Prognosis value of Chinese Ocular Fundus Diseases Society classification for proliferative diabetic retinopathy on postoperative visual acuity after pars plana vitrectomy in type 2 diabetes
2022-10-24TieZhuLinYanKongChengShiEmmanuelEricPazoGuangZhengDaiXianWeiWuLingXuLiJunShen
Tie-Zhu Lin, Yan Kong, Cheng Shi, Emmanuel Eric Pazo, Guang-Zheng Dai, Xian-Wei Wu, Ling Xu, Li-Jun Shen
1Eye Hospital and School of Ophthalmology and Optometry,Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
2He Eye Specialist Hospital, Shenyang 110034, Liaoning Province, China
3Ophthalmology Department, Zhejiang Provincial People’s Hospital, Hangzhou 310000, Zhejiang Province, China
Abstract
● KEYWORDS: proliferative diabetic retinopathy; pars plana vitrectomy; postoperative visual acuity; best-corrected visual acuity; Chinese Ocular Fundus Diseases Society
INTRODUCTION
Diabetic retinopathy (DR) is a common cause of vision loss and blindness. The global prevalence of retinopathy is estimated to be 22.27% among all diabetics[1]. The advanced form of DR, proliferative diabetic retinopathy (PDR), is a sight-threatening illness[2]. The global prevalence of PDR is estimated to be approximately 1.4% among all individuals with diabetes[3]. In a Meta-analysis, the prevalence of PDR was 6% in Asian type 2 diabetic patients, accounting for 17%DR patients[4]. Vitreous hemorrhage, severe fibrovascular proliferation, combined tractional retinal detachment (TRD),and/or rhegmatogenous retinal detachment (RRD) are the primary signs of PDR, which are also considered as indication criteria for pars plana vitrectomy (PPV). The surgical goals include reducing bleeding-related vitreous opacities, alleviating vitreoretinal tension by removing the posterior hyaloid and epiretinal membranes, and using endo-photocoagulation to achieve proliferative tissue regression.
For the first cases of PDR, the Early Treatment Diabetic Retinopathy Study (ETDRS) severity scale classified the illness as mild, moderate, or high-risk PDR. Advanced PDR was defined as the presence of traction, retinal detachment (RD), rubeosis iridis, or fundus obscuration[5]. Because of its complexity, most retinal surgeons managing diabetic patients do not use the whole ETDRS severity scale, according to several surveys[6].The number of diabetics in China is by far the highest. A common use classification for PDR in China based on retinal pathology and morphology was made by Chinese Ocular Fundus Diseases Society (COFDS) in 1985, and was kept in the modified version in 2014, including the early PDR stage(neovascular elsewhere or of the disk, with or without vitreous or preretinal hemorrhage), the fibrous proliferation stage, and the advanced PDR stage (TRD)[7]. Though this classification has fewer details compared to ETDRS grading, it is more convenient for retinal surgeons in clinical practice. Based on this classification, we would like to know the prognosis of PDR after PPV surgery. In this study, we compared the postoperative visual acuity among PDR eyes of different stages in type 2 diabetic patients.
SUBJECTS AND METHODS
Ethical ApprovalThe study design followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at He Eye Specialist Hospital [IRB (2020)K006.01]. Written informed consent was waived due to retrospective design.
Study Design and ParticipantsThis retrospective study was performed for PDR eyes undergoing PPV in patients with type 2 diabetes in He Eye Specialist Hospital between January 2017 and June 2020.
The criteria of inclusion were: 1) PDR eyes undergoing 23G PPV or phacovitrectomy treatment; 2) patients with type 2 diabetes mellitus; 3) more than 18 years old; 4) Snellen best-corrected visual acuity (BCVA) at baseline over light perception; and 5) the minimal follow-up time was 6mo.Exclusion as follows: 1) ocular pathology other than cataract and PDR that may affect visual acuity, such as pterygium,corneal opacity, glaucoma, age related macular degeneration,amblyopia; 2) history of ocular surgery; 3) cataract of grade 3 or more dense[8]; 4) postoperative anatomical success was not achieved; and 5) other systemic diseases that may influence vision acuity, such as cerebral thrombosis, hematencephalon,intracranial tumors.
Data Collection and GroupingPatients’ gender, age,HbAlc, duration of diabetes, vitreous tamponade, previous photocoagulation, the use of anti-vascular endothelial growth factor (VEGF) agents from the medical charts were recorded.The records of surgical procedures were also reviewed.All the patients underwent Snellen BCVA at distance, slitlamp examination, intraocular pressure measurement,pupil dilated fundus examination, ultra-field scanning laser ophthalmoscopy (SLO; Optos®200Tx, Optos®, Dunfermline,UK) and ultrasonographic B-scan preoperatively. All eyes were divided into three groups based on PDR classification of COFDS using preoperative SLO and ultrasonographic B-scan imaging[5]. Group A (primary vitreous hemorrhage): massive hemorrhage was seen in the vitreous on SLO image without obvious proliferative membrane noted, no vitreous tractional lesion or TRD was seen on ultrasonographic B-scan. Group B(primary fibrovascular proliferation): obvious fibroproliferative membrane on the optic disc or posterior pole area along the vascular arch was seen on SLO image, or obvious fibroproliferative traction was noted on ultrasonographic B-scan, without TRD or RRD. Vitreous hemorrhage was permitted. Group C (TRD/RRD type): TRD or RRD noted on SLO and/or ultrasound B-scan (Figure 1). That grouping would be corrected if obvious fibrovascular membrane or TRD was discovered intraoperatively, because of the low sensitivity of SLO and ultrasound B-scan for fibrovascular membrane in the presence of dense vitreous hemorrhage. The consistency analysis would be done between the real retinal condition and the initial classification. The classification was performed by two masked, experienced retina specialists (Wu XW and Kong Y). A third masked retina specialist (Lin TZ) made the final decision in cases where no agreement between the graders was achieved. The visual acuity at 6mo follow up was assigned as postoperative visual acuity for statistical analysis.
Surgical TechniquesUnder retrobulbar anesthesia, all patients received a comprehensive PPV using 23-gauge instrumentation (OS4TM, Oertli, Switzerland) and the Resight 500 (Carl Zeiss Meditec, Inc., Dublin, California, USA) for visualization. Fibrovascular membrane dissection was done according to each subject’s specific demands and requirements.During the procedure, an endolaser was used. Replacement of the vitreous with gas (C2F6), fluid, or silicone oil was left to the surgeon’s discretion. Phacoemulsification was conducted systematically before the PPV, through a 2.8 mm clean corneal incision, with the implantation of an acrylic foldable IOL in the case of a combined surgery. If eyes received intravitreal injection of anti-VEGF agents [ranibizumab (Lucentis; Genentech,South San Francisco, CA), aflibercept (Eylea; Regeneron,Tarrytown, NY), or conbercept (Kanghong Biotech Company,Chengdu, Sichuan Province, China)], that was administered intraoperatively or 3-5d before surgery[9].
Statistical AnalysisFor data analysis, Snellen visual acuity was transformed to logMAR visual acuity. The logMAR result for count-fingers was 1.9, hand motion was 2.3, light perception was 2.7, and no light perception was 3.0[10]. The inter-rater agreement for classification of groups was checked using intraclass coefficient (ICC). The agreement between reviewers was high: ICC=0.965 (P<0.001). Mean±SD and percentage (%) were used among the descriptive statistics. Categorical data was presented as percentages and analyzed using the Chi-square test. ANOVA was used to test homogeneity of variance among groups, and based on the homogeneity of variance, an independentttest was used for multiple comparison. Multiple linear regression was used to identify the factors associated with postoperative BCVA.All statistical analyses were carried out using SPSS software version 26.0 (SPSS Inc, Chicago, USA), with an alpha level of 0.05 (type I error).
RESULTS
Patient CharacteristicsIn total, 195 eyes of 195 patients were collected in this study. At first, Group A with 76 eyes, Group B with 72 eyes and Group C with 47 eyes were classified by only using SLO and ultrasound B-scan imaging. There were 71 eyes of 71 patients (35 females and 36 males with a mean age of 54.37±11.30y) in Group A, 75 eyes of 75 patients (37 females and 38 males with a mean age of 50.95±9.00y) in Group B and 49 eyes of 49 patients (27 females and 22 males with a mean age of 47.43±13.32y) in Group C after correction by reviewing surgical records. Four eyes with primary vitreous hemorrhage presented fibrovascular proliferation intraoperatively. RD was found during surgery in 1 eye with primary vitreous hemorrhage and 1 eye with fibrovascular proliferation. The agreement between preoperative and intraoperative DR classification was excellent: ICC=0.981 (P<0.001). The baseline BCVA was comparable among three groups (Table 1).
Postoperative Visual AcuityThere were significant improvements in mean postoperative visual acuity compared to baseline in all three groups (0.48±0.48vs1.55±0.61,P<0.001;0.89±0.63vs1.62±0.57,P<0.001; 1.04±0.67vs1.81±0.57,P<0.001; respectively, Figure 2). In the comparison among three groups, the eyes in Group A got better mean postoperative visual acuity compared to the eyes in Groups B and C(0.48±0.48vs0.89±0.63,P<0.001; 0.48±0.48vs1.04±0.67,P<0.001; respectively), meanwhile the eyes in Group A got more improvement of mean postoperative visual acuity compared to the eyes in Groups B and C (1.07±0.70vs0.73±0.68,P=0.004; 1.07±0.70vs0.77±0.78,P=0.024;respectively). The mean improvement of postoperative visual acuity is comparable between Groups B and C (0.73±0.68vs0.77±0.78,P=0.751). Thirty-seven (52.11%) eyes in Group A,17 (22.67%) eyes in Group B and 8 (16.33%) eyes in Group C got visual acuity of ≥20/40 after PPV therapy (P<0.001). The percentage of eyes with postoperative increase of visual acuity of ≥3 lines was 77.46%, 45.33% and 40.82% in Groups A, B and C respectively (P<0.001; Table 2).

Figure 1 Ultra-field SLO and B-scan images of 3 stages of PDR Primary vitreous hemorrhage type: massive hemorrhage noted in the vitreous on SLO image (A), no vitreous tractional lesion or traction retinal detachment on ultrasound B-scan (B). Primary fibrovascular proliferation type: obvious fibroproliferative membrane on posterior pole area along the vascular arch noted on SLO image (C), without retinal detachment on ultrasound B-scan (D). Retinal detachment type: tractional retinal detachment noted on SLO image (E) and ultrasound B-scan (F). SLO: Scanning laser ophthalmoscopy; PDR:Proliferative diabetic retinopathy.

Figure 2 The preoperative and postoperative BCVA in three groups BCVA: Best-corrected visual acuity.
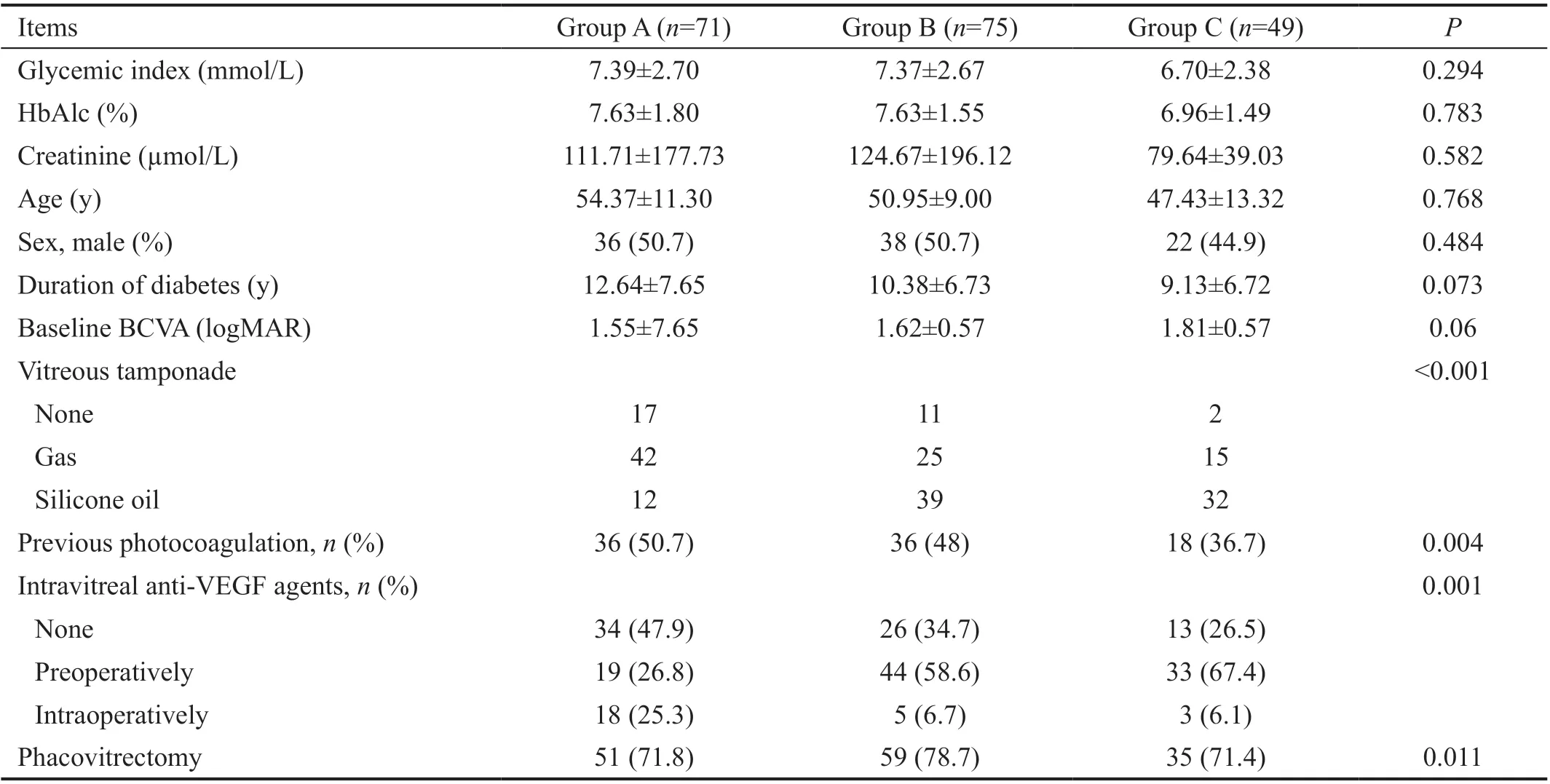
Table 1 Demographic data and surgical methods in three groups
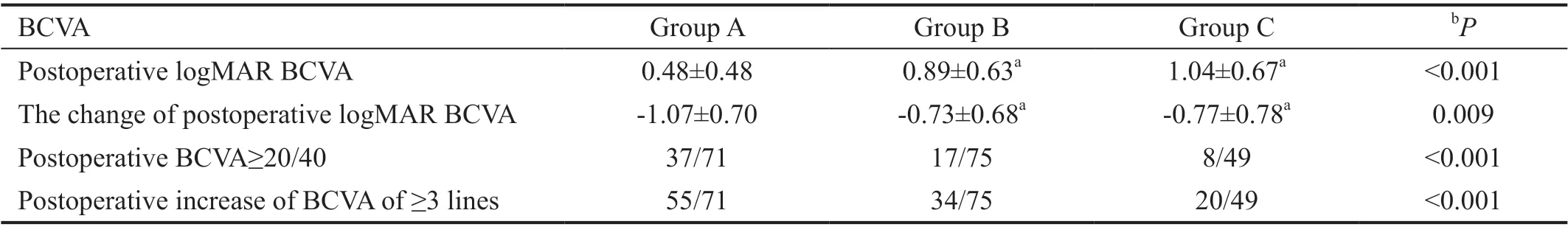
Table 2 Comparison of postoperative BCVA among three groups
Postoperative Visual Acuity Associated FactorsIn the univariate analysis, the duration of diabetes (P=0.073), baseline BCVA (P<0.001), previous photocoagulation (P=0.004),classification of PDR (P<0.001), vitreous tamponade(P<0.001), intravitreal anti-VEGF agents (P<0.001),phacovitrectomy (P=0.011), and duration of operation(P=0.001) were significantly correlated with postoperative BCVA (Table 3). The variates withP<0.2 were included in the multiple linear regression analysis, PDR of primary fibrovascular proliferation type (β=0.194, 95%CI=0.060-0.447,P=0.01), RD type (β=0.244, 95%CI=0.132-0.579,P=0.02),baseline logMAR BCVA (β=0.192, 95%CI=0.068-0.345,P=0.004), silicone oil tamponade (β=0.272, 95%CI=0.173-0.528,P<0.001) were positively correlated with postoperative logMAR BCVA, while phacovitrectomy was negatively correlated with postoperative logMAR BCVA (β=-0.144,95%CI=-0.389 to -0.027,P=0.025; Table 4).
DISCUSSION
While the proportion of people with diabetes developing PDR and severe visual loss decreased between 1980 and 2008 in populations with improved diabetes control[11], the crude prevalence of visual impairment and blindness caused by DR increased significantly between 1990 and 2015, according to the most recent report of the Vision Loss Expert Group of the Global Burden of Disease Study[12], owing largely to the increase in diabetes prevalence. PDR is characterized by the hallmark feature of pathologic preretinal neovascularization[13].The newly formed vessels could locate in the retina, or extend into vitreous or subretinal space. Structural alterations at the vitreoretinal interface encourage vasogenic cells to migrate and proliferate in the vitreous, resulting in vitreous hemorrhage and macular edema. The vitreous collagen mixes with the developing vascular endothelium to give it contractile properties. Simultaneously, fibrous tissue forming along new veins aligns with the posterior hyaloid, giving the vitreous contractile properties. Contraction of the vitreous can pull developing retinal vessels into the subhyaloid region, causing bleeding. Blood can break into the vitreous tissue concurrently or later if the hemorrhage is severe or the vitreous is substantially liquefied. TRD, secondary RRD,chronic macular edema, premacular hemorrhage, and recurrentvitreous hemorrhage can all be caused by continued vitreous contraction.Krollet al[14]established a classification of PDR that clearly distinguished different stages of the disease. Based on the hypothetic pathogenesis of PDR and clinical observations with preoperative examination techniques as well as intraoperative observations, the stages were briefly differentiated as stage A (proliferative changes with retina attachment), stage B(circumscribed TRD with macula attachment) and stage C(TRD in volving the macula), which is primarily based on retinal proliferative changes and macular status. In clinical trials, the severity of a subject’s retinopathy over time can be categorized into discrete steps on Diabetic Retinopathy Severity Scale (DRSS). The DRSS is based on grading of fundus stereo photographs of 7 fields, and classifies DR per eye into 13 complex levels (7 levels for PDR). This grade can be influenced by the presence of severe vitreous hemorrhage.The procedure of seven standard mydriatic and stereoscopic 30-degree fundus fields (seven-field ETDRS) is also timeconsuming. Ultra-field SLO is a time-saving technique that can cover up to 200 degrees of the fundus without mydriasis, and ultrasound B-scan could give the sagittal view of vitreous and retina. Though the dense vitreous hemorrhage shields the retina and could cause some bias in classification, we corrected that grouping using surgical records, and the agreement between preoperative and intraoperative classification was excellent.Only a few eyes with flat fibrovascular membrane or mild traction retinal detachment were incorrectly assigned to group A or B.

Table 3 Univariate analysis for postoperative BCVA

Table 4 Multiple analysis of linear regression for postoperative BCVA
For about 40y, PPV has been utilized in PDR. Several papers describe the visual acuity outcomes in PDR eyes following vitrectomy surgery. Ratnarajanet al[15]reported that the mean postoperative visual acuity of PDR eyes undergoing PPV was significantly improved during 44mo of follow up.Additionally, we observed a considerable improvement in postoperative visual acuity in PDR eyes at various stages following PPV surgery. A total of 62 (31.79%) eyes got postoperative visual acuity of ≥20/40, and 109 (55.90%) eyes got over 3 lines improvement of postoperative visual acuity.We classified the eyes of PDR into three groups based on the PDR classification of COFDS: primary vitreous hemorrhage,primary fibrovascular proliferation and RD. We discovered that the eyes with primary vitreous hemorrhage had significantly better postoperative visual acuity than the other two groups.Postoperative visual acuity improvement was also greater in eyes with primary vitreous hemorrhage. Early vitrectomy,according to the Diabetic Retinopathy Study, increased the likelihood of restoring visual acuity greater than 20/40. In this study, more eyes with primary vitreous hemorrhage had postoperative visual acuity of 20/40 or greater than a three-line improvement in comparison to the other two PDR types.
Additionally, in univariate and multiple linear regression analyses, the type of primary vitreous hemorrhage was identified as a positive risk factor for postoperative BCVA.Nishiet al[16]also discovered a correlation between vitreous hemorrhage and improved postoperative visual acuity in the absence of a fibrovascular membrane. Vitreous blood is typically caused by retinal neovascularization in PDR eyes.However, retinal function is not always compromised and is frequently preserved in the absence of RD. On the other hand,bleeding can enhance the vitreous contractile property by introducing vasogenic and fibrogenic materials, and chronic bleeding can result in the formation of fibrovascular tissue or even RD. This finding may suggest that undergoing surgery without reservation provides a greater therapeutic benefit.
Improved baseline BCVA predicts improved postoperative BCVA in PDR eyes undergoing PPV has been extensively reported. Ramezaniet al[17]reviewed 236 PDR eyes undergoing vitrectomy and silicone oil injection. The findings indicated that a higher baseline BCVA was associated with a greater risk of developing final visual acuity greater than 20/200. Shen and Yang[18]also discovered that the only variable associated with final visual acuity was preoperative visual acuity. Though functional failure could be caused by retinal redetachment,we included in the current study only eyes with postoperative macular attachment. We hypothesize that macular ischemia may be a significant factor influencing postoperative visual acuity recovery in PDR eyes, but this hypothesis requires further investigation in the future.
Typically, retinal surgeons prefer to choose the tamponade materials intraoperatively. We frequently use silicone oil or gas to treat retinal anatomical instability, such as TRD or extensive fibrous proliferation. Rushet al[19]demonstrated in a randomized clinical trial that vitreous substitution with C3F8gas resulted in improved visual acuity at 6mo when compared to silicone oil tamponade in PDR eyes. That result corroborated ours. In comparison to eyes with fluid or gas tamponade, eyes with silicone oil tamponade were more likely to have poor postoperative visual acuity. Numerous studies hypothesized that silicone oil posed a threat to the retina’s overall health[20].Christensen and la Cour[21]reported that after silicone oil tamponade, the inner retinal layers of the macula become thinner. This may account for the poor postoperative visual acuity observed in eyes treated with silicone oil tamponade.
Cataract formation is a common complication of vitrectomy[22],which can impair postoperative visual acuity, which is why many retinal surgeons prefer phacovitrectomy for older patients, which can save money and avoid the need for additional cataract surgery. Additionally, retinal surgeons may benefit from enhanced visibility and the ability to perform more extensive vitrectomy while in an aphakic state during surgery. To minimize the impact of cataract on visual acuity and fundus evaluation at baseline, we excluded the severe cataract (grade 3 or more dense) patients. The results indicated that eyes that had undergone phacovitrectomy were more likely to have improved postoperative visual acuity in this study.We hypothesized that the poorer postoperative visual acuity in eyes with lens preservation was due to cataract progression following vitrectomy. On the basis of the development of surgical techniques and instruments for small-gauge PPV, we advocated for phacovitrectomy in older patients.
This study has some limitations. It is a retrospective case control study with a brief follow-up period; a prospective study with a larger sample size and a longer follow-up period will be required in the future. Though we hypothesize that poor postoperative BCVA is due to macular ischemia, we lack sufficient imaging evidence to support this. In the future,quantification of macular thickness and vascular density on optical coherence tomography or optical coherence tomography angiography should be studied. A comparison of COFDS and ETDRS classifications for PDR is also necessary.In summary, we classified PDR eyes using COFDS grading and discovered that PDR eyes with primary vitreous hemorrhage typically have a better visual acuity prognosis than PDR eyes with primary fibrovascular proliferative type or RD type. These findings may aid retinal surgeons in predicting postoperative function in PDR patients. COFDS classification for PDR may have a significant predictive value for visual outcome and surgical management indications.
ACKNOWLEDGEMENTS
Foundations:Supported in part by the National Science Foundation of Liaoning Province, China (No.2020-MS-360);Shenyang Science and Technology Bureau (No.RC210267).
Conflicts of Interest: Lin TZ,None;Kong Y,None;Shi C,None;Eric Pazo E,None;Dai GZ,None;Wu XW,None;Xu L,None;Shen LJ,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Lacrimal sac lymphoma: a case series and literature review
- Age-related changes of lens thickness and density in different age phases
- Therapeutic potential of pupilloplasty combined with phacomulsification and intraocular lens implantation against uveitis-induced cataract
- Prophylaxis with intraocular pressure lowering medication and glaucomatous progression in patients receiving intravitreal anti-VEGF therapy
- Optimal timing of preoperative intravitreal anti-VEGF injection for proliferative diabetic retinopathy patients
- Visual field defects and retinal nerve fiber layer damage in buried optic disc drusen: a new insight
