Prophylaxis with intraocular pressure lowering medication and glaucomatous progression in patients receiving intravitreal anti-VEGF therapy
2022-10-24JeanetteDuJamesPatrieXiaoYuCaiBrucePrumYevgeniyShildkrot
Jeanette Du, James T Patrie, Xiao-Yu Cai, Bruce E Prum, Yevgeniy Shildkrot
1New York Eye and Ear Infirmary of Mount Sinai, 310 East 14th Street, New York, NY 10003, USA
2Department of Public Health Sciences, University of Virginia P.O. Box 800717, Charlottesville, Virginia 22908, USA
3Department of Ophthalmology, University of Virginia School of Medicine, 1300 Jefferson Park Ave, Charlottesville, Virginia 22908, USA
Abstract
● KEYWORDS: anti-vascular endothelial growth factor therapy; pretreatment; glaucomatous progression
INTRODUCTION
Intravitreal anti-vascular endothelial growth factor (VEGF)therapy is a widely used treatment for various neovascular conditions. Ranibizumab was the first agent determined to be safe and efficacious for treating wet age-related macular degeneration (ARMD) in a large-scale clinical trial[1]. Since then, the safety of bevacizumab and aflibercept has also been demonstrated[2]. While the benefits of anti-VEGF agents have substantially increased their use in recent years, some evidence suggests that a subset of patients may be susceptible to intraocular hypertension (OHT) and glaucomatous change associated with long-term treatment.
Transient intraocular pressure (IOP) elevations following intravitreal anti-VEGF injections are common and generally tolerated in the short-term[3-4]. However, sustained OHT is a more serious consequence of repeated injections and has been reported in patients undergoing long-term therapy[5-7].Compared to patients without preexisting glaucoma, those with concurrent glaucoma or OHT have higher rates of sustained pressure elevation after multiple injections[8-10]. The evidence on treatment-associated retinal nerve fiber layer (RNFL)thinning in non-glaucomatous eyes is controversial[11-15], but studies of glaucomatous eyes suggest that repetitive injections may be associated with accelerated structural change[16-18].These findings call into question the safety of long-term therapy for glaucoma patients, as they may be predisposed to OHT and associated complications.
As anti-VEGF therapy often extends many years, it is especially important to evaluate methods for controlling IOP in this specific population. Medical prophylaxis with pressurelowering agents prior to injections has emerged as one strategy.While prophylaxis appears to be effective for controlling acute post-injection pressure spikes[19-20], whether the benefits extend to long-term risk reduction in disease progression is not yet characterized.
This study investigates whether there is a difference in glaucomatous progression between patients who received or did not receive pretreatment with pressure-lowering medications prior to anti-VEGF injections. In turn, this may lead to a better understanding of whether long-term therapy is safe for treating neovascular retinal disease in eyes with concurrent glaucoma, and if injection-associated IOP elevations may underlie the previously reported deleterious effects.
SUBJECTS AND METHODS
Ethical ApprovalPermission was obtained by the Institutional Review Board for health sciences research at the University of Virginia. All research adhered to the Tenets of the Declaration of Helsinki. Informed consent to publish the manuscript was not obtained, as this report does not contain any personal information that could lead to patient identification.
Study DesignThis is a retrospective study of patients receiving six or more injections of ranibizumab, bevacizumab,aflibercept, or any combination of these agents. Data from patients with an ICD diagnosis of glaucoma or OHT, who were treated with anti-VEGF therapy for concurrent ARMD, retinal vein occlusion, or diabetic retinopathy, were collected and analyzed.
Inclusion criteria: Only patients carrying a diagnosis of glaucoma or OHT were included. Subjects identified for chart review had two or more RNFL thickness measurements by Heidelberg spectral domain optical coherent tomography (SDOCT) and/or Humphrey visual field tests, taken at least one calendar year apart. The first study obtained prior to or within 3wk of first anti-VEGF injection was designated as baseline.In cases where injections began before the subject’s first automated perimetry or Heidelberg SD-OCT test, the earliest study was taken for baseline. If patients required transition to intravitreal steroid therapy, the end of the follow up period was set as the start date of steroid therapy.
All subjects who received pretreatment were under the care of one retina specialist at our institution, while those who did not receive pretreatment were under the care of a different retina specialist. The reason for pretreating patients with a prior diagnosis of glaucoma or OHT was the practice preference of one specialist, and there were no other specific criteria for this decision. In the pretreatment group, patients were given both brimonidine and dorzolamide/timolol prior to injections unless there was allergy or intolerance to the drop. If patients experienced a significantly IOP spike to >60 mm Hg postinjection, they were also pretreated with oral acetazolamide for subsequent injections. For statistical analysis, subjects meeting the inclusion criteria were divided into two groups based on pre-treatment status. Group 1 included all eyes that were administered IOP lowering drops 30min prior to injection,with or without oral acetazolamide on injection days. Group 2 included all eyes that were not administered any pressure lowering medications. The primary outcome measures were rate of visual field loss in dB/year, rate of change in RNFL thickness in microns/year, and need for additional glaucoma medications, surgery, or laser as determined by treating glaucoma specialists. Rates of change in OCT and visual field parameters were calculated using the baseline test and subsequent test closest to 12-month from start of injections.Secondary outcome measures were change in best-corrected visual acuity (BCVA) and maximum IOP. BCVA was measured by Snellen chart and converted to logMAR scale.Maximum IOP was documented as the highest measurement by either rebound or applanation tonometry. Automated inbuilt Heidelberg software was employed for calculating mean global RNFL thickness and mean superior, inferior, temporal, and nasal quadrant RNFL thicknesses. Mean deviation (MD) and pattern standard deviation (PSD) values were generated using the Humphrey visual field analyzer (Carl Zeiss Meditec, USA).Statistical MethodsRates of change in visual acuity were comparedviaa linear mixed model (LMM) covariate adjusted linear contrast of the pre-treated and the non-pretreated group ΔlogMAR/year means. Visual acuity and IOP (mm Hg) were compared between groups at baseline, 12, 24, and 36monthsviaLMM linear contrasts of logMAR and IOP means. A two-sidedP≤0.05 decision rule was established a priori as the null hypothesis rejection criterion for the intergroup comparisons of visual acuity and IOP mean change. Change in IOP between baseline and Tmax was also comparedviaa LMM.
Rates of change in visual field parameters and OCT RNFL thicknesses were comparedviaLMM covariate adjusted linear contrasts of the pre-treated and non-pretreated groups.A two-sidedP≤0.05 decision rule was established a priori as the null hypothesis test rejection criterion for the intergroup comparisons. Covariate adjustment variables for the above analyses included baselines for each respective parameter, age at start of injections, prior comorbidities and interventions,injection number, injection indications, and follow up duration.SAS version 9.4 Mixed Procedure (SAS Institute Inc. Cary,NC, USA) was used to conduct LMM statistical analyses.
RESULTS
Patient Baseline CharacteristicsA total of 66 eyes from 54 patients were included in this study. Baseline characteristics of pretreated eyes (group 1) and non-pretreated eyes (group 2)are summarized in Table 1. No significant differences between groups were detected with regards to age at initial injection,prior diagnosis of diabetes mellitus or hypertension, IOP,visual acuity, lens status, number of prescribed anti-glaucoma medications, or number of prior glaucoma lasers or surgeries(P>0.089). Notable differences included injection indication,total number of injections, and mean follow up time, which are detailed in Table 1. Although total number of injections was greater in Group 2 (P=0.004), there was no difference in injection rate per year between groups (P=0.458).
Visual AcuityVisual acuity was similar between groups throughout the follow up period. Baseline logMAR visual acuity was 0.50 (Snellen 20/63) for group 1 and 0.48 (Snellen 20/60) for group 2 (Table 1). Rate of visual acuity change from baseline to last follow up was not significantly negative for either group 1 (-0.0078 logMAR units/year; 95%CI: -0.0434,0.0277;P=0.658) or group 2 (0.0045 logMAR units/year;95%CI: -0.0480, 0.0570;P=0.865). There was no significant difference between groups in annual rate of visual acuity decline (P=0.990) after adjustment for potential confounders.Analyses of logMAR trends over the first 36mo of follow up also showed no differences between groups at 12, 24, or 36mo(Table 2), as shown in the Figure 1.
而那按风琴的人,因为越按越快,到后来也许是已经找不到琴键了,只是那踏脚板越踏越快,踏的呜呜地响,好象有意要毁坏了那风琴,而想把风琴撕裂了一般地。
Intraocular PressureIOP did not differ between groups throughout the follow up period, but mean maximum IOP was significantly higher than baseline IOP in both groups(P<0.001). Average baseline IOP was 18.1 mm Hg (95%CI:16.7, 19.8 mm Hg) for group 1 and 17.2 mm Hg (95%CI:15.7, 18.6 mm Hg) for group 2. After potential confounder adjustment, there was no difference between groups (P=0.191). Mean maximum IOP over the follow up period was 27.1 mm Hg (95%CI: 24.0, 30.0 mm Hg) for group 1 and 26.1 mm Hg (95%CI: 21.8, 30.2 mm Hg) for group 2, with no difference between groups after potential confounder covariate adjustment (P=0.939). Analyses of IOP trends over the first 36mo of follow up also revealed no differences at 12, 24, or 36mo (Table 2), as shown in the Figure 1.
Visual FieldTable 3 summarizes the visual field outcomes.Mean baseline MD was -8.24 dB (95%CI: -10.6, -5.85)and -5.04 dB (95%CI: -6.84, -3.23 dB) in groups 1 and 2,respectively, with a marginal difference between groups after potential confounder adjustment (P=0.060). Mean baseline PSD was 5.43 dB (95%CI: 3.78, 7.08 dB) and 4.96 dB(95%CI: 3.71, 6.21 dB) in groups 1 and 2, respectively, with no difference between groups after potential confounder adjustment (P=0.853). Estimated mean decline in MD was -1.08 dB/year and -0.29 dB/year for groups 1 and 2,respectively (P=0.002 andP=0.549, respectively), with no between group differences after potential confounder adjustment (P=0.122). Estimated mean change in PSD was-0.64 dB/y and 0.06 dB/y for groups 1 and 2, respectively(P=0.073 andP=0.890, respectively), with no between group differences (P=0.332).
Retinal Nerve Fiber Layer Thickness AnalysesAnalyses of OCT study outcomes are summarized in Table 4. Baseline average global RNFL thickness was 79.8 μm (95%CI: 55.2,104.4 μm) and 95.3 μm (95%CI: 74.0, 116.6 μm) in groups 1 and 2, respectively, with no difference between groups after potential confounder adjustment (P=0.859). Baseline mean thicknesses of the superior, inferior, temporal, and nasal quadrants ranged from 55.3-100.0 and 72.3-115.6 μm for groups 1 and 2, respectively. There were no significant differences in global or superior, inferior, temporal, and nasal quadrant thicknesses detected between groups at baseline(P>0.154). Estimated mean rate of global RNFL thinning was-4.22 μm/y and -5.29 μm/y for groups 1 and 2, respectively,and although both groups showed significant change from baseline (P=0.005,P=0.011), there was no difference detected between groups (P=0.467). On quadrant analyses, there was significant RNFL thinning from baseline only in the inferior quadrant of group 2 (P=0.049), and this rate of thinning was marginally different from the rate of inferior quadrant thinning of group 1 (P=0.057). There were no detectable differences in mean RNFL thinning between groups for any of the remaining quadrants.
Glaucoma TherapiesThe average number of prescribed anti-glaucoma medications prior to initiation of injections was 2.1 and 1.9 for groups 1 and 2, respectively (P=0.519). Prior to first injection, 7 of 20 (35%) eyes in the pretreated group and 7 of 46 (15.2%) eyes in the non-pretreated group had a history of glaucoma laser or surgery (P=0.089). Five of 20(25.0%) pretreated eyes and 14 of 46 (30.4%) non-pretreated eyes required the addition of at least one anti-glaucoma medication over the follow up period. The non-pretreated to pretreated odds ratio was 1.33 (95%CI: 0.25, 7.20), but was not statistically significant after potential confounder adjustment (P=0.740). The number of eyes requiring glaucoma laser or surgery over the follow up period was 4 of 20 (20.0%)and 13 of 46 (28.3%) for the pretreated and non-pretreated groups respectively. The non-pretreated to pretreated odds ratio for further intervention was 1.93 (95%CI: 0.55, 6.84), but was also not statistically significant after potential confounder adjustment (P=0.306).
DISCUSSION

Figure 1 Visual acuity (A) and intraocular pressure (B) at baseline and at 12, 24, and 36mo Vertical lines identify the 95%CI for the mean of the distribution.
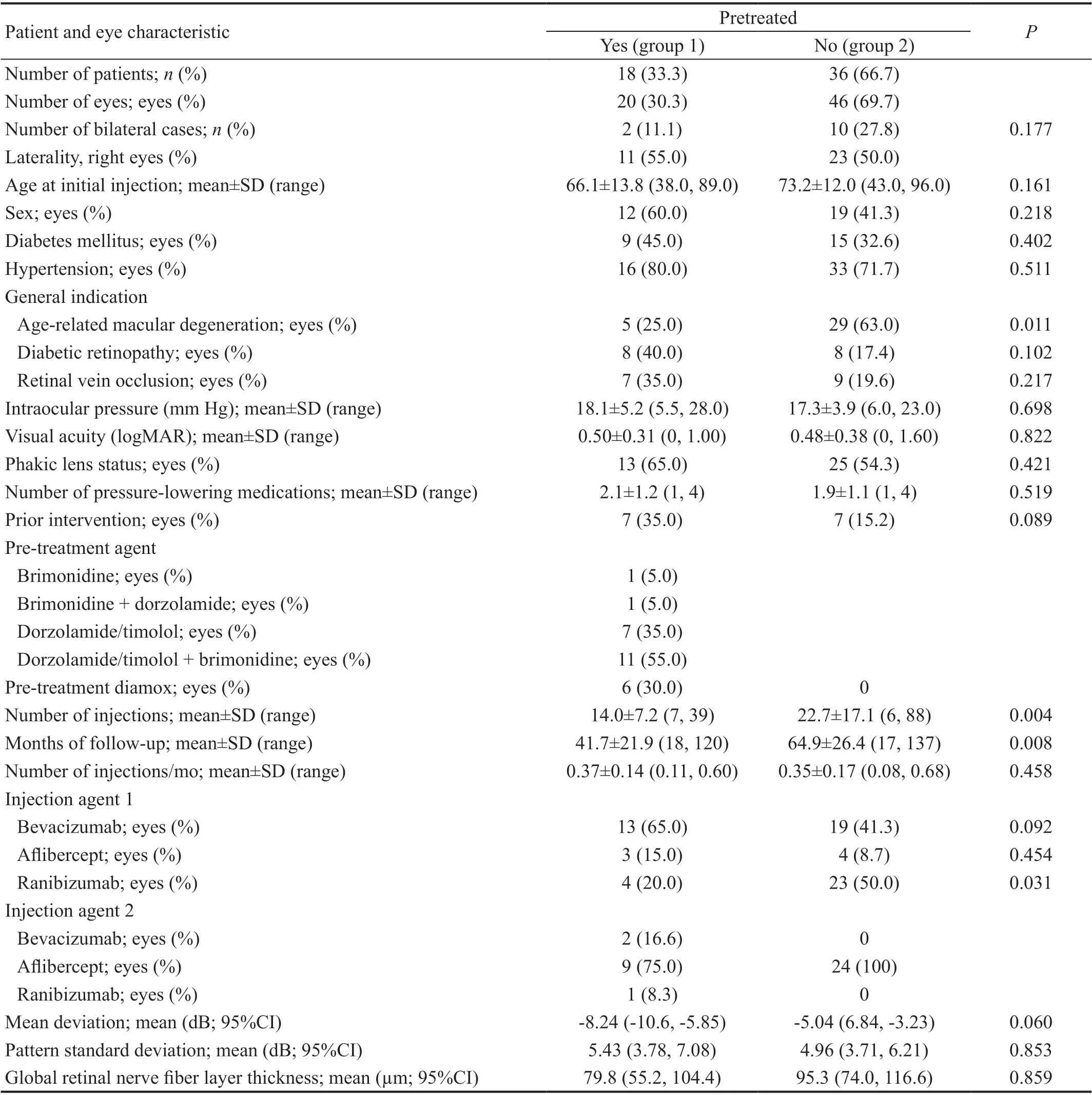
Table 1 Baseline patient and eye characteristics

Table 2 Trends in visual acuity (logMAR) and intraocular pressure (mm Hg) over 36mo of follow up
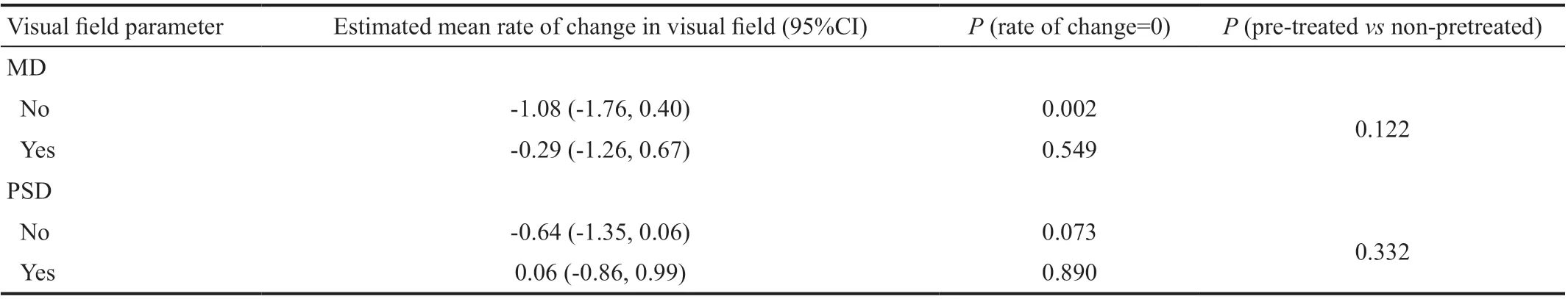
Table 3 Mean rate of change in visual field parameters dB/y
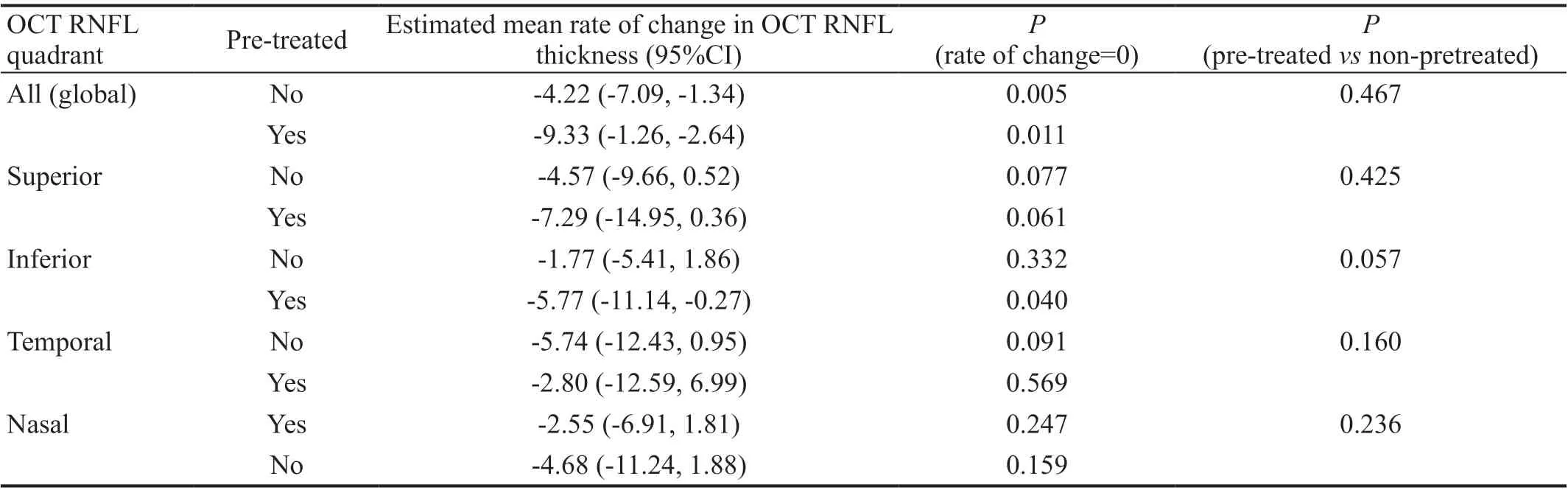
Table 4 Mean rate of change in retinal nerve fiber layer thickness mm/y
IOP elevation following intravitreal anti-VEGF injections is well characterized and generally a transient effect[3-4]. Although large-scale clinical trials supported the safety of anti-VEGF therapy[1-2], sustained OHT has been reported in association with greater injection frequency, higher total number of injections,IOP: Intraocular pressure.and concurrent glaucoma[21]. Goodet al[8]defined sustained OHT as IOP >22 mm Hg lasting >30d, recorded on at least two separate visits and a change from baseline >6 mm Hg. They showed that eyes with concurrent glaucoma were more likely to meet these criteria. In our study, glaucomatous eyes experienced mean maximum pressures of 26.1 and 27.1 mm Hg in the pretreated and non-pretreated groups, which were significantly higher than baseline means of 17.3 and 18.1 mm Hg,respectively. These values are in line with prior reports that glaucoma patients treated with repetitive anti-VEGF injections may be at risk for prolonged OHT.
The need for anti-glaucoma medications or more invasive interventions during the course of treatment may reflect this risk. Bressleret al[5]found that patients receiving repeated ranibizumab injections had a 9.5% composite probability of experiencing sustained OHT or requiring augmentation of pressure-lowering medication during 3y of follow up,compared to 3.4% in the sham group. Eadieet al[22]showed that a greater number of annual injections significantly increased the risk of later requiring a glaucoma drainage device, trabeculectomy, or cycloablative procedure. Over 3y of follow up, 44.6% of patients who received 7 or more injections per year required glaucoma surgery, with an adjusted rate ratio of 2.48 (95%CI: 1.25-4.93) for individuals receiving surgery versus matched controls not requiring surgery. Similarly, our previous study of glaucomatous eyes found that a significantly greater proportion of anti-VEGF treated eyes later underwent invasive glaucoma intervention[17]. In the present study, 30.4%of non-pretreated patients and 25% of pretreated patients required additional pressure-lowering medication over the follow up period. The 28.3% of non-pretreated patients and 20% of pretreated patients experienced pressures uncontrolled on drops alone and further received glaucoma laser or surgery.These findings support the importance of closely following patients to assess whether augmentation of glaucoma medication is needed. Moreover, patients with preexisting OHT or glaucoma may require laser or surgical intervention over the duration of therapy.
Prophylaxis with pressure-lowering medications has emerged as one strategy for increasing the safety of repeated injections.It is well supported that administering topical agents prior to injections is effective for controlling transient IOP elevations[19-20]. In this study, we questioned whether medical prophylaxis to dampen post-injection IOP spikes had an effect on structural or functional glaucomatous progression. To our knowledge, Parket al[18]are the only other group to examine the effects of pretreatment on structural RNFL changes. They found that non-glaucomatous eyes showed no change in RNFL thickness, while glaucomatous eyes exhibited significant thinning only in the non-pretreated group[18]. Thus, they concluded that pretreatment was protective against structural progression. Similarly, we found that non-pretreated eyes with underlying glaucoma or OHT demonstrated a significant change in global RNFL thickness (P=0.005). However, the pretreated group also showed significant thinning (P=0.011),and there was no difference detected between groups. This is in contrast to Parket al[18], as we observed RNFL thinning regardless of whether pressure-lowering medication was administered prior to injections. One explanation is that our inclusion criteria included patients with OHT but not necessarily evident glaucomatous disease. Thus, patients suffering RNFL loss in their study could have been at higher risk at baseline. The lack of significant difference between pretreated and non-pretreated groups in our study could also point to the possibility that RNFL thinning in this population of patients may be more related to underlying glaucoma or another effect of injections, rather than post-injection pressure spikes.
This study contributes to the ongoing discussion of whether intravitreal therapy has the potential to accelerate glaucomatous change. Parlaket al[12]and Valverde-Megíaset al[13]reported that anti-VEGF treated eyes demonstrated significant RNFL thinning, but that comparable thinning was also observed in non-treated fellow eyes. In contrast, Martinez-de-la-Casaet al[11]reported that treated eyes showed significant thinning compared to controls. A Meta-analysis combining six studies revealed no significant decrease in RNFL thickness from baseline, but noted in subgroup analyses that significant RNFL loss was demonstrated in the controlled experimental studies[15].Another reason proposed for these discrepant findings is the component of macular edema in neovascular retinal disease,which could obscure true gain or loss of peripapillary RNFL[24].Despite the varied findings in non-glaucomatous eyes, we questioned the nature of RNFL thinning specifically in patients with concurrent glaucoma or OHT. In our previous study, anti-VEGF injections were associated with significantly greater change in superior quadrant RNFL thickness in a glaucomalike pattern[17]. Elevated IOP and pressure fluctuations are known risk factors for glaucoma. Thus, it could be speculated that controlling transient post-injection IOP spikes would slow any deleterious effects of repetitive injections. Here we found that glaucomatous eyes exhibited significant decrease in global RNFL thickness, but no difference in thinning whether pretreated or not. If pretreatment is effective in dampening transient IOP spikes, then the observed structural change may not be attributable to injection-associated acute pressure fluctuations.
Other than transient pressure spikes, it is possible that some process underlying sustained OHT, whether related to anti-VEGF treatment or predisposing anatomy, is a more important driver of glaucomatous change. Some recent studies align with the mechanical theory that injections could damage the outflow apparatus through repeated compressions of the anterior chamber (AC) volume, straining the outflow system particularly in phakic eyes. Wingardet al[25]reported that an elevated risk of glaucomatous disease was associated with higher injection frequency and phakic lens status. Studies by Wenet al[26]and Arslanet al[27]showed that post-injection AC angle narrowing and decrease in AC depth were related to phakic lens status, and that outflow facility was reduced by 12% in eyes receiving 20 or more injections compared to fellow untreated eyes. Likewise, Cuiet al[28]discuss a “tipping point” for elevated risk of IOP-related changes. In their study,patients receiving >14 or >20 injections had increased risk of needing new ocular hypertensive medication, but that pseudophakia had a protective effect. Conversely, the study by Sternfeldet al[29]reported that pseudophakia with history of Nd:YAG capsulotomy was associated with increased risk of sustained IOP elevation, lending support to the hypothesis that introduction of injected particles to the trabecular meshwork could also affect outflow facility[30]. In our study, there was no significant difference in phakic lens status between groups,and the average rate of injections was 0.37 and 0.35 injections/month in pretreated and non-pretreated eyes, respectively.Thus, it is possible that the number or frequency of injections was not enough to detect a change in glaucoma parameters within the first year. It is also possible that the effectiveness of pretreatment may be related to lens status, which is a question that should be explored in future studies.
Class of anti-VEGF agent is another factor to consider in assessing glaucomatous change. The prior study by Goodet al[8]found a higher prevalence of requiring IOP lowering intervention in eyes receiving bevacizumab compared to ranibizumab[8]. The authors suggested this could be related to a post-injection immunological reaction after intravitreal bevacizumab or to the mode of bevacizumab storage in plastic syringes, which may produce protein aggregates that both deposit in the trabecular meshwork and further incite a significant immunological response. In the present study,outcomes were not analyzed separately based on anti-VEGF agent. If there is some component of the anti-VEGF molecule that differentially influences either IOP spikes or trabecular meshwork integrity, then the lack of difference in treatment groups may be explained by this. Given the retrospective design and the significant number of patients who were switched to a different anti-VEGF agent over the course of therapy, assessing outcome measures based on injection type was not feasible, but should also be addressed in future investigations.
Our subject sample is representative of the varied demographics and conditions seen in a typical retina practice. However, the inclusion of multiple vitreoretinal conditions and the different proportions of these conditions represented in the pretreated and non-pretreated groups may be a limitation of the study,as it is difficult to parse out their effects from each other and from glaucoma progression. For example, visual deterioration following retinal vein occlusion could affect the observed changes in glaucoma parameters. Parameters used to assess glaucomatous progression may also be influenced by patients’underlying maculopathies. Changes in OCT measurements may be related to improvement in macular edema rather than nerve fiber layer loss. Although it is unclear why only the pretreatment group showed a significant decline in MD, one possibility is that differing macular pathology affected the analyses. Despite these limitations, our study is one of the few to examine visual field changes in conjunction with structural change, and carries particular clinical relevance for patients with preexisting glaucoma or OHT. Larger prospective studies with subgroup analyses to separate subjects by underlying retinal pathology are needed to further understand the nature and extent of the association between anti-VEGF therapy and disease progression.
In conclusion, prophylactic pressure-lowering medication is a method for controlling IOP fluctuations secondary to intravitreal anti-VEGF injections. In this sample population of glaucomatous patients receiving long-term anti-VEGF therapy, pretreatment had no detectable effect on structural or functional glaucomatous progression. However, 28.3%of non-pretreated eyes and 20% of pretreated eyes required glaucoma laser or surgery. Given that our study numbers are small and progression parameters were assessed only for the approximate first year after start of injections, larger subject numbers and longer follow up may yield differing conclusions,and providers should not discount pretreatment as an option for predisposed eyes. It is advisable for ophthalmologists to monitor for glaucomatous complications and exercise caution when administering repeated injections in this population of patients.
ACKNOWLEDGEMENTS
Conflicts of Interest:Du J,None;Patrie JT,None;Cai X,None;Prum BE,None;Shildkrot Y,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Lacrimal sac lymphoma: a case series and literature review
- Age-related changes of lens thickness and density in different age phases
- Therapeutic potential of pupilloplasty combined with phacomulsification and intraocular lens implantation against uveitis-induced cataract
- Optimal timing of preoperative intravitreal anti-VEGF injection for proliferative diabetic retinopathy patients
- Prognosis value of Chinese Ocular Fundus Diseases Society classification for proliferative diabetic retinopathy on postoperative visual acuity after pars plana vitrectomy in type 2 diabetes
- Visual field defects and retinal nerve fiber layer damage in buried optic disc drusen: a new insight
