 via miR-516a-5p[J]. Cell Signal, 2021, 84: 109992. DOI: 10.1016/j.cellsig.2021.109992.
[16]XU X, SONG F, JIANG X, et al. Long non-coding RNA SNHG14 contributes to the development of hepatocellular carcinoma via sponging miR-217[J]. Onco Targets Ther, 2020, 13: 4865-4876. DOI: 10.2147/OTT.S244530.
[17]LI S, PENG F, NING Y, et al. SNHG16 as the miRNA let-7b-5p sponge facilitates the G2/M and epithelial-mesenchymal transition by regulating CDC25B and HMGA2 expression in hepatocellular carcinoma[J]. J Cell Biochem, 2020, 121(3): 2543-2558. DOI: 10.1002/jcb.29477.
[18]MA T, ZHOU X, WEI H, et al. Long non-coding RNA SNHG17 upregulates RFX1 by sponging miR-3180-3p and promotes cellular function in hepatocellular carcinoma[J]. Front Genet, 2020, 11: 607636. DOI: 10.3389/fgene.2020.607636.
[19]TU W, YANG Y, SONG Y, et al. Hepatitis B virus x protein accelerated the proliferation of hepatocellular carcinoma cell through lncRNA SNHG20/PTEN pathway[J]. J Biochem, 2019, 165(5): 423-431. DOI: 10.1093/jb/mvy120.
[20]SHIGETA K, DATTA M, HATO T, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma[J]. Hepatology, 2020, 71(4): 1247-1261. DOI: 10.1002/hep.30889.
[21]ZHANG H, XU HB, KURBAN E, et al. LncRNA SNHG14 promotes hepatocellular carcinoma progression via H3K27 acetylation activated PABPC1 by PTEN signaling[J]. Cell Death Dis, 2020, 11(8): 646. DOI: 10.1038/s41419-020-02808-z.
[22]LUO Y, LIN J, ZHANG J, et al. LncRNA SNHG17 contributes to proliferation, migration, and poor prognosis of hepatocellular carcinoma[J]. Can J Gastroenterol Hepatol, 2021, 2021: 9990338. DOI: 10.1155/2021/9990338.
[23]ZHAN T, GAO X, WANG G, et al. Construction of novel lncRNA-miRNA-mRNA network associated with recurrence and identification of immune-related potential regulatory axis in hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 626663. DOI: 10.3389/fonc.2021.626663.
[24]FANG P, XIANG L, CHEN W, et al. LncRNA GAS5 enhanced the killing effect of NK cell on liver cancer through regulating miR-544/RUNX3[J]. Innate Immun, 2019, 25(2): 99-109. DOI: 10.1177/1753425919827632.
[25]WANG G, XU J, ZHAO J, et al. Arf1-mediated lipid metabolism sustains cancer cells and its ablation induces anti-tumor immune responses in mice[J]. Nat Commun, 2020, 11(1): 220. DOI: 10.1038/s41467-019-14046-9.
[26]LI Y, HU J, GUO D, et al. LncRNA SNHG5 promotes the proliferation and cancer stem cell-like properties of HCC by regulating UPF1 and Wnt-signaling pathway[J]. Cancer Gene Ther, 2022, 29(10): 1373-1383. DOI: 10.1038/s41417-022-00456-3.
[27]GAO S, XU X, WANG Y, et al. Diagnostic utility of plasma lncRNA small nucleolar RNA host gene 1 in patients with hepatocellular carcinoma[J]. Mol Med Rep, 2018, 18(3): 3305-3313. DOI: 10.3892/mmr.2018.9336.
[28]SHUAI Y, MA Z, LU J, et al. LncRNA SNHG15: A new budding star in human cancers[J]. Cell Prolif, 2020, 53(1): e12716. DOI: 10.1111/cpr.12716.
[29]ZHANG Z, WANG F, ZHANG J, et al. An m6A-related lncRNA signature predicts the prognosis of hepatocellular carcinoma[J]. Front Pharmacol, 2022, 13: 854851. DOI: 10.3389/fphar.2022.854851.
[30]ZHU Q, YANG H, CHENG P, et al. Bioinformatic analysis of the prognostic value of the lncRNAs encoding snoRNAs in hepatocellular carcinoma[J]. Biofactors, 2019, 45(2): 244-252. DOI: 10.1002/biof.1478.
[31]HUANG W, HUANG F, LEI Z, et al. LncRNA SNHG11 promotes proliferation, migration, apoptosis, and autophagy by regulating hsa-miR-184/AGO2 in HCC[J]. Onco Targets Ther, 2020, 13: 413-421. DOI: 10.2147/OTT.S237161.
[32]GUO D, LI Y, CHEN Y, et al. DANCR promotes HCC progression and regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1 pathway[J]. Cell Prolif, 2019, 52(4): e12628. DOI: 10.1111/cpr.12628.
[33]LIU Y, CHEN L, YUAN H, et al. LncRNA DANCR promotes sorafenib resistance via activation of IL-6/STAT3 signaling in hepatocellular carcinoma cells[J]. Onco Targets Ther, 2020, 13: 1145-1157. DOI: 10.2147/OTT.S229957.
[34]LIU XF, THIN KZ, MING XL, et al. Small nucleolar RNA host gene 18 acts as a tumor suppressor and a diagnostic indicator in hepatocellular carcinoma[J]. Technol Cancer Res Treat, 2018, 17: 1533033818794494. DOI: 10.1177/1533033818794494.
[35]SANGRO B, SAROBE P, HERVS-STUBBS S, et al. Advances in immunotherapy for hepatocellular carcinoma[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(8): 525-543. DOI: 10.1038/s41575-021-00438-0.
[36]LI W, DONG X, HE C, et al. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR-21 in hepatocellular carcinoma cells[J]. J Exp Clin Cancer Res, 2019, 38(1): 183. DOI: 10.1186/s13046-019-1177-0.
[37]WANG C, KE S, LI M, et al. Downregulation of LncRNA GAS5 promotes liver cancer proliferation and drug resistance by decreasing PTEN expression[J]. Mol Genet Genomics, 2020, 295(1): 251-260. DOI: 10.1007/s00438-019-01620-5.
[38]ZHAO P, CUI X, ZHAO L, et al. Overexpression of growth-arrest-specific transcript 5 improved cisplatin sensitivity in hepatocellular carcinoma through sponging miR-222[J]. DNA Cell Biol, 2020, 39(4): 724-732. DOI: 10.1089/dna.2019.5282.
[39]ZHANG PF, WANG F, WU J, et al. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma[J]. J Cell Physiol, 2019, 234(3): 2788-2794. DOI: 10.1002/jcp.27095.
[40]YE J, ZHANG R, DU X, et al. Long noncoding RNA SNHG16 induces sorafenib resistance in hepatocellular carcinoma cells through sponging miR-140-5p[J]. Onco Targets Ther, 2019, 12: 415-422. DOI: 10.2147/OTT.S175176.
收稿日期:2022-11-08;錄用日期:2023-02-20
本文编辑:王莹
引证本文:LI YH, GUO BC, ZHOU F, et al. Research advances in long non-coding small nucleolar RNA host gene in hepatocellular carcinoma[J]. J Clin Hepatol, 2023, 39(8): 1977-1982.