环状RNA在肝细胞癌发生发展及诊疗中的作用
2023-04-29苗淑莹杨军管文燕张标何璐樊智文
苗淑莹 杨军 管文燕 张标 何璐 樊智文
摘要:肝细胞癌(HCC)作为原发性肝癌最常见的类型,是一种具有侵袭性且致命的恶性肿瘤,其发生发展是一个多基因参与、多步骤、多阶段的过程。环状 RNA(circRNA)作為一类内源性非编码RNA,主要通过吸附微小RNA(miRNA)或者RNA结合蛋白(RBP)发挥“海绵作用”,进而调控下游靶基因表达。本文全面介绍了circRNA在HCC信号转导、免疫、代谢、耐药、HBV相关HCC中的作用及意义,及其作为HCC的生物标志物或治疗靶点的潜在价值,为HCC的诊断和治疗提供新思路。关键词:RNA, 环状; 癌, 肝细胞; 诊断; 治疗学基金项目:国家自然科学基金(81700554, 82170592)
Role of circular RNA in the development, progression, diagnosis, and treatment of hepatocellular carcinoma
MIAO Shuying, YANG Jun, GUAN Wenyan, ZHANG Biao, HE Lu, FAN Zhiwen. (Department of Pathology, Nanjing Drum Tower Hospital, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing 210008, China)
Corresponding author:FAN Zhiwen, fanzhiwenfff@126.com (ORCID:0000-0002-3465-4622)
Abstract:As the most common type of primary liver cancer, hepatocellular carcinoma (HCC) is an invasive and fatal malignant tumor, and its development and progression involve multiple genes, steps, and stages. Circular RNA (circRNA), as a class of endogenous non-coding RNAs, mainly acts as a sponge by absorbing microRNA or RNA-binding proteins to regulate the expression of downstream target genes. This article comprehensively introduces the role and significance of circRNA in signal transduction, immunity, metabolism, drug resistance, and hepatitis B virus-related HCC and its potential value as a biomarker or therapeutic target for HCC, so as to provide new ideas for the diagnosis and treatment of HCC.
Key words:RNA, Circular; Carcinoma, Hepatocellular; Diagnosis; Therapeutics
Research funding: The National Natural Science Foundation of China (81700554, 82170592)
肝细胞癌(HCC)是肝癌最常见和最致命的组织学类型,是世界范围内癌症相关死亡的第二大原因[1]。每年全世界HCC的发病率超过50万,并且逐年上升。慢性HBV/HCV感染、酒精性损伤、非酒精性脂肪性肝病、黄曲霉毒素、肥胖、糖尿病和肝硬化被认为是HCC的主要危险因素[2]。原位肝移植和手术切除是目前治疗肝癌最有效的方法,虽然索拉非尼和瑞戈非尼已被用于HCC的一线二线全身化疗,但对其耐药导致高病死率的担忧日益增加。大多数HCC患者被确诊时已为疾病晚期,错过了最好的治疗时机。另一方面,HCC易扩散转移、术后易复发,导致HCC患者术后5年的转移或复发率仍然很高,生存期较短[3]。因此,需要更多可靠的生物标志物用于HCC的诊断、治疗和监测。越来越多的研究发现环状RNA (circular RNA,circRNA)在HCC的发生发展中发挥着重要的调控作用。
1circRNA概述
circRNA于1976年在类病毒颗粒上被首次发现[4]。其通过反式剪接使3端和5端以共价键相连接形成1个闭合环状结构。得益于其闭合的环状结构,circRNA对核酸外切酶不敏感。已知的circRNA的功能机制可以大致分为四类: (1)作为miRNA海绵;(2)调控转录过程;(3)与RNA结合蛋白(RNA-binding proteins,RBP)相互作用;(4)参与肽或蛋白质翻译。circRNA具有丰富性、动态性、保守性、稳定性,这些特性使circRNA在作为新型临床诊断相关生物标志物的开发应用上具有明显优势。新的证据表明,circRNA在HCC的发生和发展中发挥重要作用,并参与细胞增殖、肿瘤转移、免疫逃逸、代谢和耐药[5]。
2circRNA在HCC发生发展中的作用
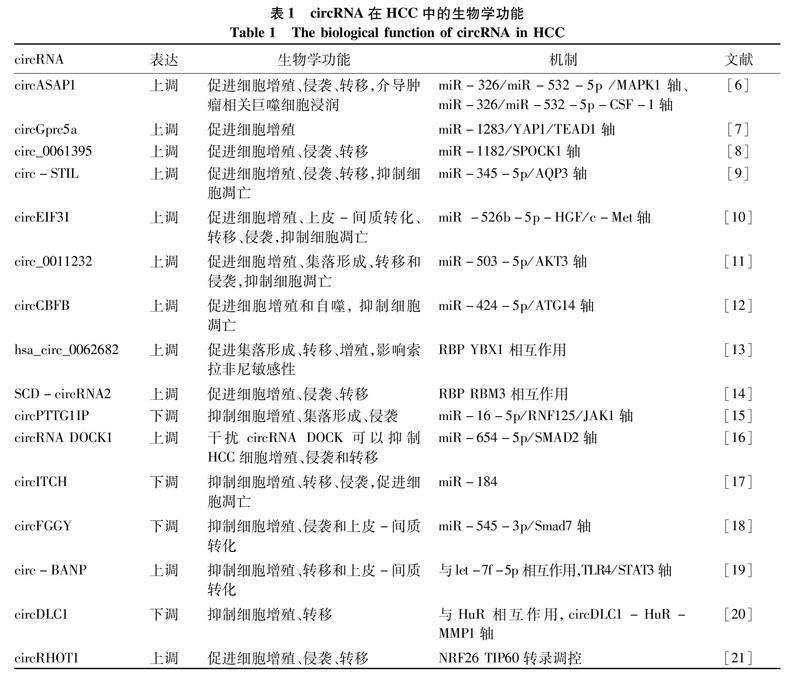
2.1circRNA在信号转导方面对HCC发生发展的影响HCC患者晚期表现之一是肿瘤细胞的侵袭和转移,有侵袭和转移的HCC患者预后不佳,circRNA表达失衡可能是HCC患者侵袭和转移发生的原因之一。在HCC中,circASAP1 (hsa_circ_0085616)通过调控miR-326/miR-532-5p/MAPK1轴增强HCC细胞的增殖和侵袭能力,此外,通过调节 miR-326/miR-532-5p-CSF-1通路介导肿瘤相关巨噬细胞浸润[6]。Lin等[7]发现circGprc5a可以通过海绵化miR-1283激活Hippo信号通路关键下游蛋白YAP1/TEAD1,促进HCC的进展。circ_0061395通过调控miR-1182/SPOCK1通路,促进HCC细胞的发展,为HCC提供了一种新的靶向治疗方法[8]。Liu等[9]发现CircSTIL在HCC组织和细胞中表达上调。CircSTIL敲除通过调控miR-345-5p/AQP3通路减少细胞增殖、迁移和侵袭,抑制HCC进展。与此类似,circEIF3I在HCC中是一种致癌circRNA,下调circEIF3I可以通过circEIF3I/miR-526b-5p/HGF/c-Met通路延缓HCC肿瘤生长[10]。circ_0011232通过miR-503-5p/AKT3轴促进HCC进展,可能为HCC提供一种新的治疗策略[11]。circCBFB通过抑制miR-424-5p,使ATG14表达上调,从而促进HCC细胞增殖和自噬[12]。上述研究表明,致癌circRNA在HCC中通常上调,主要通过充当miRNA海绵促进肿瘤细胞的增殖、迁移和侵袭等进程。hsa_circ_0062682的上调促进了HCC细胞增殖、迁移和侵袭,其功能通过与YBX1及其他RBP相互作用实现[13]。SCD-circRNA2在HCC组织中表达上调,其中RBP RBM3以SCD-circRNA2依赖的方式促进HCC细胞增殖[14]。已有诸多研究表明,circRNA能够起到抑制HCC进展的作用。circPTTG1IP是HCC中的一种新型肿瘤抑制circRNA,低水平的circPTTG1IP通过miR-16-5p/RNF125/JAK1轴促进HCC的发展[15]。在HCC中,circRNA DOCK1和SMAD2表达升高,miR-654-5p表达降低,干扰circRNA DOCK1可通过调控miR-654-5p/SMAD2轴抑制HCC细胞的增殖、侵袭和迁移[16]。在体内外实验中,过表达circITCH可通过海绵化miR-184抑制细胞增殖、迁移、侵袭,促进细胞凋亡,而敲低circITCH则相反[17]。circFGGY通过调控miR-545-3p/Smad7轴抑制细胞生长、侵袭和肝细胞上皮-间充质转化[18]。在不充分射频消融后残留HCC中,circ-BANP通过与let-7f-5p结合,抑制HCC细胞的增殖、迁移和上皮-间叶细胞转化形成[19]。研究[20]表明,circDLC1可与RNA结合蛋白HuR结合,进而减少HuR与MMP1 mRNA的相互作用,从而抑制MMP1的表达,最终抑制HCC的进展。致癌circRHOT1还通过将TIP60(也称为KAT5)招募到NR2F6的启动子并增强其转录,从而抑制HCC的增殖和转移[21]。抑癌circRNA表达降低是肝癌的主要危险因素,对肿瘤细胞的增殖、侵袭和转移均有不利影响。表1列举了部分在 HCC 中失调的circRNA的表达和功能。
2.2circRNA在免疫方面对HCC发生发展的影响在病毒感染过程中,circRNA表达谱发生变化,可调节免疫系统功能。例如,最近的一项研究[22]发现,HCC细胞通过外泌体分泌circUHRF1。临床生理表型显示circUHRF1表达高的患者中肿瘤体积较大,血液中NK细胞比例较低,微血管浸润较多。Kaplan-Meier生存分析显示,circUHRF1高表达患者伴随临床预后不良。circUHRF1可以通过上调NK细胞TIM-3的表达来抑制NK细胞分泌IFN-γ和TNF-α。该研究显示,肿瘤中血浆外泌体circUHRF1水平与NK细胞浸润水平呈负相关。研究者甚至提出了circUHRF1可能促进肝癌患者对程序性死亡受体1免疫治疗产生耐药的假设,但是证据仍然不足。来自HCC细胞的外泌体circGSE1通过调控miR324-5p/TGFBR1/Smad3轴诱导Treg扩增,从而促进HCC的进展[23]。据报道[24],hsa_circ_0003410在HCC中明显上调,通过调节miR-139-3p/CCL5轴增加M2/M1巨噬细胞比率,促进HCC的进展。下调hsa_circ_0074854通过与HuR相互作用和抑制外泌体介导的巨噬细胞M2极化,从而在体内外抑制肝癌的迁移和侵袭[25]。上述研究证实了circRNA可以通过调节HCC患者的免疫系统来影响HCC的发展和预后,未来circRNA也许会成为理想的免疫治疗靶点。
2.3circRNA在代谢方面对HCC发生发展的影响近年来,circRNA与HCC代谢的相互作用引起了广泛关注。通过circRNA调控HCC细胞的代谢,可促进或抑制物质代谢的某些关键酶,从而改变HCC的增殖、侵袭、分化和转移等进展过程。因此,在一定程度上,一些参与代谢调控的circRNA可以作为HCC的潜在生物标志物。在缺氧条件下,circMAT2B通过海绵介导miR-338-3p上调PKM2的表达,增强糖酵解,从而促进HCC的进展[26]。在氧化应激条件下,circ-SPECC1通过miR-33a调控TGFβ2和自噬,促进HCC发生[27]。同样的,HCC细胞中circ_0091579部分通过miR-490-5p/CASC3轴促进细胞增殖、迁移、侵袭和糖酵解[28]。circRPN2通过加速烯醇化酶1 (ENO1)降解和调控miR-183-5p/FOXO1轴抑制HCC有氧糖酵解和转移,表明circRPN2可能是肝癌的治疗靶点[29]。在HCC中,下调circ-CFH通过调控miR-377-3p/RNF38轴,可抑制细胞增殖、迁移、侵袭和糖酵解,从而抑制HCC的发展[30]。hsa_circ_0001806在HCC组织和细胞中表达上调,过表达hsa_circ_0001806通过调控miR-125b/HK2轴促进肝癌细胞增殖、迁移和糖酵解,抑制细胞凋亡[31]。
3circRNA与HCC耐药
目前,多激酶抑制剂、单克隆抗体和免疫检查点抑制剂是治疗晚期HCC的主要分子靶向治疗方法。然而,治疗结果却差强人意,主要问题是难以避免的耐药。越来越多的证据表明,circRNA在HCC耐药的发展中起关键作用。circ-001241在HCC组织和细胞中显著上调,通过调节miR-21-5p/TIMP3轴促进肝癌索拉非尼耐药[32]。circARNT2通过靶向miR-155-5p/PDK1轴抑制肝癌细胞对顺铂的敏感性[33]。hsa_circRNA_102049过表达可以通过海绵化hsa-miR-214-3p上调RELN基因的表达,增加HepG2细胞和Huh-7细胞对索拉非尼的敏感性[34]。Lu等[35]研究发现,在抗PD-1治疗反应不良和HCC术后预后不良的患者中circTMEM181表达升高。HCC细胞通过外泌体circTMEM181作用于巨噬细胞,从而增加其CD39的表达。这一过程与肿瘤细胞上的CD73协同激活eATP-腺苷通路,导致肿瘤环境中的腺苷升高,从而损害CD8+ T淋巴细胞功能,引起抗PD-1免疫治疗的耐药性。circUBE2D2的高表达与HCC患者的低生存率显著相关,体外实验[36]证明,circUBE2D2可通过miR-889-3p/LDHA轴加速HCC的糖酵解和索拉非尼的耐药,这为HCC治疗提供了一种新的方法。Weng等[37]研究通过RNA测序(RNA-seq) 在索拉非尼耐药的HCC组织中鉴定出了一种新型circRNA,circFOXM1。在功能上,circFOXM1显著抑制HCC的生长,调控索拉非尼耐药。circFOXM1下调可能通过释放更多的游离miR-1324和抑制MECP2的表达来调控索拉非尼耐药。circFBXO11在HCC组织中显著上调,通过海绵化miR-605,从而靶向FOXO3蛋白,FOXO3靶向ABCB1的啟动子区,促进ABCB1的表达。总之,本研究揭示了circFBXO11/miR-605/FOXO3/ABCB1在HCC 中介导奥沙利铂耐药的机制[38]。上述研究阐明了circRNA在介导HCC耐药中的作用,为晚期肝癌患者克服耐药提供了新的见解。必须进一步明确耐药的机制,并探索circRNA在分子靶向药物耐药中的作用。
4HBV相关HCC
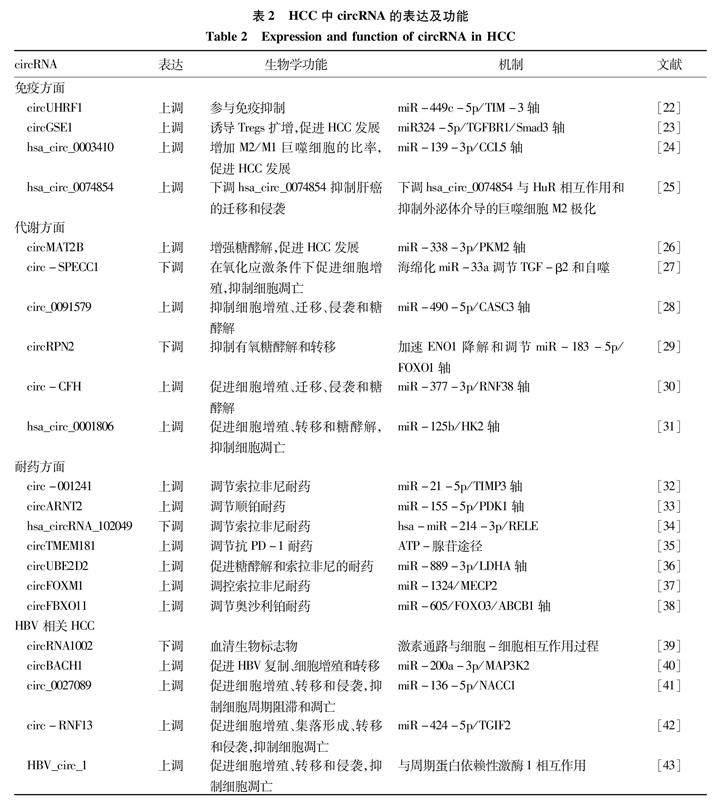
HBV是导致肝癌的主要因素。circRNA已被证实与HBV诱导的肝癌密切相关。有研究[39]从50 327个circRNA中,鉴别出1 187个circRNA在 HBV相关HCC和HBV无症状携带者之间的表达存在显著差异。其中circRNA1002在HCC血清和组织中均显著下调,提示circRNA1002可以作为HBV相关HCC的生物标志物。在HCC组织和HBV转染的肝癌细胞中,circBACH1和MAP3K2表达升高,而miR-200a-3p表达降低。circBACH1缺失或miR-200a3p过表达可抑制HBV转染肝癌细胞中的HBV复制、增殖和转移[40]。circ_0027089作为一种致癌基因,通过竞争性靶向miR-136-5p调控NACC1的表达,促进HBV相关HCC的发生发展[41]。circ-RNF13可能通过调控miR-424-5p/ TGIF2轴抑制HBV相关HCC恶性进展和HBV感染[42]。HBV可以产生circRNA,但是其功能尚未明确。Zhu等[43]研究发现了一种由HBV产生的新型circRNA HBV_circ_1。生存分析显示,HBV_circ_1阳性患者的生存率明显低于HBV_circ_1阴性患者。并且,瞬时表达HBV_circ_1可以增强肝癌细胞增殖、迁移和侵袭能力,抑制细胞凋亡。此外,HBV_circ_1还与周期蛋白依赖性激酶1的相互作用,调节细胞增殖。血清外泌体hsa_circ_0028861在HCC中的表达低于慢性HBV和肝硬化,并且,hsa_circ_0028861联合AFP鉴别HCC与慢性HBV和肝硬化的ROC曲线下面积(AUC)为0.86,具有更好的诊断能力[44]。上述数据不仅为了解HBV相关HCC的发生机制和进展提供了新的线索,而且为治疗药物的开发提供了新的靶点。表2概括了circRNA在免疫、代谢、耐药及HBV相关HCC中的生物学功能。
5生物标志物
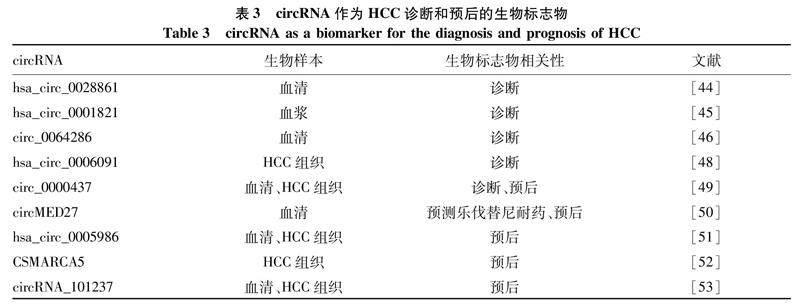
虽然HCC是原发性肝癌最常见的类型,但早期缺乏准确的生物标志物,导致HCC确诊往往较晚。随着分子生物标志物研究的进展和基因组学的发展,circRNA已作为一种新型的液体活检生物标志物被人们所认识。circRNA可在组织、外泌体、血浆、血清、唾液、尿液、脑脊液和乳汁等样本中检测到,其表达谱表现为细胞特异性或阶段特异性(表3)。
5.1诊断标志物研究[45]表明,hsa_circ_0001821在HCC的血浆中表达上调,AUC为0.692,提示血浆hsa_circ_0001821可能是一种新的HCC诊断标志物。其他研究[46]表明,hsa_circ_0064286和hsa_circ_0000475在HCC患者中均显著下调,与ALP、ALT、AST、AFP、胆红素水平呈负相关。circ_0064286的敏感度和特异度较高,分别为88.3%和96%,可能作为HCC诊断的潜在生物标志物。一项研究[47]对血清/血浆circ RNA或circRNA联合AFP检测在HCC诊断中的准确性进行了荟萃分析,结果显示,circRNA的敏感度为0.82(95%CI: 0.78~0.85),特异度为0.82(95%CI: 0.78~0.86)。AFP的敏感度为0.65(95%CI: 0.61~0.68),特异度为0.90(95%CI: 0.85~0.93)。circRNA的AUC为0.89(95%CI: 0.86~0.91),AFP的AUC为0.77(95%CI: 0.74~0.81)。circRNA和AFP联合检测的敏感度为0.88(95%CI: 0.84~0.92),特异度为0.86(95%CI: 0.80~0.91),AUC为0.94(95%CI: 0.91~0.96)。Zhang等[48]也证明hsa_circ_0006091&AFP与hsa_circ_0006091&RGS12联合诊断具有重要意义,可作为HCC诊断的分子标志物。以上结果说明,血清/血浆circRNA是适合临床诊断HCC的生物标志物,circRNA与AFP的联合检测提高了HCC诊断的准确性,circRNA可作为监测HCC发生发展的生物标志物。然而,目前还没有一种方便可靠的血清circRNA生物标志物。
5.2预后标志物Chen等[49]发现,无论在HCC肿瘤组织还是血清中,肿瘤组circ_0000437表达显著上调,且与TNM分型、分化程度、肿瘤大小、BCLC分期相关(P<0.05)。此外,较差的总生存期与circ_0000437的高表达相关,circ_0000437在血清中诊断HCC的AUC为0.928 1,上述结果提示 circ_0000437可能作为HCC患者诊断和预后的一种新的生物标志物。最近一项研究[50]表明,circMED27在HCC血清中显著升高,与HCC患者不良临床特征和不良预后相关,并且促进肝癌细胞对乐伐替尼的耐药,提示circMED27可作为接受乐伐替尼治疗的HCC患者的潜在治疗靶点,并可能作为预测乐伐替尼耐药HCC的一种潜在的生物标志物。除此之外,hsa_circ_0005986的高表达与生存改善相关,是总体生存率和无进展生存率的独立预后因素[51]。此外,DHX9在HCC中表达显著上调,并抑制cSMARCA5的产生(hsa_circ_0001445)。DHX9是一种RNA解旋酶,可结合并抑制两侧反向互补序列的配对,从而阻止circRNA的产生。CSMARCA5通过SMARCA5/miR-17-3p/miR-181b-5p/TIMP3通路抑制HCC的生长。HCC组织中cSMARCA5的降低与肿瘤生长和转移的增加有关,使其成为肿瘤切除后患者的独立预后指标[52]。circRNA_101237在HCC患者的肿瘤组织和血清中表达上调,且与circRNA_101237的表达与肿瘤大小、淋巴结转移、远处转移及TNM分期有关。单因素和多因素分析显示,血清circRNA_101237水平是HCC患者生存预后的独立预测因素[53]。
6小结
circRNA已经成为肿瘤分子生物学领域研究的新热点,目前circRNA在HCC发展、诊疗方面的研究尚处于初步阶段,仍存在许多问题。首先,通过RNA测序已在HCC中鉴定出了上千种circRNA,但只有少量circRNA功能被研究。如何从大量的候选circRNA 中挑选发挥关键作用且具有临床价值的circRNA 是一项巨大的工程。其次,目前对于circRNA的研究主要集中于肝癌组织,对于外周血、外泌体、尿液和唾液等分泌的circRNA研究较少。对circRNA进行多种类样本的全方位研究,有利于提高对circRNA复杂的调控网络的认知。重视体液中circRNA的研究,有利于开发用于肝癌筛查和预后监测的circRNA检测试剂盒。综上所述,筛选出发挥关键调控功能的circRNA,阐明其靶向分子和信号通路,将有助于发掘circRNA作为 HCC 治疗靶点的巨大临床价值。
利益冲突声明:本文不存在任何利益冲突。作者贡献声明:苗淑莹负责课题设计,资料分析,撰写论文;杨军、管文燕、张标、何璐参与修改论文;樊智文负责拟定写作思路,修改论文并最后定稿。
參考文献:
[1]ZENG C, ZHANG L, LUO C, et al. A stratification model of hepatocellular carcinoma based on expression profiles of cells in the tumor microenvironment[J]. BMC Cancer, 2022, 22(1): 613. DOI: 10.1186/s12885-022-09647-5.
[2]CAI P, ZHENG H, SHE J, et al. Molecular mechanism of aflatoxin-induced hepatocellular carcinoma derived from a bioinformatics analysis[J]. Toxins (Basel), 2020, 12(3): 203. DOI: 10.3390/toxins12030203.
[3]KHASHKHASHI MOGHADAM S, BAKHSHINEJAD B, KHALAFIZADEH A, et al. Non-coding RNA-associated competitive endogenous RNA regulatory networks: Novel diagnostic and therapeutic opportunities for hepatocellular carcinoma[J]. J Cell Mol Med, 2022, 26(2): 287-305. DOI: 10.1111/jcmm.17126.
[4]SANGER HL, KLOTZ G, RIESNER D, et al. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures[J]. Proc Natl Acad Sci U S A, 1976, 73(11): 3852-3856. DOI: 10.1073/pnas.73.11.3852.
[5]LOUIS C, LECLERC D, COULOUARN C. Emerging roles of circular RNAs in liver cancer[J]. JHEP Rep, 2022, 4(2): 100413. DOI: 10.1016/j.jhepr.2021.100413.
[6]HU ZQ, ZHOU SL, LI J, et al. Circular RNA sequencing identifies CircASAP1 as a key regulator in hepatocellular carcinoma metastasis[J]. Hepatology, 2020, 72(3): 906-922. DOI: 10.1002/hep.31068.
[7]LIN Y, HUANG G, JIN H, et al. Circular RNA Gprc5a promotes HCC progression by activating YAP1/TEAD1 signalling pathway by sponging miR-1283[J]. Onco Targets Ther, 2020, 13: 4509-4521. DOI: 10.2147/OTT.S240261.
[8]WU W, ZHOU Z, CHEN C, et al. Circ_0061395 functions as an oncogenic gene in hepatocellular carcinoma by acting as a miR-1182 sponge[J]. Cell Cycle, 2022, 21(20): 2192-2205. DOI: 10.1080/15384101.2022.2092177.
[9]LIU J, HE X, ZOU Y, et al. Circular RNA circ-STIL contributes to cell growth and metastasis in hepatocellular carcinoma via regulating miR-345-5p/AQP3 axis[J]. Dig Dis Sci, 2022, 67(6): 2269-2282. DOI: 10.1007/s10620-021-07054-7.
[10]LIU Y, XIAO X, WANG J, et al. Silencing circEIF3I/miR-526b-5p axis epigenetically targets HGF/c-Met signal to hinder the malignant growth, metastasis and angiogenesis of hepatocellular carcinoma[J]. Biochem Genet, 2023, 61(1): 48-68. DOI: 10.1007/s10528-022-10239-y.
[11]JU A, SHEN Y, YUE A. Circ_0011232 contributes to hepatocellular carcinoma progression through miR-503-5p/AKT3 axis[J]. Hepatol Res, 2022, 52(6): 532-545. DOI: 10.1111/hepr.13758.
[12]ZHAO Z, HE J, FENG C. CircCBFB is a mediator of hepatocellular carcinoma cell autophagy and proliferation through miR-424-5p/ATG14 axis[J]. Immunol Res, 2022, 70(3): 341-353. DOI: 10.1007/s12026-021-09255-8.
[13]RAZPOTNIK R, VIDMAR R, FONOVIC' M, et al. Circular RNA hsa_circ_0062682 binds to YBX1 and promotes oncogenesis in hepatocellular carcinoma[J]. Cancers (Basel), 2022, 14(18): 4524. DOI: 10.3390/cancers14184524.
[14]DONG W, DAI ZH, LIU FC, et al. The RNA-binding protein RBM3 promotes cell proliferation in hepatocellular carcinoma by regulating circular RNA SCD-circRNA 2 production[J]. EBioMedicine, 2019, 45: 155-167. DOI: 10.1016/j.ebiom.2019.06.030.
[15]PENG R, CAO J, SU BB, et al. Down-regulation of circPTTG1IP induces hepatocellular carcinoma development via miR-16-5p/RNF125/JAK1 axis[J]. Cancer Lett, 2022, 543: 215778. DOI: 10.1016/j.canlet.2022.215778.
[16]LU Y, ZHANG J, WU Y. Interference with circRNA DOCK1 inhibits hepatocellular carcinoma cell proliferation, invasion and migration by regulating the miR-654-5p/SMAD2 axis[J]. Mol Med Rep, 2021, 24(2): 609. DOI: 10.3892/mmr.2021.12247.
[17]GUO X, WANG Z, DENG X, et al. Circular RNA CircITCH (has-circ-0001141) suppresses hepatocellular carcinoma (HCC) progression by sponging miR-184[J]. Cell Cycle, 2022, 21(15): 1557-1577. DOI: 10.1080/15384101.2022.2057633.
[18]FENG KL, DIAO N, ZHOU ZW, et al. CircFGGY inhibits cell growth, invasion and epithelial-mesenchymal transition of hepatocellular carcinoma via regulating the miR-545-3p/Smad7 axis[J]. Front Cell Dev Biol, 2022, 10: 850708. DOI: 10.3389/fcell.2022.850708.
[19]LI G, KONG J, DONG S, et al. Circular BANP knockdown inhibits the malignant progression of residual hepatocellular carcinoma after insufficient radiofrequency ablation[J]. Chin Med J (Engl), 2022. DOI: 10.1097/CM9.00000000000001822. [Online ahead of print]
[20]LIU H, LAN T, LI H, et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR[J]. Theranostics, 2021, 11(3): 1396-1411. DOI: 10.7150/thno.53227.
[21]WANG L, LONG H, ZHENG Q, et al. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression[J]. Mol Cancer, 2019, 18(1): 119. DOI: 10.1186/s12943-019-1046-7.
[22]ZHANG PF, GAO C, HUANG XY, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma[J]. Mol Cancer, 2020, 19(1): 110. DOI: 10.1186/s12943-020-01222-5.
[23]HUANG M, HUANG X, HUANG N. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells[J]. Cancer Sci, 2022, 113(6): 1968-1983. DOI: 10.1111/cas.15365.
[24]CAO P, MA B, SUN D, et al. hsa_circ_0003410 promotes hepatocellular carcinoma progression by increasing the ratio of M2/M1 macrophages through the miR-139-3p/CCL5 axis[J]. Cancer Sci, 2022, 113(2): 634-647. DOI: 10.1111/cas.15238.
[25]WANG Y, GAO R, LI J, et al. Downregulation of hsa_circ_0074854 suppresses the migration and invasion in hepatocellular carcinoma via interacting with HuR and via suppressing exosomes-mediated macrophage M2 polarization[J]. Int J Nanomedicine, 2021, 16: 2803-2818. DOI: 10.2147/IJN.S284560.
[26]LI Q, PAN X, ZHU D, et al. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress[J]. Hepatology, 2019, 70(4): 1298-1316. DOI: 10.1002/hep.30671.
[27]ZHANG B, LIU Z, CAO K, et al. Circ-SPECC1 modulates TGFβ2 and autophagy under oxidative stress by sponging miR-33a to promote hepatocellular carcinoma tumorigenesis[J]. Cancer Med, 2020, 9(16): 5999-6008. DOI: 10.1002/cam4.3219.
[28]LIU W, YIN C, LIU Y. Circular RNA circ_0091579 promotes hepatocellular carcinoma proliferation, migration, invasion, and glycolysis through miR-490-5p/CASC3 axis[J]. Cancer Biother Radiopharm, 2021, 36(10): 863-878. DOI: 10.1089/cbr.2019.3472.
[29]LI J, HU ZQ, YU SY, et al. CircRPN2 inhibits aerobic glycolysis and metastasis in hepatocellular carcinoma[J]. Cancer Res, 2022, 82(6): 1055-1069. DOI: 10.1158/0008-5472.CAN-21-1259.
[30]CHEN Z, DU J, YANG C, et al. circ-CFH promotes the development of HCC by regulating cell proliferation, apoptosis, migration, invasion, and glycolysis through the miR-377-3p/RNF38 axis[J]. Open Life Sci, 2022, 17(1): 248-260. DOI: 10.1515/biol-2022-0029.
[31]CHEN X, SHE P, WANG C, et al. Hsa_circ_0001806 promotes glycolysis and cell progression in hepatocellular carcinoma through miR-125b/HK2[J]. J Clin Lab Anal, 2021, 35(12): e23991. DOI: 10.1002/jcla.23991.
[32]YANG Q, WU G. CircRNA-001241 mediates sorafenib resistance of hepatocellular carcinoma cells by sponging miR-21-5p and regulating TIMP3 expression[J]. Gastroenterol Hepatol, 2022, 45(10): 742-752. DOI: 10.1016/j.gastrohep.2021.11.007.
[33]LI Y, ZHANG Y, ZHANG S, et al. circRNA circARNT2 suppressed the sensitivity of hepatocellular carcinoma cells to cisplatin by targeting the miR-155-5p/PDK1 axis[J]. Mol Ther Nucleic Acids, 2021, 23: 244-254. DOI: 10.1016/j.omtn.2020.08.037.
[34]WANG S, LIU D, WEI H, et al. The hsa_circRNA_102049 mediates the sorafenib sensitivity of hepatocellular carcinoma cells by regulating Reelin gene expression[J]. Bioengineered, 2022, 13(2): 2272-2284. DOI: 10.1080/21655979.2021.2024332.
[35]LU JC, ZHANG PF, HUANG XY, et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma[J]. J Hematol Oncol, 2021, 14(1): 200. DOI: 10.1186/s13045-021-01207-x.
[36]HUANG H, PENG J, YI S, et al. Circular RNA circUBE2D2 functions as an oncogenic factor in hepatocellular carcinoma sorafenib resistance and glycolysis[J]. Am J Transl Res, 2021, 13(6): 6076-6086.
[37]WENG H, ZENG L, CAO L, et al. circFOXM1 contributes to sorafenib resistance of hepatocellular carcinoma cells by regulating MECP2 via miR-1324[J]. Mol Ther Nucleic Acids, 2021, 23: 811-820. DOI: 10.1016/j.omtn.2020.12.019.
[38]LI J, QIN X, WU R, et al. Circular RNA circFBXO11 modulates hepatocellular carcinoma progress and oxaliplatin resistance through miR-605/FOXO3/ABCB1 axis[J]. J Cell Mol Med, 2020, 24(9): 5152-5161. DOI: 10.1111/jcmm.15162.
[39]LI Y, LI R, CHENG D, et al. The potential of CircRNA1002 as a biomarker in hepatitis B virus-related hepatocellular carcinoma[J]. PeerJ, 2022, 10: e13640. DOI: 10.7717/peerj.13640.
[40]DU N, LI K, WANG Y, et al. CircRNA circBACH1 facilitates hepatitis B virus replication and hepatoma development by regulating the miR-200a-3p/MAP3K2 axis[J]. Histol Histopathol, 2022, 37(9): 863-877. DOI: 10.14670/HH-18-452.
[41]HE W, ZHU X, TANG X, et al. Circ_0027089 regulates NACC1 by targeting miR-136-5p to aggravate the development of hepatitis B virus-related hepatocellular carcinoma[J]. Anticancer Drugs, 2022, 33(1): e336-e348. DOI: 10.1097/CAD.0000000000001211.
[42]CHEN Y, LI S, WEI Y, et al. Circ-RNF13, as an oncogene, regulates malignant progression of HBV-associated hepatocellular carcinoma cells and HBV infection through ceRNA pathway of circ-RNF13/miR-424-5p/TGIF2[J]. Bosn J Basic Med Sci, 2021, 21(5): 555-568. DOI: 10.17305/bjbms.2020.5266.
[43]ZHU M, LIANG Z, PAN J, et al. Hepatocellular carcinoma progression mediated by hepatitis B virus-encoded circRNA HBV_circ_1 through interaction with CDK1[J]. Mol Ther Nucleic Acids, 2021, 25: 668-682. DOI: 10.1016/j.omtn.2021.08.011.
[44]WANG Y, PEI L, YUE Z, et al. The potential of serum exosomal hsa_circ_0028861 as the novel diagnostic biomarker of HBV-derived hepatocellular cancer[J]. Front Genet, 2021, 12: 703205. DOI: 10.3389/fgene.2021.703205.
[45]SONG Y, CAO P, LI J. Plasma circular RNA hsa_circ_0001821 acts as a novel diagnostic biomarker for malignant tumors[J]. J Clin Lab Anal, 2021, 35(11): e24009. DOI: 10.1002/jcla.24009.
[46]EL SHARKAWI FZ, AWAD MS, ELAGAWY W, et al. Circular RNAs 0064286 and 0000475: potential diagnostic biomarkers in hepatocellular carcinoma[J]. Asian Pac J Cancer Prev, 2021, 22(9): 3039-3044. DOI: 10.31557/APJCP.2021.22.9.3039.
[47]NIE G, PENG D, LI B, et al. Diagnostic accuracy of serum/plasma circular RNAs and the combination of circular RNAs and α-fetoprotein for detecting hepatocellular carcinoma: A Meta-analysis[J]. Front Genet, 2021, 12: 722208. DOI: 10.3389/fgene.2021.722208.
[48]ZHANG Y, LI J, CUI Q, et al. Circular RNA hsa_circ_0006091 as a novel biomarker for hepatocellular carcinoma[J]. Bioengineered, 2022, 13(2): 1988-2003. DOI: 10.1080/21655979.2021.2006952.
[49]CHEN G, XIE D, ZHANG P, et al. Circular RNA hsa_circ_0000437 may be used as a new indicator for the diagnosis and prognosis of hepatocellular carcinoma[J]. Bioengineered, 2022, 13(6): 14118-14124. DOI: 10.1080/21655979.2022.2081458.
[50]ZHANG P, SUN H, WEN P, et al. circRNA circMED27 acts as a prognostic factor and mediator to promote lenvatinib resistance of hepatocellular carcinoma[J]. Mol Ther Nucleic Acids, 2022, 27: 293-303. DOI: 10.1016/j.omtn.2021.12.001.
[51]KIM G, HAN JR, PARK SY, et al. Circular noncoding RNA hsa_circ_0005986 as a prognostic biomarker for hepatocellular carcinoma[J]. Sci Rep, 2021, 11(1): 14930. DOI: 10.1038/s41598-021-94074-y.
[52]LEE T, PAQUET M, LARSSON O, et al. Tumor cell survival dependence on the DHX9 DExH-box helicase[J]. Oncogene, 2016, 35(39): 5093-5105. DOI: 10.1038/onc.2016.52.
[53]ZHOU S, WEI J, WANG Y, et al. Cisplatin resistance-associated circRNA_101237 serves as a prognostic biomarker in hepatocellular carcinoma[J]. Exp Ther Med, 2020, 19(4): 2733-2740. DOI: 10.3892/etm.2020.8526.
收稿日期:2022-10-27;錄用日期:2022-12-17
本文编辑:王莹
引证本文:MIAO SY, YANG J, GUAN WY, et al. Role of circular RNA in the development, progression, diagnosis, and treatment of hepatocellular carcinoma[J]. J Clin Hepatol, 2023, 39(8): 1983-1991.