转录中介体复合物在心血管发育和疾病中的转录调控作用
2022-05-24张元赵语婷庄乐南贺津
张元,赵语婷,庄乐南,2,,贺津
综 述
转录中介体复合物在心血管发育和疾病中的转录调控作用
张元1,赵语婷1,庄乐南1,2,3,贺津3
1. 浙江大学动物科学学院动物医学系,杭州 310058 2. 浙江大学医学院附属邵逸夫医院心内科,浙江省心血管介入与再生修复研究重点实验室,杭州 310016 3. 浙江大学动物科学学院动物遗传育种与繁殖研究所,杭州 310058
哺乳动物心血管系统发育过程中,各分子、细胞和组织器官形态发生过程的精细协调对于形成成熟且功能齐全的心血管系统是不可或缺的,这些过程出现异常通常会导致严重的先天性心血管发育缺陷。多细胞生物中细胞命运的决定和维持在很大程度上依赖于对RNA聚合酶II (Pol II)转录活性的时空精确调控,而转录中介体(Mediator)在Pol II转录过程中起着重要的协同作用。Mediator是一种进化上保守的多亚基蛋白质复合体,包括头部、中部、尾部和激酶部四个部分,是转录因子和基础转录机器之间的功能联系的桥梁。近年来,鉴于Mediator在基因表达中的关键作用,越来越多的人类心血管疾病被证实与特定的Mediator基因突变相关,如心脏瓣膜缺陷、大动脉转位、DiGeorge综合征及一些与能量稳态失衡相关的心血管疾病。本文就Mediator在心血管系统发育和疾病中的作用进行综述,重点讨论Mediator对转录调控的影响在心血管疾病发生发展中的作用,旨在为与Mediator相关的心血管系统发育和疾病的研究提供广阔的研究思路。
转录中介体复合物;转录调控;心血管系统发育;心血管系统疾病
转录中介体(Mediator)是从芽殖酵母()中提纯出来的一个大的多亚基复合体,通过与DNA结合的转录因子(transcription factor, TF)的相互作用介导基因的转录激活。目前,研究发现Mediator几乎涉及到真核生物基因转录的所有方面[1]。除此之外,Mediator还调节染色质循环[2]和染色质高阶折叠[3],以及mRNA加工[4]和出核[5]、转录记忆[6]和再启动[7]。Mediator除调控基因表达之外还有其他作用,如DNA修复[8]和类开关重组(class switch recombination, CSR)[9]等。近年来,在多种发育障碍和癌症等疾病中发现Mediator某个亚基发生了突变或异常表达[10],进一步引起大家的广泛关注。虽然已经有很多文章阐明了Mediator的组成、结构及其在转录方面的作用,但是关于Mediator的生物学意义及涉及其突变引发疾病的分子机制仍存在许多有待探究的地方。本文主要综述了Mediator近年来相关的研究进展,重点介绍了其在调控心血管发育和疾病发生发展中的重要作用。
1 Mediator的发现与相关研究
1.1 Mediator的组成与结构
在2000年,酵母[11]和哺乳动物细胞[12,13]中的Mediator被通过遗传学和生物化学方法鉴定为转录的共同调节因子,同时其结构也被解析。研究发现酵母和人类()间Mediator的结构和亚基组织是保守的(在芽殖酵母中含有25个亚基,在高等生物中含有30个以上的亚基)。对酵母Mediator结构的研究表明,其各亚单位稳定组合成复合物,整个复合物的结构可以分成明显不同的模块,包括头部、中部、尾部和激酶。正常情况下,Mediator的头部、中部和尾部是关联在一起的,但与激酶模块的结合不太稳定[14]。Mediator的这种模块化的组成方式也同时反映了Mediator组件的不同功能。在酵母和人类中,Mediator头部和中部模块构成了转录调控所必需的核心,而尾部和CDK8激酶模块则起着调节功能[15,16]。
尽管Mediator的许多亚单位呈现相对较高的序列多样性,但研究表明Mediator具有较为保守的组织结构[17],且在人类中得到了验证[18,19]。一项利用冷冻电镜对小鼠CH12细胞中Mediator结构解析的研究详细地阐述了小鼠Mediator的分子模型[20]。首先是头部模块,小鼠Mediator头部模块的结构与酵母非常相似,MED17充当头部模块的中心;中部模块可以分为由长螺旋束形成的顶部和底部,顶部由MED7和MED21组成,底部由MED4和MED9组成,其中MED14充当了连接其他模块的枢纽[15]。图1显示了人类Mediator的亚单位结构组成,各个亚基模块在复合体中的相对位置基于已发表的蛋白质相互作用研究和部分结构生物学研究数据[21]。
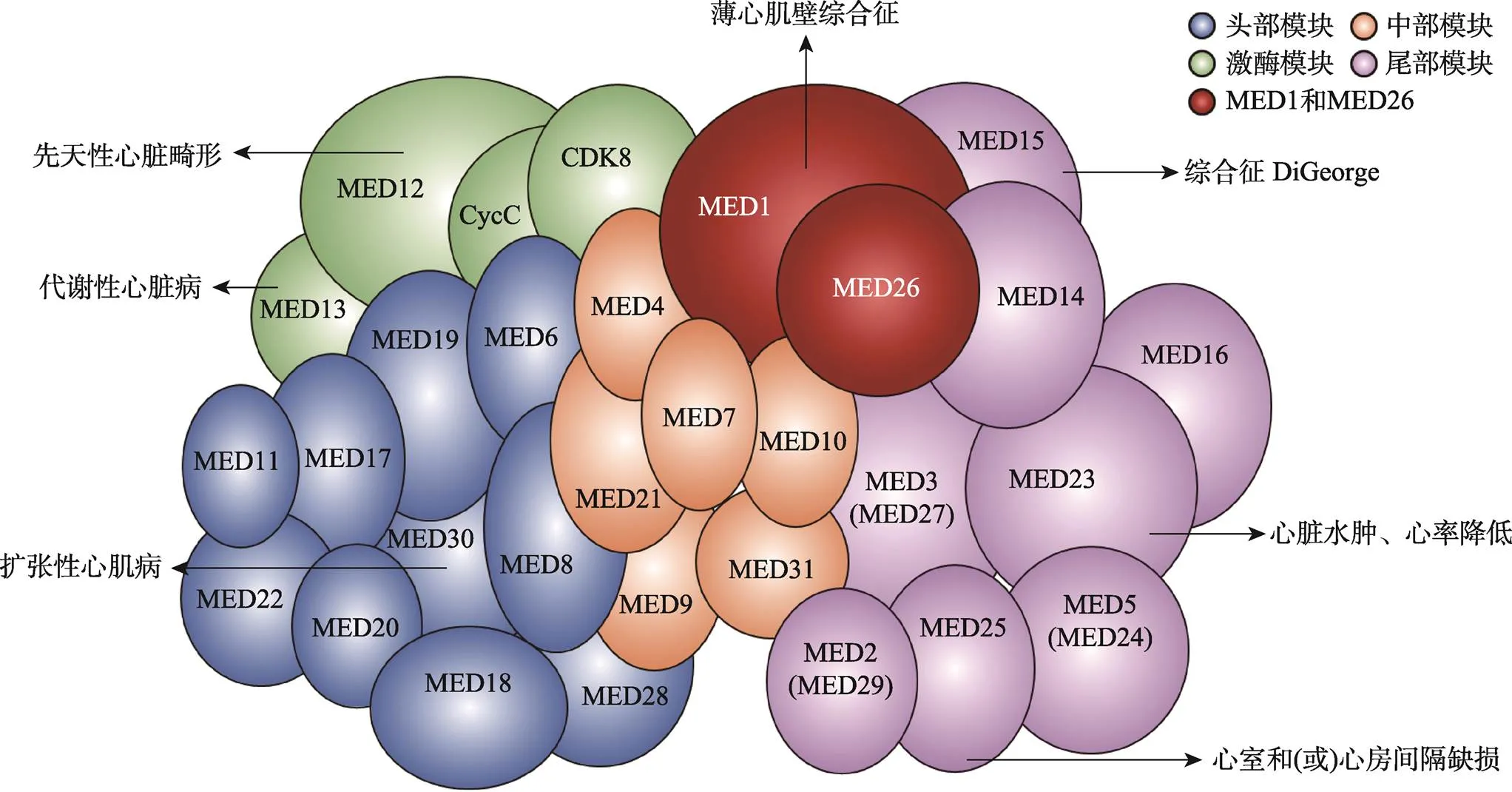
图1 人类Mediator的模块化结构示意图
箭头表示对应亚基突变或缺失导致的心脏发育异常或心脏疾病。
1.2 Mediator与转录调控
1.2.1 转录调控
所有真核细胞中与DNA相关的过程,包括转录和DNA修复,都发生在染色质拥挤的环境中,而基因表达启始于转录。真核生物中编码蛋白质的基因大多是由RNA聚合酶II(Pol II)转录的。由Pol II调控的转录需要各种转录激活因子和抑制因子的协同作用,包括转录激活因子、一组通用转录因子(general transcription factors, GTFs)和转录辅助因子[22]。它们与转录调节区的靶序列相结合,通过直接的蛋白质-蛋白质相互作用或通过影响染色质(进行染色质结构重塑、组蛋白修饰等)来调节转录机器的组装和活性。TF的这些功能进一步受到转录协同调节因子的调节。而Mediator复合物是真核生物中Pol II转录的关键调节因子,是转录所必需的。这一基本、保守且极其复杂的生物过程受到严格监管以确保遗传程序适应细胞的要求。转录调控的失衡会导致严重的疾病,包括癌症和心脏疾病[23]。
1.2.2 Mediator在转录过程中的协同作用
Mediator的发现彻底改变了人们之前对转录调控的研究。在经典的Mediator功能模型中,Mediator充当适配器,将转录信号从激活子传递到一般的转录机器,以帮助Pol II启动转录。在酵母和哺乳动物中,一些Mediator亚单位被证明与各种激活物相互作用[21,24,25],头部模块的特定亚基也被证实与Pol II和其他GTF相互作用[26,27]。除了作为TF和预起始复合物(包括Pol II和GTF)之间桥梁的经典功能外,Mediator还参与转录的许多其他方面,包括转录延伸[28~30]、终止[31]、RNA加工[32]、染色质在启动子中的重塑[33~35]以及表观遗传调控[36]等。25个酵母Mediator亚基中的10个亚基(MED4、MED6、MED7、MED8、MED10、MED11、MED14、MED17、MED21和MED22)的单独缺失是致死的;并且各种哺乳动物Mediator亚基的敲除也是胚胎致死的[37~41]。以上这些研究证实了Mediator在转录过程中的重要协同作用。
1.2.3 Mediator在预起始复合体(preinitiation complex, PIC)上游招募的功能
在转录过程中,Mediator被招募到酵母的上游激活序列(upstream activation sequence, UAS)区域或哺乳动物中增强子的调节区域,作为结合到这些区域的特定TF和核心启动子等基础转录机器之间的功能纽带。因此,Mediator是TF和基础转录机器之间的功能联系的桥梁[42]。真核转录需要组装多亚基PIC,PIC由Pol II、Mediator和TFIIA、TFIIB、TFIID、TFIIE、TFIIF和TFIIH组成。在转录调控中,Mediator主要的“中介桥梁”功能是刺激PIC的形成。为了促进PIC在体内的组装,Mediator与PIC的各组分通过特定的蛋白-蛋白相互作用连接[29,43]。根据这些不同的相互作用,Mediator在体内的不同阶段协调PIC组装,并且Mediator的头部和中间模块都参与了这些功能[44]。Mediator足够大,足以作为PIC的支架,支撑PIC在核心启动子上的组装[26,45]。
1.2.4 Mediator与TREX-2在转录启动和mRNA输出中的作用
目前有研究证实TREX-2与Pol II的重要调节因子Mediator相互作用[46,47]。核孔复合物(nuclear pore complex, NPC)是细胞质与细胞核内物质输送活动相关的一种大型超级分子复合物,既是分子转运的门户,又是染色体的附着位点。除了在核运输中的既定功能外,NPC还会影响基因表达[48]。而酵母TREX-2定位于NPC,由Sac3、Thp1、Sem1、Sus1和Cdc31组成,可分为PCI结构域部分和NPC锚定元件,TREX-2可影响基因与NPC的相互作用[49]、转录和mRNA输出[50]。
而MED31/MED7N亚基不仅位于Mediator头部的Pol II C端结构域(C-terminal domain, CTD)结合位点处,同时也在Mediator中间模块上的CKM结合位点处,所以Mediator中间模块和头部模块之间的接口由MED31通过MED7中的非结构化接头连接而成。因此,MED31缺失会导致Mediator架构的重排[18]。在这种情况下,Sac3 PCI域与MED31/MED7N亚基复合体的对接可以局部改变介体构象,从而调节与Pol II的相互作用,因为Sac3、MED31和CDK8都是正常Ser5-P水平所必需的[51,52]。同样的原理可能延伸到CDK8激酶模块(CDK8 kinase module, CKM)[53],主要通过与中间模块MED19亚基之间的接触和Mediator头部的区域与核心Mediator相互作用。酵母MED19位于灵活的MED7/MED21亚基复合体的末端,该亚基复合体反过来锚定MED31。因此,相关结合位点的紧密连接可以解释TREX-2对Pol II和CKM的双重作用。同时,TREX-2还利用其与Mediator相互作用表面来调节mRNA输出,提示了一种将转录启动和mRNA处理的早期步骤相耦合的机制[46]。根据现有的转录启动模型,Mediator和染色质重塑复合物SAGA共同占据了许多酵母启动子[54]。酵母TREX-2主要定位于NPC,考虑到SAGA调节TREX-2的C端结构域和Mediator与PCI的相互作用,TREX-2可能可以与SAGA和Mediator联系[55]。综上所述,TREX-2的小部分可以在核孔穿梭,从而扩大其工作范围。而TREX-2通过与Mediator相结合,利用Mediator的已知整合外部信号能力(如结合TF和共激活因子并将它们直接与Pol II相连)。对于NPC靶向的高诱导性基因,Mediator可以通过TREX-2与NPC优化转录输出,以此作为对基因位置做出反应的一种方式。
2 Mediator与心血管发育和疾病
心血管疾病(cardiovascular disease, CVD)是中国人死亡和过早死亡的主要原因[56],心血管病死亡约占城乡居民总死亡原因的40%[57]。研究表明,先天性心脏病是最常见的先天性疾病,影响约0.8%的活产儿[58]。Mediator作为TF和基础转录机器之间的结构和功能联系的桥梁,其突变已被证实与许多人类疾病,包括心血管疾病(表1)。下面主要介绍与Mediator相关的心血管系统发育异常及心血管系统疾病。
2.1 Mediator与心血管发育
在心血管系统发育过程中,各分子、细胞和组织器官形态发生过程的精细协调对于形成成熟且功能齐全的心血管系统是不可或缺的,这些过程的异常通常会导致严重的先天性心血管发育缺陷。但驱动心血管系统发育的确切分子和细胞机制以及在发育过程中导致心血管系统缺陷的相关病理机制仍未完全理解。目前已有多项研究证实,Mediator的改变会影响心血管系统发育。
心脏瓣膜的缺陷是较为常见的先天性和获得性心脏病,但目前只有少数心脏瓣膜病变相关的遗传和分子机制被探究。已有研究证实,MED12功能缺陷与人类先天性心脏病有关,并对小鼠和斑马鱼心脏发育产生影响。对小鼠的早期发育和正确的Wnt和Wnt/PCP信号转导是必不可少的,表达异常的小鼠在神经管闭合、体细胞发育和心脏形成方面存在严重缺陷,且缺陷胚胎的凋亡细胞主要存在于心脏中,表现为心脏增大及心功能不全[60]。有研究证实,的心脏特异性缺失小鼠表现为进行性扩张型心肌病[70]。一项有关缺陷的斑马鱼突变体的研究发现在斑马鱼心脏房室管发育、心内膜垫发育和心脏瓣膜形成过程中发挥重要作用,并且首次证明了可能在调节和的表达和心脏的发育中发挥重要作用,从而使斑马鱼胚胎心脏内膜垫的形成不依赖于[71]。

表1 与Mediator相关的心血管疾病
大动脉转位(transposition of the great arterie, TGA)是新生儿中最常见的紫绀型心脏缺陷,占所有先天性心脏病的5%~7%。TGA患者心脏主动脉和肺动脉与正常心脏流出相反,导致缺氧血液返回身体而不是肺部。研究表明,(也被称为或)在胎儿和成人组织中广泛表达,在心脏及主动脉中高度表达[72]。对97名TGA患者的筛选分析显示(p.E251G、p.R1872H、p.D2023G)中有3个错义突变[72]。这些发现表明突变会导致先天性心脏病TGA[62,63],并表明该Mediator亚基在心脏正常发育中发挥重要作用。
染色体22q11.2缺失综合征又称为DiGeorge综合征(digeorge syndrome, DGS)或velocardiofacial综合征(velocardiofacial syndrome, VCFS),是人类最常见的多发性先天性异常综合征之一,在儿童中的发病率约为1/3900。DGS常见的临床表现包括心脏缺陷、腭异常、特征性面部畸形、免疫功能障碍、胸腺和甲状旁腺发育不全引起的低钙血症[64,65]。22q11.2缺失综合征相关的复杂临床症状表明其涉及多个基因,而就是其中之一。人类是甾醇调节元件结合蛋白1α ()和激活的的物理靶标和功能传感器,它们分别控制脂质代谢和发育程序[73,74]。作为多种生物学上重要的信号转导途径中的关键枢纽,其表达降低将会导致与22q11.2缺失综合征相关的临床异质表型[75]。
有研究证实,过氧化物酶体增殖物激活受体γ辅激活因子1α ()通过与过氧化物酶体增殖物激活受体γ ()相互作用的Mediator亚基MED1,直接与Mediator相互作用[76],并在DNA模板上刺激Mediator依赖的功能。是氧化磷酸化基因表达的关键转录调节因子,可以刺激p300依赖的组蛋白乙酰化和转录,以响应[77]。根据研究报道,条件性缺失小鼠会出现类似于薄心肌壁综合征的心脏表型,心肌壁发育不良,小梁发育异常,并且心肌致密层变薄,在E11.5左右开始出现死亡[38,78],30%发育不全的小鼠可存活到E13.5,但胚胎心脏发育迟缓,最终死于E14.0[59]。
2.2 Mediator与心血管疾病
众所周知,能量平衡失调是心血管病的一个危险因素[62]。另外,心脏和骨骼肌等在传统意义上不被认为是新陈代谢调节器的多个器官被证实可调控全身能量消耗[79~81]。例如,心脏的传统功能是为身体其他部位运送血液。维持心肌收缩力需要很高的ATP生成能力,据估计,心脏自己储存的ATP仅能维持其功能搏动几次。因此,心肌必须高度调节其自身代谢以应对生理、病理和发育条件的变化[82]。心脏主要是以脂肪酸氧化的形式高速消耗能量,并通过微调心脏新陈代谢和改变基因表达来适应能量供应的变化,以最大限度地提高能量效率[82,83]。一旦发生心血管疾病,线粒体功能减弱,心脏能量代谢就会从β-氧化转变为糖酵解[84]。例如通过介导核激素受体依赖的转录来调节脂肪酸代谢等。Mediator在新陈代谢中的作用也在关于秀丽隐杆线虫的研究中得到证实[85]。
心脏收缩力由钙信号在分子水平上调控,细胞内钙的释放和再摄取驱动收缩和放松,并且这一过程在心肌细胞中受到严格调节。钙离子(Ca2+)调控基因的破坏将会导致心脏收缩功能改变。例如,肌质网(sarcoplasmic reticulum, SR)中Ca2+摄取减少或衰竭心脏发生SR钙渗漏[86,87],钙离子调控基因的突变会导致心律失常和扩张型心肌病(dilated cardiomyopathy, DCM)[88]。已有研究证实定位于心脏中钙离子调控基因的TF结合序列,与心肌细胞中的TF共同结合钙离子调控基因的启动子[70]。在心脏中的缺失会改变钙离子调控基因的表达,从而改变心肌细胞中的钙循环并中断心肌细胞电活动,影响心脏收缩,导致成年小鼠扩张型心肌病,但的缺失不影响-DNA结合或Pol II的募集[70]。
在心脏中抑制许多甲状腺激素受体(thyroid hormone receptor, TR)反应基因,及心脏相关的研究已证实心脏调节全身能量稳态的能力[79]。甲状腺激素(thyroid hormone, TH)是心脏转录稳态的关键调节因子,主要调控与维持心脏正常结构和功能相关的基因转录。已知低TH水平与加速心力衰竭进程和预后不良有关[89,90],而高水平的TH会导致快速性心律失常和心室功能障碍[91,92]。心脏过表达小鼠由于能量消耗增加,表现出对高脂饮食诱导肥胖的抵抗力,而心脏特异性缺失的小鼠在高脂饮食刺激下出现肥胖的现象。同时,在甲状腺功能减退症小鼠的心脏中过表达维持了心脏中的表达,可以在一定程度上缓解心脏损伤[61]。此研究揭示了心脏转录通路在甲状腺功能减退中的调节作用,并确定了由调节的响应TH信号的分子通路在心脏疾病中发挥的作用。
与线粒体氧化磷酸化(oxidative phosphorylation, OXPHOS)和脂肪酸氧化(fatty acid oxidation, FAO)代谢程序相关,进而对心脏功能产生特定影响[10]。心肌的高能量需求需要充足的三磷酸腺苷(adenosine triphosphate, ATP)供应,其主要可从线粒体OXYPHOS中获得。因此,心脏线粒体功能障碍会导致ATP生成减少,进而影响心脏收缩功能,严重时会导致心力衰竭和死亡[68]。已有研究证实,连接Mediator头部和尾部模块的MED30亚基缺失潜在地影响Mediator核心的稳定性。(p.I44F)上的异亮氨酸到苯丙氨酸的纯合突变小鼠断奶后患致命的扩张性心肌病,线粒体氧化磷酸化能力急剧下降、呼吸链功能下降且氧化磷酸化基因mRNA表达水平降低,但可以通过生酮饮食在一定程度上挽救,延长一定的存活时间。
此外,其他Mediator也被证实影响代谢。如可通过介导核激素受体依赖的转录来调节脂肪酸代谢[85];在脂肪形成和平滑肌细胞分化过程中都参与了胰岛素信号的调节和细胞命运的决定[32,93];作为Mediator复合体的核心蛋白,MED1可直接与核激素受体结合以维持骨骼肌能量稳态[94]。也有研究表明,在心肌肥厚时被激活[95],的激活抑制的表达,导致线粒体功能障碍,从而导致凋亡性心肌病[96]。
目前,虽然一些研究发现了Mediator的部分亚基与特定的基因启动子共定位,进而在心血管系统与心脏代谢过程中的发挥作用。但关于Mediator在心脏特异性发育和功能中的具体机制尚未见报道。
2.3 Mediator与人类其他疾病
多细胞生物中细胞命运的决定和维持在很大程度上依赖于对Pol II转录活性的时空精确调控,以此影响细胞命运决定信号。因此,扰乱生理转录控制的遗传或环境因素可以改变细胞命运决定,导致各种病理状况,包括发育缺陷和癌症[10]。鉴于Mediator在基因表达中的关键作用,除心血管疾病外,越来越多的人类其他疾病被证实与特定的Mediator基因突变相关,如行为障碍、神经发育异常、多种形式的癌症等。例如人类多种神经发育疾病与[66]、[97]、[98]、[99]、[100]、[101]、[102]和[103]的突变或改变有关。MED20通过桥接C/EBPβ和Pol II来组织早期脂肪形成复合物,以促进中央脂肪形成因子的转录,在促进脂肪生成、脂肪组织发育和饮食诱导的肥胖中的关键作用[104]。此外,[78,105]突变导致乳腺癌及肝脏肿瘤、[4]通过调节TGF-β受体信号来控制对多种抗癌药物的反应、[106]在甲状腺乳头状癌中表达下调、[107]在膀胱癌组织中的表达上调、[108]缺陷的自然杀伤细胞表现出抗肿瘤活性受损、[109]表达升高预示乳腺癌女性预后不佳、[110]在胰腺癌发展过程中具有致癌特性、的治疗干预有益于结肠癌的临床治疗[111]。
3 Mediator的潜在治疗作用
在临床上转录激活因子的异常激活是导致很多疾病发生发展的重要原因,编码Mediator亚基的基因表达水平变化与许多人类疾病有关[23,112],因此,Mediator亚基可能被用作疾病治疗靶点。由于Mediator的活性受含有CDK8、CycC、MED12和MED13的四亚单位激酶模块的调节[2,113],目前与Mediator相关的大部分疾病治疗研究与激酶模块相关[114]。
3.1 CDK8亚基与癌症治疗
Mediator的CDK8亚基位于激酶模块,也代表了Mediator的唯一特征酶活性,显示出与癌症的强烈相关性,并且突变也被证实是重要的致癌基因[111,112]。目前大部分关于Mediator的治疗作用的研究集中在通过对癌症的治疗。
CDK8亚基抑制剂:癌症治疗可以通过抑制Mediator中的完成。FDA批准的索拉非尼(Sorafenib)是一种比较成熟的ATP竞争性的II型抑制剂。高亲和力结合CycC-CDK8,延长了停留时间是体外化合物优化和体内药效提高的关键成功因素[115]。除此之外,如选择性ATP结合位点抑制剂Senexin A[116]、奶蓟草的活性成分水飞蓟素、汉黄芩、麦芽酮、TX522和TX527等化合物正在进行开发或测试[117~120]。
干扰RNA (RNA interference, RNAi)疗法:RNAi也被证实为癌症的新治疗方法提供了方案[121]。已有研究确定了一些对具有特异性的内源性microRNAs,如和可靶向降解CDK8,与依赖的肿瘤发生有关[122,123]。因此,开发基于RNAi的针对异常活性的治疗药物可能具有临床价值。
Mediator亚基间的连接阻断:Mediator中激酶模块各个亚基相互作用发挥功能,因此依赖于活性的亚基为抑制提供了替代靶点[114]。例如能够特异性破坏依赖于CDK8活性的CycC-CDK8和MED12-CycC间的相互作用的化合物能提供较强的特异性抑制作用[124~126]。
3.2 Mediator与超级增强子
增强子结构域由主要TF占据,并与编码细胞特性关键调节因子的基因相关,包括许多致癌基因在内的细胞身份关键决定基因的表达均受超级增强子的调控。超级增强子是紧密排列的增强子的集合,可以共同促进相邻基因的高水平转录。有研究表明,在多发性骨髓瘤细胞中有3%的增强子非常大,并且被大量的BRD4和Mediator占据。这些超级增强子通常比普通的增强子大一个数量级,并且含有的与增强子(H3K27Ac)相关的BRD4、Mediator和组蛋白标记也比普通的增强子多一个数量级[127]。另外,在小细胞肺癌和多形性胶质母细胞瘤中也同样发现了具有相似特征的超级增强子,它们跨越大的基因组区域并包含大量的Mediator和BRD4[127]。
事实上,超级增强子并不局限于肿瘤细胞,并且已经在其他几种细胞类型中被鉴定出来,它们同样与关键细胞识别基因相关[128]。肿瘤细胞依赖于致癌基因的高水平表达[129~131],所以优先破坏超增强子功能可能是选择性抑制许多肿瘤细胞的致癌驱动因素的方法。因此,有研究人员提出,通过破坏超级增强子来选择性的抑制致病基因表达可能作为开发疾病治疗药物的一种策略[128,132]。
3.3 Mediator与心血管疾病治疗可行性
在Mediator与心血管疾病治疗方面,有研究证实甲状腺功能减退小鼠的心脏中过表达可以在一定程度上缓解心脏损伤[61]。除过表达导致的小鼠进行性扩张型心肌病、心力衰竭外,目前已报道的大多数与Mediator相关的心脏疾病均与Mediator亚基缺失或突变相关,并不适应于上述降低Mediator亚基表达或抑制其活性的治疗方法。针对这种基因缺失或突变的心脏疾病,已有研究者提出CRISPR/Cas9介导的替换或矫正突变基因的基因治疗方案,利用同源重组(homologous recombination, HDR)修复突变基因[133,134],研究者使用腺相关病毒等工具将CRISPR/Cas9系统和功能性DNA模板(例如基因)递送到组织细胞中。当CRISPR/Cas9切割特定的基因组序列时,细胞会以导入的DNA序列为模板修复断裂基因组DNA,同时将功能性DNA模板插入靶向基因组,用于替换或矫正突变基因。然而基于HDR的基因编辑效率非常低,尤其是HDR很可能只发生在分裂的细胞中[135]。因此,HDR的基因治疗方法用于治疗Mediator亚基缺失或突变相关的心脏疾病具有一定的局限性。鉴于Mediator是一个多亚基共同发挥作用的复合物,且与其靶基因的转录及其下游信号通路高度相关,有关Mediator亚基间的相互连接或协调作用也可能成为心血管疾病治疗的重要研究方向。
4 结语与展望
本文主要阐述了Mediator及其部分亚基在基因转录调控中的关键作用,以及Mediator作为介导TF和基础转录机器之间功能联系的桥梁对心血管系统发育和疾病的影响。鉴于Mediator部分亚基的突变或缺失会影响Mediator核心的稳定性[68],且与Mediator亚基相关的基因表达水平变化与许多人类疾病密切相关[23,112],Mediator部分亚基可作为一些疾病的治疗靶点。但由于Mediator不具有组织特异性,且其调控基因转录表达的作用方式与传统的药物靶标不同[136],导致化学药物等直接干预Mediator的手段相对有限[137]。因此,进一步关于Mediator在转录调控相关的心血管发育和疾病中的研究还应致力于探索针对Mediator参与转录调控具体机制的研究,包括TF与Mediator不同亚基的结合和下游基因启动子、增强子上表观遗传学修饰的改变等。
[1] Jeronimo C, Robert F. The mediator complex: at the nexus of RNA polymerase II transcription., 2017, 27(10): 765–783.
[2] Allen BL, Taatjes DJ. The mediator complex: a central integrator of transcription.,2015, 16(3): 155–166.
[3] Hsieh THS, Weiner A, Lajoie B, Dekker J, Friedman N, Rando OJ. Mapping nucleosome resolution chromosome folding in yeast by Micro-C.,2015, 162(1): 108– 119.
[4] Huang SD, Hölzel M, Knijnenburg T, Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C, Prahallad A, Groenendijk FH, Mittempergher L, Nijkamp W, Neefjes J, Salazar R, Ten Dijke P, Uramoto H, Tanaka F, Beijersbergen RL, Wessels LFA, Bernards R. Med12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling.,2012, 151(5): 937–950.
[5] Pelish HE, Liau BB, Nitulescu, II, Tangpeerachaikul A, Poss ZC, Da Silva DH, Caruso BT, Arefolov A, Fadeyi O, Christie AL, Du K, Banka D, Schneider EV, Jestel A, Zou G, Si C, Ebmeier CC, Bronson RT, Krivtsov AV, Myers AG, Kohl NE, Kung AL, Armstrong SA, Lemieux ME, Taatjes DJ, Shair MD. Mediator kinase inhibition further activates super-enhancer-associated genes in AML.,2015, 526(7572): 273–276.
[6] D'Urso A, Takahashi YH, Xiong B, Marone J, Coukos R, Randise-Hinchliff C, Wang JP, Shilatifard A, Brickner JH. Set1/COMPASS and mediator are repurposed to promote epigenetic transcriptional memory.,2016, 5: e16691.
[7] Tantale K, Mueller F, Kozulic-Pirher A, Lesne A, Victor JM, Robert MC, Capozi S, Chouaib R, Bäcker V, Mateos-Langerak J, Darzacq X, Zimmer C, Basyuk E, Bertrand E. A single-molecule view of transcription reveals convoys of RNA polymerases and multi-scale bursting.,2016, 7: 12248.
[8] Eyboulet F, Cibot C, Eychenne T, Neil H, Alibert O, Werner M, Soutourina J. Mediator links transcription and DNA repair by facilitating Rad2/XPG recruitment.,2013, 27(23): 2549–2562.
[9] Thomas-Claudepierre AS, Robert I, Rocha PP, Raviram R, Schiavo E, Heyer V, Bonneau R, Luo VM, Reddy JK, Borggrefe T, Skok JA, Reina-San-Martin B. Mediator facilitates transcriptional activation and dynamic long-range contacts at the IgH locus during class switch recombination.,2016, 213(3): 303–312.
[10] Krebs P, Fan WW, Chen YH, Tobita K, Downes MR, Wood MR, Sun L, Li XH, Xia Y, Ding N, Spaeth JM, Moresco EMY, Boyer TG, Lo CWY, Yen J, Evans RM, Beutler B. Lethal mitochondrial cardiomyopathy in a hypomorphic Med30 mouse mutant is ameliorated by ketogenic diet.,2011, 108(49): 19678–19682.
[11] Malik S, Roeder RG. Transcriptional regulation through mediator-like coactivators in yeast and metazoan cells.,2000, 25(6): 277–283.
[12] Lee TI, Young RA. Transcription of eukaryotic protein- coding genes.,2000, 34: 77–137.
[13] Myers LC, Kornberg RD. Mediator of transcriptional regulation.,2000, 69: 729–749.
[14] Kornberg RD. Mediator and the mechanism of transcriptional activation.,2005, 30(5): 235–239.
[15] Cevher MA, Shi Y, Li D, Chait BT, Malik S, Roeder RG. Reconstitution of active human core mediator complex reveals a critical role of the MED14 subunit.,2014, 21(12): 1028–1034.
[16] Plaschka C, Larivière L, Wenzeck L, Seizl M, Hemann M, Tegunov D, Petrotchenko EV, Borchers CH, Baumeister W, Herzog F, Villa E, Cramer P. Architecture of the RNA polymerase II-mediator core initiation complex.,2015, 518(7539): 376–380.
[17] Lariviere L, Plaschka C, Seizl M, Wenzeck L, Kurth F, Cramer P. Structure of the mediator head module.,2012, 492(7429): 448–451.
[18] Tsai KL, Tomomori-Sato C, Sato S, Conaway RC, Conaway JW, Asturias FJ. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex.,2014, 157(6): 1430–1444.
[19] Sato S, Tomomori-Sato C, Tsai KL, Yu XD, Sardiu M, Saraf A, Washburn MP, Florens L, Asturias FJ, Conaway RC, Conaway JW. Role for the MED21-MED7 hinge in assembly of the mediator-RNA polymerase II holoenzyme.,2016, 291(52): 26886–26898.
[20] Zhao HY, Young N, Kalchschmidt J, Lieberman J, El Khattabi L, Casellas R, Asturias FJ. Structure of mammalian mediator complex reveals tail module architecture and interaction with a conserved core.,2021, 12(1): 1355.
[21] Malik S, Roeder RG. The metazoan mediator co- activator complex as an integrative hub for transcriptional regulation.,2010, 11(11): 761– 772.
[22] Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control.,2000, 14(20): 2551–2569.
[23] Soutourina J. Transcription regulation by the mediator complex.,2018, 19(4): 262–274.
[24] Brzovic PS, Heikaus CC, Kisselev L, Vernon R, Herbig E, Pacheco D, Warfield L, Littlefield P, Baker D, Klevit RE, Hahn S. The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex.,2011, 44(6): 942–953.
[25] Vojnic E, Mourão A, Seizl M, Simon B, Wenzeck L, Larivière L, Baumli S, Baumgart K, Meisterernst M, Sattler M, Cramer P. Structure and VP16 binding of the mediator Med25 activator interaction domain.,2011, 18(4): 404–409.
[26] Soutourina J, Wydau S, Ambroise Y, Boschiero C, Werner M. Direct interaction of RNA polymerase II and mediator required for transcription.,2011, 331(6023): 1451–1454.
[27] Bernecky C, Grob P, Ebmeier CC, Nogales E, Taatjes DJ. Molecular architecture of the human mediator-RNA polymerase II-TFIIF assembly.,2011, 9(3): e1000603.
[28] Kremer SB, Kim S, Jeon JO, Moustafa YW, Chen A, Zhao J, Gross DS. Role of mediator in regulating pol II elongation and nucleosome displacement in.,2012, 191(1): 95–106.
[29] Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CAS, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, Florens L, Seidel CW, Lin CQ, Smith ER, Shilatifard A, Conaway RC, Conaway JW. Human mediator subunit MED26 functions as a docking site for transcription elongation factors.,2011, 146(1): 92–104.
[30] Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network.,2010, 17(2): 194–201.
[31] Mukundan B, Ansari A. Novel role for mediator complex subunit Srb5/Med18 in termination of transcription.,2011, 286(43): 37053–37057.
[32] Huang Y, Li WC, Yao X, Lin QJ, Yin JW, Liang Y, Heiner M, Tian B, Hui JY, Wang G. Mediator complex regulates alternative mRNA processing via the MED23 subunit.,2012, 45(4): 459–469.
[33] Khorosjutina O, Wanrooij PH, Walfridsson J, Szilagyi Z, Zhu XF, Baraznenok V, Ekwall K, Gustafsson CM. A chromatin-remodeling protein is a component of fission yeast mediator.,2010, 285(39): 29729– 29737.
[34] Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, Taatjes DJ, Dekker J, Young RA. Mediator and cohesin connect gene expression and chromatin architecture.,2010, 467(7314): 430–435.
[35] Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory.,2019, 15(6): 327–345.
[36] Harper TM, Taatjes DJ. The complex structure and function of mediator.,2018, 293(36): 13778–13785.
[37] Ito M, Okano HJ, Darnell RB, Roeder RG. The TRAP100 component of the TRAP/mediator complex is essential in broad transcriptional events and development.,2002, 21(13): 3464–3475.
[38] Ito M, Yuan CX, Okano HJ, Darnell RB, Roeder RG. Involvement of the TRAP220 component of the TRAP/ SMCC coactivator complex in embryonic development and thyroid hormone action.,2000, 5(4): 683–693.
[39] Stevens JL, Cantin GT, Wang G, Shevchenko A, Shevchenko A, Berk AJ. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit.,2002, 296(5568): 755–758.
[40] Tudor M, Murray PJ, Onufryk C, Jaenisch R, Young RA. Ubiquitous expression and embryonic requirement for RNA polymerase II coactivator subunit Srb7 in mice.,1999, 13(18): 2365–2368.
[41] Westerling T, Kuuluvainen E, Makela TP. Cdk8 is essential for preimplantation mouse development.,2007, 27(17): 6177–6182.
[42] Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome.,1998, 95(5): 717–728.
[43] Eychenne T, Novikova E, Barrault MB, Alibert O, Boschiero C, Peixeiro N, Cornu D, Redeker V, Kuras L, Nicolas P, Werner M, Soutourina J. Functional interplay between mediator and TFIIB in preinitiation complex assembly in relation to promoter architecture.,2016, 30(18): 2119–2132.
[44] Eyboulet F, Wydau-Dematteis S, Eychenne T, Alibert O, Neil H, Boschiero C, Nevers MC, Volland H, Cornu D, Redeker V, Werner M, Soutourina J. Mediator independently orchestrates multiple steps of preinitiation complex assembly.,2015, 43(19): 9214–9231.
[45] Thompson CM, Young RA. General requirement for RNA polymerase II holoenzymes.,1995, 92(10): 4587–4590.
[46] Schneider M, Hellerschmied D, Schubert T, Amlacher S, Vinayachandran V, Reja R, Pugh BF, Clausen T, Köhler A. The nuclear pore-associated TREX-2 complex employs mediator to regulate gene expression.,2015, 162(5): 1016–1028.
[47] Schubert T, Kohler A. Mediator and TREX-2: emerging links between transcription initiation and mRNA export.,2016, 7(2): 126–131.
[48] Ibarra A, Hetzer MW. Nuclear pore proteins and the control of genome functions.,2015, 29(4): 337–349.
[49] Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin JC, Hurt EC, Nehrbass U. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope.,2006, 441(7094): 770–773.
[50] Gallardo M, Luna R, Erdjument-Bromage H, Tempst P, Aguilera A. Nab2p and the Thp1p-Sac3p complex functionally interact at the interface between transcription and mRNA metabolism.,2003, 278(26): 24225–24232.
[51] Jeronimo C, Robert F. Kin28 regulates the transient association of mediator with core promoters.,2014, 21(5): 449–455.
[52] Wong KH, Jin Y, Struhl K. TFIIH phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape.,2014, 54(4): 601–612.
[53] Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes.,2000, 407(6800): 102–106.
[54] Venters BJ, Wachi S, Mavrich TN, Andersen BE, Jena P, Sinnamon AJ, Jain P, Rolleri NS, Jiang CZ, Hemeryck- Walsh C, Pugh BF. A comprehensive genomic binding map of gene and chromatin regulatory proteins in saccharomyces.,2011, 41(4): 480–492.
[55] Köhler A, Schneider M, Cabal GG, Nehrbass U, Hurt E. Yeast Ataxin-7 links histone deubiquitination with gene gating and mRNA export.,2008, 10(6): 707–715.
[56] Zhou MG, Wang HD, Zhu J, Chen WQ, Wang LH, Liu SW, Li YC, Wang LJ, Liu YN, Yin P, Liu JM, Yu SC, Tan F, Barber RM, Coates MM, Dicker D, Fraser M, González-Medina D, Hamavid H, Hao YT, Hu GQ, Jiang GH, Kan HD, Lopez AD, Phillips MR, She J, Vos T, Wan X, Xu GL, Yan LL, Yu CH, Zhao Y, Zheng YF, Zou XN, Naghavi M, Wang Y, Murray CJL, Yang GH, Liang XF. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the global burden of disease study 2013.,2016, 387(10015): 251–272.
[57] The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on cardiovascular health and diseases burden in china: an updated summary of 2020.,2021, 36: 521–545.
中国心血管健康与疾病报告编写组. 中国心血管健康与疾病报告2020概要. 中国循环,2021, 36: 521– 545.
[58] Bouma BJ, Mulder BJ. Changing landscape of congenital heart disease.,2017, 120(6): 908–922.
[59] Landles C, Chalk S, Steel JH, Rosewell I, Spencer-Dene B, Lalani EN, Parker MG. The thyroid hormone receptor-associated protein TRAP220 is required at distinct embryonic stages in placental, cardiac, and hepatic development.,2003, 17(12): 2418–2435.
[60] Rocha PP, Scholze M, Bleiss W, Schrewe H. Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling.,2010, 137(16): 2723–2731.
[61] Minerath RA, Dewey CM, Hall DD, Grueter CE. Regulation of cardiac transcription by thyroid hormone and Med13.,2019, 129: 27–38.
[62] Asadollahi R, Oneda B, Sheth F, Azzarello-Burri S, Baldinger R, Joset P, Latal B, Knirsch W, Desai S, Baumer A, Houge G, Andrieux J, Rauch A. Dosage changes of MED13L further delineate its role in congenital heart defects and intellectual disability.,2013, 21(10): 1100–1104.
[63] Chen CP, Chen YY, Chern SR, Wu PS, Su JW, Chen YT, Chen LF, Wang W. Prenatal diagnosis and molecular cytogenetic characterization ofpartial trisomy 12q (12q24.21→qter) and partial monosomy 6q (6q27→qter) associated with coarctation of the aorta, ventriculomegaly and thickened nuchal fold.,2013, 516(1): 138–142.
[64] Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, Digeorge syndrome: the chromosome 22q11.2 deletion syndromes.,2007, 370(9596): 1443–1452.
[65] Shprintzen RJ. Velo-cardio-facial syndrome: 30 years of study.,2008, 14(1): 3–10.
[66] Yin JW, Liang Y, Park JY, Chen DR, Yao X, Xiao Q, Liu Z, Jiang B, Fu Y, Bao MH, Huang Y, Liu YT, Yan J, Zhu MS, Yang ZZ, Gao PJ, Tian B, Li DS, Wang G. Mediator MED23 plays opposing roles in directing smooth muscle cell and adipocyte differentiation.,2012, 26(19): 2192–2205.
[67] Basel-Vanagaite L, Smirin-Yosef P, Essakow JL, Tzur S, Lagovsky I, Maya I, Pasmanik-Chor M, Yeheskel A, Konen O, Orenstein N, Weisz Hubshman M, Drasinover V, Magal N, Peretz Amit G, Zalzstein Y, Zeharia A, Shohat M, Straussberg R, Monté D, Salmon-Divon M, Behar DM. Homozygous MED25 mutation implicated in eye-intellectual disability syndrome.,2015, 134(6): 577–587.
[68] Fosslien E. Review: mitochondrial medicine--cardiomyopathy caused by defective oxidative phosphorylation.,2003, 33(4): 371–395.
[69] Hall DD, Ponce JM, Chen BY, Spitler KM, Alexia A, Oudit GY, Song LS, Grueter CE. Ectopic expression of CDK8 induces eccentric hypertrophy and heart failure.,2017, 2(15): e92476.
[70] Baskin KK, Makarewich CA, DeLeon SM, Ye W, Chen BB, Beetz N, Schrewe H, Bassel-Duby R, Olson EN. MED12 regulates a transcriptional network of calcium-handling genes in the heart.,2017, 2(14).
[71] Segert J, Schneider I, Berger IM, Rottbauer W, Just S. Mediator complex subunit Med12 regulates cardiac jelly development and AV valve formation in zebrafish.,2018, 138: 20–31.
[72] Muncke N, Jung C, Rüdiger H, Ulmer H, Roeth R, Hubert A, Goldmuntz E, Driscoll D, Goodship J, Schön K, Rappold G. Missense mutations and gene interruption in PROSIT240, a novel TRAP240-like gene, in patients with congenital heart defect (transposition of the great arteries).,2003, 108(23): 2843– 2850.
[73] Yang FJ, Vought BW, Satterlee JS, Walker AK, Jim Sun ZY, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang SS, Macol C, Iyer L, Tjian R, van den Heuvel S, Hart AC, Wagner G, Näär AM. An ARC/mediator subunit required for SREBP control of cholesterol and lipid homeostasis.2006; 442(7103): 700–704.
[74] Kato Y, Habas R, Katsuyama Y, Näär AM, He X. A component of the ARC/mediator complex required for TGF beta/Nodal signalling.,2002, 418(6898): 641–646.
[75] Spaeth JM, Kim NH, Boyer TG. Mediator and human disease.,2011, 22(7): 776–787.
[76] Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha.,2003, 12(5): 1137–1149.
[77] Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor- associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion.,1998, 95(14): 7939– 7944.
[78] Matsumoto K, Yu ST, Jia YZ, Ahmed MR, Viswakarma N, Sarkar J, Kashireddy PV, Rao MS, Karpus W, Gonzalez FJ, Reddy JK. Critical role for transcription coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein/TRAP220 in liver regeneration and PPARalpha ligand-induced liver tumor development.,2007, 282(23): 17053–17060.
[79] Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi XX, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13.,2012, 149(3): 671–683.
[80] Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α- dependent myokine that drives brown-fat-like development of white fat and thermogenesis.,2012, 481(7382): 463–468.
[81] Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang ZY, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton.,2007, 130(3): 456–469.
[82] Carvajal K, Moreno-Sánchez R. Heart metabolic disturbances in cardiovascular diseases.,2003, 34(2): 89–99.
[83] Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics.,2004, 95(6): 568–578.
[84] Rider OJ, Francis JM, Ali MK, Holloway C, Pegg T, Robson MD, Tyler D, Byrne J, Clarke K, Neubauer S. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity.,2012, 125(12): 1511–1519.
[85] Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. Elegans.,2006, 20(9): 1137– 1149.
[86] Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction.,2014, 76: 107–127.
[87] Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics.,2013, 123(1): 46–52.
[88] Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment.,2015, 21(2): 183–194.
[89] Wang WY, Guan HX, Fang W, Zhang K, Gerdes AM, Iervasi G, Tang YD. Free triiodothyronine level correlates with myocardial injury and prognosis in idiopathic dilated cardiomyopathy: Evidence from cardiac MRI and SPECT/PET imaging.,2016, 6: 39811.
[90] Mitchell JE, Hellkamp AS, Mark DB, Anderson J, Johnson GW, Poole JE, Lee KL, Bardy GH. Thyroid function in heart failure and impact on mortality.,2013, 1(1): 48–55.
[91] Freedberg AS, Papp JG, Williams EM. The effect of altered thyroid state on atrial intracellular potentials.,1970, 207(2): 357–369.
[92] Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart.,2005, 26(5): 704–728.
[93] Wang W, Huang L, Huang Y, Yin JW, Berk AJ, Friedman JM, Wang G. Mediator MED23 links insulin signaling to the adipogenesis transcription cascade.,2009, 16(5): 764–771.
[94] Chen W, Zhang XT, Birsoy K, Roeder RG. A muscle-specific knockout implicates nuclear receptor coactivator MED1 in the regulation of glucose and energy metabolism.,2010, 107(22): 10196–10201.
[95] Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, Michael LH, DeMayo FJ, Schneider MD. Activation and function of cyclin t-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy.,2002, 8(11): 1310–1317.
[96] Sano M, Wang SC, Shirai M, Scaglia F, Xie M, Sakai S, Tanaka T, Kulkarni PA, Barger PM, Youker KA, Taffet GE, Hamamori Y, Michael LH, Craigen WJ, Schneider MD. Activation of cardiac CDK9 represses PGC-1 and confers a predisposition to heart failure.,2004, 23(17): 3559–3569.
[97] Snijders Blok L, Hiatt SM, Bowling KM, Prokop JW, Engel KL, Cochran JN, Bebin EM, Bijlsma EK, Ruivenkamp CAL, Terhal P, Simon MEH, Smith R, Hurst JA, study DDD, McLaughlin H, Person R, Crunk A, Wangler MF, Streff H, Symonds JD, Zuberi SM, Elliott KS, Sanders VR, Masunga A, Hopkin RJ, Dubbs HA, Ortiz-Gonzalez XR, Pfundt R, Brunner HG, Fisher SE, Kleefstra T, Cooper GM.mutations in MED13, a component of the mediator complex, are associated with a novel neurodevelopmental disorder.,2018, 137(5): 375–388.
[98] Kaufmann R, Straussberg R, Mandel H, Fattal-Valevski A, Ben-Zeev B, Naamati A, Shaag A, Zenvirt S, Konen O, Mimouni-Bloch A, Dobyns WB, Edvardson S, Pines O, Elpeleg O. Infantile cerebral and cerebellar atrophy is associated with a mutation in the MED17 subunit of the transcription preinitiation mediator complex.,2010, 87(5): 667–670.
[99] Hashimoto S, Boissel S, Zarhrate M, Rio M, Munnich A, Egly JM, Colleaux L. MED23 mutation links intellectual disability to dysregulation of immediate early gene expression.,2011, 333(6046): 1161–1163.
[100] Leal A, Huehne K, Bauer F, Sticht H, Berger P, Suter U, Morera B, Del Valle G, Lupski JR, Ekici A, Pasutto F, Endele S, Barrantes R, Berghoff C, Berghoff M, Neundörfer B, Heuss D, Dorn T, Young P, Santolin L, Uhlmann T, Meisterernst M, Sereda MW, Sereda M, Stassart RM, Meyer zu Horste G, Nave KA, Reis A, Rautenstrauss B. Identification of the variant Ala335Val of MED25 as responsible for CMT2B2: molecular data, functional studies of the SH3 recognition motif and correlation between wild-type MED25 and PMP22 RNA levels in CMT1A animal models.,2009, 10(4): 275–287.
[101] Meng LY, Isohanni P, Shao YR, Graham BH, Hickey SE, Brooks S, Suomalainen A, Joset P, Steindl K, Rauch A, Hackenberg A, High FA, Armstrong-Javors A, Mencacci NE, Gonzàlez-Latapi P, Kamel WA, Al-Hashel JY, Bustos BI, Hernandez AV, Krainc D, Lubbe SJ, Van Esch H, De Luca C, Ballon K, Ravelli C, Burglen L, Qebibo L, Calame DG, Mitani T, Marafi D, Pehlivan D, Saadi NW, Sahin Y, Maroofian R, Efthymiou S, Houlden H, Maqbool S, Rahman F, Gu S, Posey JE, Lupski JR, Hunter JV, Wangler MF, Carroll CJ, Yang YP. MED27 variants cause developmental delay, dystonia, and cerebellar hypoplasia.,2021, 89(4): 828–833.
[102] Mukhopadhyay A, Kramer JM, Merkx G, Lugtenberg D, Smeets DF, Oortveld MA, Blokland EAW, Agrawal J, Schenck A, van Bokhoven H, Huys E, Schoenmakers EF, van Kessel AG, van Nouhuys CE, Cremers FPM. CDK19 is disrupted in a female patient with bilateral congenital retinal folds, microcephaly and mild mental retardation.,2010, 128(3): 281–291.
[103] Ueberham U, Hessel A, Arendt T. Cyclin C expression is involved in the pathogenesis of Alzheimer's disease.,2003, 24(3): 427–435.
[104] Tang WS, Weng L, Wang X, Liu CQ, Hu GS, Yin ST, Tao Y, Hong NN, Guo HL, Liu W, Wang HR, Zhao TJ. The mediator subunit MED20 organizes the early adipogenic complex to promote development of adipose tissues and diet-induced obesity.,2021, 36(1): 109314.
[105] Zhu Y, Qi C, Jain S, Le Beau MM, Espinosa R, 3rd, Atkins GB, Lazar MA, Yeldandi AV, Rao MS, Reddy JK. Amplification and overexpression of peroxisome proliferator-activated receptor binding protein (PBP/ PPARBP) gene in breast cancer.,1999, 96(19): 10848–10853.
[106] Gao HW, Bai PR, Xiao L, Shen MJ, Yu QX, Lei YY, Huang WT, Lin X, Zheng XY, Wei T, Jiang Y, Ye F, Bu H. Mediator complex subunit 16 is down-regulated in papillary thyroid cancer, leading to increased transforming growth factor-β signaling and radioiodine resistance.,2020, 295(31): 10726–10740.
[107] Zhang H, Jiang HW, Wang W, Gong J, Zhang LM, Chen ZQ, Ding Q. Expression of Med19 in bladder cancer tissues and its role on bladder cancer cell growth.,2012, 30(6): 920–927.
[108] Xu Y, Sun Y, Shen H, Dai YL, Liu HF, Li RH, Zhang HD, Wu LG, Zhu XY, Liu XL. Regulation of the terminal maturation of iNKT cells by mediator complex subunit 23.,2018, 9(1): 3875.
[109] Yoon NK, Maresh EL, Elshimali Y, Li A, Horvath S, Seligson DB, Chia D, Goodglick L. Elevated MED28 expression predicts poor outcome in women with breast cancer.,2010, 10: 335.
[110] Kuuselo R, Savinainen K, Sandström S, Autio R, Kallioniemi A. Med29, a component of the mediator complex, possesses both oncogenic and tumor suppressive characteristics in pancreatic cancer.,2011, 129(11): 2553–2565.
[111] Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, Chheda MG, Tamayo P, Finn S, Shrestha Y, Boehm JS, Jain S, Bojarski E, Mermel C, Barretina J, Chan JA, Baselga J, Tabernero J, Root DE, Fuchs CS, Loda M, Shivdasani RA, Meyerson M, Hahn WC. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity.,2008, 455(7212): 547–551.
[112] Schiano C, Casamassimi A, Rienzo M, de Nigris F, Sommese L, Napoli C. Involvement of mediator complex in malignancy.,2014, 1845(1): 66–83.
[113] Poss ZC, Ebmeier CC, Taatjes DJ. The mediator complex and transcription regulation.,2013, 48(6): 575–608.
[114] Clark AD, Oldenbroek M, Boyer TG. Mediator kinase module and human tumorigenesis.,2015, 50(5): 393–426.
[115] Schneider EV, Bottcher J, Huber R, Maskos K, Neumann L. Structure-kinetic relationship study of CDK8/Cycc specific compounds.,2013, 110(20): 8081–8086.
[116] Porter DC, Farmaki E, Altilia S, Schools GP, West DK, Chen MQ, Chang BD, Puzyrev AT, Lim CU, Rokow- Kittell R, Friedhoff LT, Papavassiliou AG, Kalurupalle S, Hurteau G, Shi J, Baran PS, Gyorffy B, Wentland MP, Broude EV, Kiaris H, Roninson IB. Cyclin-dependent kinase 8 mediates chemotherapy-induced tumor-promoting paracrine activities.,2012, 109(34): 13799–13804.
[117] Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin- dependent signaling.,2010, 12(5): 415–424.
[118] Guerzoni C, Amatori S, Giorgi L, Manara MC, Landuzzi L, Lollini PL, Tassoni A, Balducci M, Manfrini M, Pratelli L, Serra M, Picci P, Magnani M, Fusi V, Fanelli M, Scotlandi K. An aza-macrocycle containing maltolic side-arms (maltonis) as potential drug against human pediatric sarcomas.,2014, 14: 137.
[119] He LC, Lu N, Dai QS, Zhao Y, Zhao L, Wang H, Li ZY, You QD, Guo QL. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells.,2013, 312: 36–47.
[120] Verlinden L, Verstuyf A, Van Camp M, Marcelis S, Sabbe K, Zhao XY, De Clercq P, Vandewalle M, BouillonR. Two novel 14-Epi-analogues of 1,25-dihydroxyvitamin D3 inhibit the growth of human breast cancer cells in vitro and.,2000, 60(10): 2673– 2679.
[121] Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects.,2012, 19(1): 60–71.
[122] Li XY, Luo QF, Wei CK, Li DF, Li J, Fang L. MiRNA-107 inhibits proliferation and migration by targeting CDK8 in breast cancer.,2014, 7(1): 32–40.
[123] Li J, Li XY, Kong XJ, Luo QF, Zhang JF, Fang L. MiRNA-26b inhibits cellular proliferation by targeting CDK8 in breast cancer.,2014, 7(3): 558–565.
[124] Rajender PS, Vasavi M, Vuruputuri U. Identification of novel selective antagonists for cyclin C by homology modeling and virtual screening.,2011, 48(2): 292–300.
[125] Hoeppner S, Baumli S, Cramer P. Structure of the mediator subunit cyclin C and its implications for CDK8 function.,2005, 350(5): 833–842.
[126] Schneider EV, Böttcher J, Blaesse M, Neumann L, Huber R, Maskos K. The structure of CDK8/Cycc implicates specificity in the CDK/cyclin family and reveals interaction with a deep pocket binder.,2011, 412(2): 251–266.
[127] Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers.,2013, 153(2): 320–334.
[128] Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes.,2013, 153(2): 307–319.
[129] Cheung HW, Cowley GS, Weir BA, Boehm JS, Rusin S, Scott JA, East A, Ali LD, Lizotte PH, Wong TC, Jiang GZ, Hsiao J, Mermel CH, Getz G, Barretina J, Gopal S, Tamayo P, Gould J, Tsherniak A, Stransky N, Luo B, Ren Y, Drapkin R, Bhatia SN, Mesirov JP, Garraway LA, Meyerson M, Lander ES, Root DE, Hahn WC. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer.,2011, 108(30): 12372–12377.
[130] Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Sustained loss of a neoplastic phenotype by brief inactivation of MYC.,2002, 297(5578): 102– 104.
[131] Weinstein IB. Cancer. Addiction to oncogenes–the Achilles heal of cancer.,2002, 297(5578): 63–64.
[132] Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease.,2013, 155(4): 934–947.
[133] He XJ, Tan CL, Wang F, Wang YF, Zhou R, Cui DX, You WX, Zhao H, Ren JW, Feng B. Knock-in of large reporter genes in human cells via CRISPR/Cas9- induced homology-dependent and independent DNA repair.,2016, 44(9): e85.
[134] Platt RJ, Chen SD, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, Graham DB, Jhunjhunwala S, Heidenreich M, Xavier RJ, Langer R, Anderson DG, Hacohen N, Regev A, Feng GP, Sharp PA, Zhang F. CRISPR-Cas knockin mice for genome editing and cancer modeling.,2014, 159(2): 440–455.
[135] Devkota S. The road less traveled: strategies to enhance the frequency of homology-directed repair (HDR) for increased efficiency of CRISPR/Cas-mediated transgenesis.,2018, 51(9): 437–443.
[136] Vogelstein B, Papadopoulos N, Velculescu VE, Zhou SB, Diaz Jr LA, Kinzler KW. Cancer genome landscapes.,2013, 339(6127): 1546–1558.
[137] Moustaqil M, Gambin Y, Sierecki E. Biophysical techniques for target validation and drug discovery in transcription-targeted therapy.,2020, 21(7): 2301.
Transcriptional regulation of transcriptional Mediator complexes in cardiovascular development and disease
Yuan Zhang1, Yuting Zhao1, Lenan Zhuang1,2,3, Jin He3
During the development of the mammalian cardiovascular system, the formation of a mature and fully functional cardiovascular system needs the fine coordination of the morphogenesis of various molecules, cells, tissues, and organs. Abnormalities in these processes usually lead to serious congenital heart defects. The determination and maintenance of cell fate in multicellular organisms depend to a large extent on the precise timing and control of RNA polymerase II (Pol II) transcription, and the transcription Mediator complex plays an irreplaceable role in the Pol II transcription process. Mediator is an evolutionarily conserved multi-subunit protein complex, including four parts: head, middle, tail, and kinase. It is a functional bridge between transcription factors and basic transcription machines. In recent years, due to the key role of Mediator in the transcriptional regulation of gene expression, many of human heart diseases have been confirmed to be related to specific Mediator gene mutations, such as heart valve defects, translocation of the great arteries, DiGeorge syndrome and some cardiovascular diseases related to energy homeostasis. In this review, we summarize the role of Mediator in cardiovascular development and disease, focusing on the role of Mediator in the development of cardiovascular disease, and provides a broad idea for the research on Mediator-related cardiovascular system development and diseases.
Mediator complex; transcriptional regulation; cardiovascular development; cardiovascular disease
2022-01-18;
2022-03-03;
2022-03-28
国家重点研发计划(编号:2019YFE0117400, 2021YFA0805902),国家自然科学基金项目(编号:81941003)和中央高校基本科研业务费专项基金(编号:2020XZZX002-20)资助[Supported by the National Key Research and Development Program (Nos. 2019YFE0117400, 2021YFA0805902), the National Natural Science Foundation of China (No. 81941003), and the Fundamental Research Funds for the Central Universities (No. 2020XZZX002-20)]
张元,博士研究生,研究方向:转录调控。E-mail: zhangyuan2020@zju.edu.cn
庄乐南,博士,研究员,研究方向:表观遗传。E-mail: zhuangln@zju.edu.cn
贺津,博士,副教授,研究方向:家畜遗传育种。E-mail: hejin@zju.edu.cn
10.16288/j.yczz.21-411
(责任编委: 刘峰)