镉胁迫对不同植烟土壤硝化速率及N2O排放的影响
2022-03-21许琳刘跃东高加明李方明彭玉龙刘明宏林伟徐茜孙朝辉张继光
许琳 刘跃东 高加明 李方明 彭玉龙 刘明宏 林伟 徐茜 孙朝辉 张继光




摘 要:为明确镉(Cd)胁迫对不同植烟土壤的硝化速率及氧化亚氮(N2O)排放的影响,采用外源镉添加试验,设置两个Cd浓度梯度(10和100 mg/kg),以探究Cd胁迫下中性(中性土1和2)和酸性(酸性土1和2)植烟土壤的净硝化速率以及N2O排放特征。结果表明:1)随培养时间增加,植烟土壤铵态氮含量逐渐减少,而硝态氮含量逐渐增加,且低Cd胁迫和高Cd胁迫均未显著改变这一变化趋势;2)植烟土壤净硝化速率在培养前期(1~7 d)发生较大波动变化,Cd胁迫明显增加了酸性土1在第1天的净硝化速率;3)整体上各处理酸性土的N2O排放速率和累积排放量高于中性土,高Cd胁迫增加了酸性土1的N2O初始排放速率,培养结束后酸性土1的N2O累积排放量达355.42 μg/kg;4)各植烟土壤N2O排放速率和累积排放量与土壤pH和全氮含量极显著负相关,而与有机质和铵态氮含量极显著正相关。本研究结果表明植烟土壤的N2O排放受土壤酸碱性和氮底物的影响,酸性土的N2O累积排放量高于中性土,且高Cd胁迫增加其N2O排放量,后续可通过土壤酸化改良以达到N2O减排目的。
关键词:植烟土壤;氧化亚氮;镉胁迫;净硝化速率
Abstract: In order to determine the effect of cadmium stress on nitrification rate and N2O emission traits of different tobacco planting soils, an incubation experiment of acid and neutral soils under two concentrations (10 and 100 mg/kg) of Cd addition were conducted. The results showed that, 1) The concentration of ammonium in acid and neutral tobacco soils decreased gradually with the extension of incubation time, while the concentration of nitrate increased throughout the whole incubation periods, and this tendency was not affected by low and high concentrations of Cd stress; 2) Soil net nitrification rate fluctuated strongly during the 1-7 d of incubation and Cd stress could stimulate net nitrification rate of acid soil type 1 on the 1st day; 3) The maximum of N2O emission rate and cumulative emission of acid soils were higher than that of neutral soils in general, and the high Cd stress could stimulate the initial N2O emission rate of acid soil 1, and the cumulative N2O emission of acid soil 1 was 355.42 μg/kg at the end of the incubation; 4) Soil pH and total nitrogen had significantly negative correlations with soil N2O emission rate and cumulative emission, but soil organic matter and ammonium had significantly positive correlations with soil N2O emission rate and the cumulative emission. These results indicated that N2O emission of tobacco planting soils was affected by soil pH and N substrate. The N2O cumulative emission of acid soils was much higher than neutral soils and they could be stimulated by the high Cd stress. So, the reduction of N2O emission could be achieved by improving soil acidification in the future.
Keywords: tobacco planting soil; nitrous oxide; cadmium stress; net nitrification rate
溫室气体排放增加是导致全球气温上升的主要原因。在所有温室气体中,氧化亚氮(N2O)的增温效应和增温潜势最大[1]。农田土壤是农业N2O的最大排放源[2],农田土壤中的硝化和反硝化反应所释放的N2O约占生物圈释放到大气中N2O总量的70%。烟草是我国重要的经济作物之一[3],近年来,不合理施肥与土壤管理及产地环境问题制约着烟草农业的可持续发展[4],如植烟土壤长期大量施用氮肥导致土壤酸化、土壤板结和营养流失等问题[5],同时也增加烟田生态系统N2O排放,加剧温室效应[6]。
N2O产生途径主要包括硝化和反硝化过程,主要受土壤理化性质、微生物和重金属等因素的影响[7-8]。其中土壤有机碳增加可增强土壤反硝化作用,从而促进N2O排放[9],土壤硝态氮含量增加也会促进酸性土壤N2O排放[10];在高铵态氮条件下,中性土壤的硝化作用由氨氧化细菌主导[11],而在低铵态氮条件下,酸性土壤的硝化作用则由氨氧化古菌主导[12]。在一定pH范围内,土壤N2O排放量会随着pH增加而逐渐降低[13],过量施氮导致农田土壤酸化会增加土壤N2O排放[14-15]。而在碱性土壤中,因反硝作用产物变化导致N2O排放降低[15]。此外,重金属等环境胁迫,可以通过影响参与硝化/反硝化过程的细菌从而影响N2O排放[16]。有研究认为,重金属对N2O反硝化还原过程中微生物的抑制作用强于生成作用,一定浓度范围内可促进N2O排放[17-19]。Cd作为我国农田土壤最主要的无机污染物之一,前期研究发现[20],不同程度Cd胁迫对土壤氮转化过程的影响并不一致,低浓度(2~5 mg/kg)Cd胁迫可显著促进氨氧化作用和硝化作用,而当Cd浓度达10~20 mg/kg时,对两个过程具有抑制作用。因此,农田土壤N2O排放受不同土壤条件及Cd胁迫的重要影响。
当前,受地质背景、施肥管理及人类活动的影响,部分烟区存在土壤酸化及不同程度的Cd污染[21],而Cd污染胁迫对不同植烟土壤的硝化作用和N2O排放的影响尚不清楚。因此,本研究通过外源Cd添加试验,以不同酸碱性的植烟土壤(中性及酸性)为研究对象,探究Cd胁迫下不同植烟土壤的硝化作用、N2O排放特征及其影响因素,以期为植烟土壤N2O减排管理提供理论依据。
1 材料与方法
1.1 供试材料
供试土壤采集时间为2017年6月至10月,采自全国4个典型优质烟叶产区,分别为潍坊诸城、南平邵武、恩施宣恩和遵义湄潭,按照土壤酸碱属性可划分为2种中性土和2种酸性土(表1)。为了减少自然环境及耕作措施对土壤样品的影响,土壤样品采集均在晴天且未开展农艺活动时进行。每个产区均选择当地同一区域的3块代表性烟田,采用“S”型取样法,取土深度为0~20 cm,每块样地取10~15点,并混合均匀作为一个混合土样,每个土壤类型采集3个混合土样(约50 kg)。将采集的土壤样品立刻带回实验室,并剔除可见的石块及动植物残体,自然风干并过2 mm筛备用。供试土壤的基本理化性质如表1所示。
1.2 试验设计
胁迫培养试验在中国农业科学院烟草研究所进行,采用预培养后的植烟土壤开展试验。镉在土壤中具有较强的积累和迁移特征,我国部分区域农田土壤的镉含量最高达25.7 mg/kg,而矿区土壤的最高量还要高一个数量级[22],因此本试验设置10 mg/kg与100 mg/kg两个外源镉浓度梯度,分别模拟两个污染场景的情况,并描述为低浓度Cd胁迫处理与高浓度镉胁迫处理。不同Cd浓度梯度通过向土壤中添加乙酸Cd溶液来实现,且每种土壤均设置对照处理(CK,0 mg/kg)。每个处理设置3个重复,在25 ℃恒温条件下培养28 d,并在Cd添加后的第1、4、7、14、28 天等5个时间节点采集N2O气体。在采集完气体后,土壤样品进行破坏性采样处理,并密封保存于−40 ℃冰箱中用于土壤铵态氮和硝态氮含量测定。
1.3 试验方法
1.3.1 试验操作 预培养:测定风干土样的含水率,称取相当于40 g干土的样品加入锥形瓶中,用去离子水调节土壤含水量为最大田间持水量(WHC)的45%,在瓶口套上封口膜并固定(以减少水分的蒸发),同时在封口膜上扎几个小孔使土壤与外界进行气体交换。将锥形瓶放入25 ℃恒温室进行预培养7 d,采用重量法定期加水以维持土壤含水量。
Cd胁迫培养试验:预培养后按照试验设计加入不同浓度的乙酸镉溶液,并调节土壤含水量为最大田间持水量的60%,对土壤进行称重记录,将土壤样品继续放入25 ℃恒温室中进行为期28 d的培养,培养期间添加去离子水保持土壤含水量为60% WHC,并定期进行气体采样。
气体采集:取样前24 h将瓶口塑料膜换成丁基橡胶塞进行密封,24 h后用带有三通阀的注射器垂直插入丁基橡胶塞,来回推拉以使瓶內气体混匀,抽取30 mL气体垂直打入18 mL的真空顶空瓶,将收集好的气体样品于48 h内上机测定。
1.3.2 指标测定 土壤的基础理化性质指标按鲍士旦方法测定[23]。土壤铵态氮和硝态氮含量采用2 mol/L的KCl溶液浸提,振荡30 min后过滤,通过AA3流动注射分析仪(Skalar,Breda,荷兰)分析测定[24]。N2O气体采用气相色谱仪(7890A,安捷伦公司)测定。
1.3.3 土壤净硝化速率计算[25]
NR=(C1-C2)/(t1-t2)
NR为土壤净硝化速率[mg/(kg·d)],C1和C2分别为培养t2 d与t1 d时NO3--N含量(mg/kg)。
1.3.4 N2O累积排放量计算[10,26]
C样品=C标准×PA样品/PA标准
C样品为N2O浓度(cm3/m3);C标准为N2O标准气体样品浓度;PA标准为气相色谱仪测定出的峰面积;PA样品为气体样品的峰面积。
F为培养瓶内N2O排放速率[μg N/(kg·d)];Cs为N2O浓度(cm3/m3);Ca为室外新鲜空气中N2O浓度(空白培养瓶内N2O浓度);ρ(N2O)为N2O在25 ℃时的密度,其值为1.12 kg/m3;V为培养瓶体积(mL);m为培养瓶内相当于干土的土壤质量(g);T为两个相邻采样时间间隔(d)。
CE为N2O累积排放量(μg/kg);i为第i次气体采样;F为N2O排放速率[μg N/(kg·d)];ti+1-ti为两个相邻采样日期间隔(d);n为累积排放量观测时间内总的测定次数。
1.4 数据分析
采用SPSS 22.0软件对数据进行方差分析(ANOVA),采用Duncan法进行多重比较,运用Origin9.0软件绘图,图中数据均为平均值±标准误。
2 结 果
2.1 Cd脅迫下不同植烟土壤铵态氮和硝态氮含量变化
如图1所示,各处理土壤铵态氮含量随培养时间呈快速下降并趋于平缓趋势。在整个培养时段,在无胁迫CK下,酸性土1铵态氮含量显著高于酸性土2和中性土1,除培养第1天及第4天外,其铵态氮与中性土2差异不显著。培养结束时,各土壤铵态氮的减少量表现为:酸性土1(11.74 mg/kg)>酸性土2(10.51 mg/kg)>中性土1(8.05 mg/kg)>中性土2(4.25 mg/kg)(图1 a)。在低Cd胁迫下,各土壤铵态氮的变化与CK处理趋势一致,培养结束时,酸性土1的铵态氮减少量(12.84 mg/kg)分别是酸性土2和中性土1、2的1.11、1.61和2.13倍(图1 b)。在高Cd胁迫下,酸性土1在培养后期(14~28 d)的铵态氮含量显著高于其他3种土壤,且培养结束时,各土壤铵态氮减少量表现为:酸性土1(11.03 mg/kg)>酸性土2(10.10 mg/kg)>中性土1(6.12 mg/kg)>中性土2(5.04 mg/kg)(图1 c)。
与铵态氮变化趋势相反,在培养前期(1~7 d),各处理土壤的硝态氮含量呈快速增加,并在培养后期(14~28 d)趋于平缓(图1 d-f)。在CK处理下,各土壤硝态氮含量整体呈现:中性土2>酸性土2>中性土1>酸性土1。在培养结束时,各土壤硝态氮
的增加量表现为:酸性土2(24.92 mg/kg)>中性土2(24.30 mg/kg)>中性土1(18.34 mg/kg)>酸性土1(11.00 mg/kg)(图1 d)。低Cd胁迫下各土壤硝态氮的变化趋势与CK处理一致(图1 e);而在高Cd胁迫下,与CK相比,中性土1、中性土2和酸性土2硝态氮增加量减少,而酸性土1增加量变大。
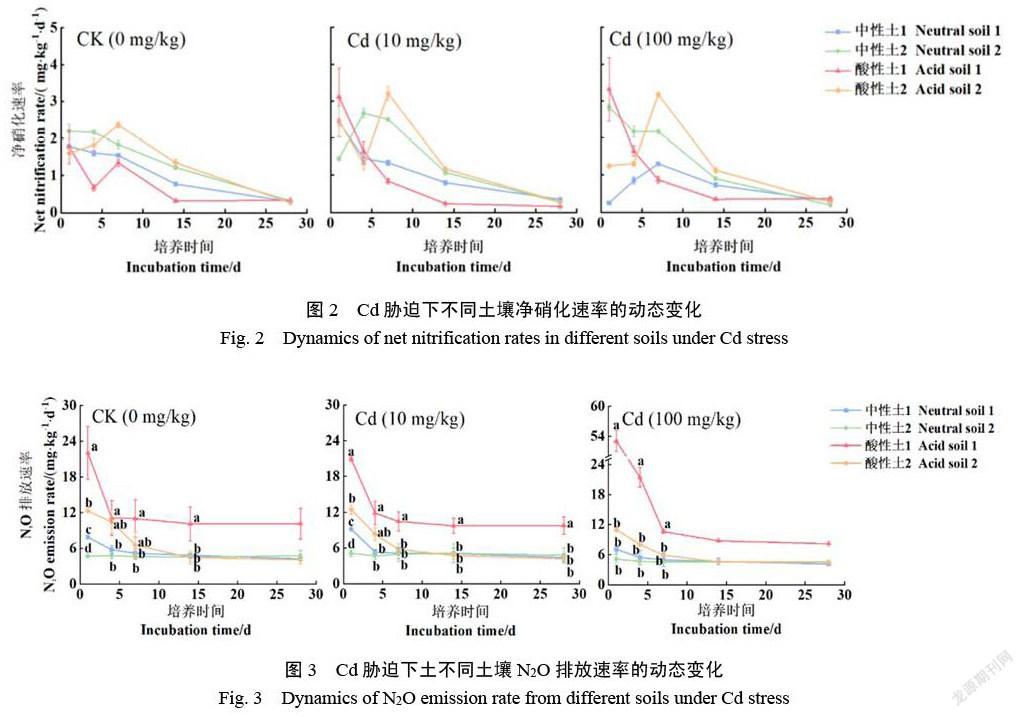
2.2 Cd胁迫下不同植烟土壤净硝化速率变化
如图2所示,各处理土壤的净硝化速率随培养时间总体呈波动降低趋势,其中在培养前期(1~7 d)波动较大并在培养结束趋于一致。在CK处理中,整个培养阶段2个中性土的净硝化速率始终呈降低趋势,在第1天达最大值,分别为1.78 mg/(kg·d)和2.19 mg/(kg·d),随后降低;而2个酸性土的净硝化速率均呈波动降低趋势,其中酸性土1和2的净硝化速率最大值分别出现在第1天[1.77 mg/(kg·d)]和第7天2.36 mg/(kg·d)](图2 a)。在低Cd胁迫下,中性土1和2分别在第1天和4天达最大值,分别是2.83 mg/(kg·d)和2.67 mg/(kg·d),酸性土1和2分别在第1天和第7天达到最大值,分别为3.31 mg/(kg·d)和3.20 mg/(kg·d)。与CK相比,低Cd胁迫后第1天明显降低了中性土2的净硝化速率,但增加了中性土1和2个酸性土的净硝化速率,培养结束时4个土壤的净硝化速率无显著差异(图2 a和b)。在高Cd胁迫下,中性土和酸性土的净硝化速率变化趋势与低Cd胁迫类似,与CK相比,高Cd胁迫后第1天,中性土1和酸性土2的净硝化速率明显降低,而中性土2和酸性土1的净硝化速率明显增加(图2 a和c)。
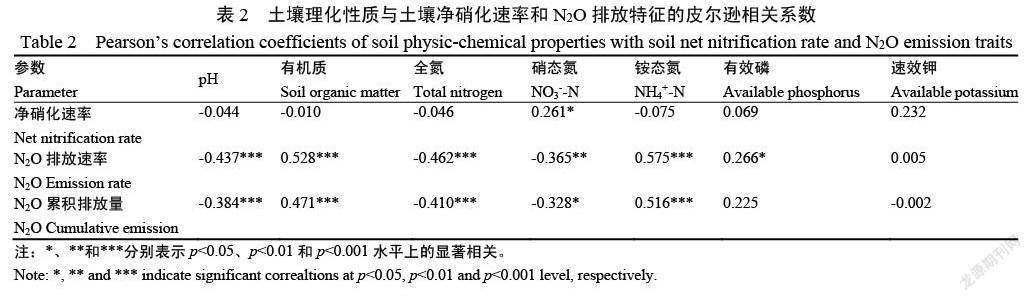
2.3 Cd胁迫下不同植烟土壤N2O排放速率变化
由图3可知,各处理土壤的N2O排放速率随培养时间逐渐降低并趋于平缓,且酸性土的N2O排放速率整体高于中性土。低Cd胁迫下对N2O排放速率的影响与CK处理一致,各土壤的N2O排放速率在第1天达最大值,且4种土壤的N2O排放速率大小为:酸性土1>酸性土2>中性土1>中性土1(图3 a~b)。在高Cd胁迫下,中性土1和2及酸性土1和2的N2O排放速率均在第1天达最大值,分别为7.04、5.12、53.05和10.97 μg/(kg·d),其中酸性土1在整个培养时段内的N2O排放速率显著高于其他3种土壤。酸性土1在Cd胁迫后第1天的N2O排放速率显著增加,分别是CK和低Cd胁迫处理的2.41和2.54倍。
2.4 Cd胁迫下不同植烟土壤N2O累积排放量变化
在培养时段内,4种土壤的N2O累积排放量随着培养时间而逐渐增加,酸性土的N2O累积排放量始终高于中性土,且酸性土1的N2O累积排放量显著高于其他3种土壤(图4 a-c)。此外,高Cd胁迫促进酸性土1在各取样时段的N2O累积排放量(53.05~355.42 μg/kg)增加,分别是同期CK和低Cd胁迫处理的1.16~2.43倍和1.17~2.54倍。
2.5 植烟土壤理化性质与净硝化速率及N2O排放特征的相关性
如表2所示,土壤净硝化速率仅与硝态氮含量呈显著正相关,N2O排放速率和累积排放量与土壤pH和全氮含量呈极显著负相关,与土壤硝态氮含量呈显著负相关,而与土壤有机质和铵态氮含量均呈极显著正相关。此外,N2O排放速率还与土壤的有效磷呈显著正相关。
3 讨 论
在农田土壤中,由反硝化作用产生的N2O占主要地位[27],其中N2O还原酶对N2O的产生具有关键调控作用,该酶通常在中性环境下起作用并在酸性环境具有较强活性[28]。彭艳等[17]研究发现,酸性土壤中初始硝态氮含量增加会促进N2O排放,与该研究结果相似,本研究中酸性土的N2O排放速率在培养初期(1~7 d)明显高于中性土,由于土壤pH
下降會抑制反硝化过程中N2O还原酶形成,进而促进N2O的积累[29]。此外,在酸性土壤条件下,真菌反硝化作用对N2O排放也产生重要促进作用[30-32]。许多研究已表明[10,13,33],土壤硝态氮和铵态氮含量同样影响土壤N2O排放。随着土壤硝化作用进程,土壤铵态氮不断向硝态氮转化,硝态氮不断累积。本研究中2个中性土的铵态氮含量处于较低水平,而土壤硝态氮含量始终处于较高水平,而且同期土壤N2O排放速率降低。KHALIQ等[33]研究表明,在一定pH范围内(4.78~6.83)硝态氮含量增加后土壤N2O排放反而减少,这主要原因是在高硝态氮条件下,微生物不需要耗能产生N2O还原酶,进而促使N2O向N2转变[13]。此外,本研究中发现酸性土1的N2O累积排放量最高,这可能与其较高的有机质含量有关,低pH可增加土壤有机碳的可利用性,进而增强土壤反硝化潜势,导致N2O排放增加[34-35]。
重金属胁迫也对土壤氮转化及N2O排放具有重要影响,GUI等[20]研究表明,低浓度的Cd(2~5 mg/kg)可以显著促进土壤氨氧化作用和硝化作用,使得土壤铵态氮含量和硝态氮含量增加,而Cd浓度达10~20 mg/kg时,则对这两个过程具有明显抑制作用。本研究结果显示,低Cd胁迫(10 mg/kg)和高Cd胁迫(100 mg/kg)对培养初期不同土壤净硝化速率的影响不一致,低Cd胁迫明显降低了中性土2的初始净硝化速率(第1 d),但增加了中性土1和两种酸性土的净硝化速率,而高Cd胁迫则明显降低了中性土1和酸性土2的初始净硝化速率,但增加了中性土2和酸性土1的净硝化速率。目前,有关重金属胁迫对土壤N2O排放的影响及机制尚未有一致结论。一般认为随着重金属浓度增加,反硝化功能基因nosZ表达受到抑制,需氧反硝化活动降低,导致硝酸盐离子不断积累和N2O排放速率增强[36]。与其他重金属相比,Cd对土壤反硝化作用的抑制最强[37]。本研究中发现,高Cd胁迫更明显促进酸性土N2O累积排放量增加,这是由于在酸性土壤环境中,土壤Cd的有效性增加[38],从而加剧了Cd对N2O转化过程的关键微生物的抑制作用,如抑制了N2O反硝化还原酶活性[9,18],进而促进N2O在土壤中的积累。因此,后续针对一些具有Cd污染风险的酸性植烟土壤,可以通过施用生石灰、白云石粉及碱性肥料等酸化改良措施,来改善土壤酸碱性以降低Cd的有效性,从而起到减少土壤氮素损失及N2O减排的目的[14,39]。
4 结 论
随Cd胁迫培养进程,4个植烟土壤的铵态氮含量减少,硝态氮含量逐渐增加,且低Cd和高Cd胁迫均未显著改变这一变化趋势。Cd胁迫对不同土壤培养初期(1~7 d)的硝化速率影响不一致,低Cd胁迫增加了2个酸性土和中性土1的净硝化速率,而高Cd胁迫增加了酸性土1和中性土2的净硝化速率。酸性土N2O排放潜力最大,高Cd胁迫促进酸性土N2O的累积排放量的增加,而且土壤N2O排放速率和累积排放量与土壤pH、全氮及硝态氮含量呈显著负相关。因此,为减少植烟特别是酸化及Cd污染风险植烟的土壤N2O排放,进行土壤酸化改良是行之有效的重要措施。
参考文献
[1]AMELOOT N, MAENHOUT P, DE N, et al. Biochar-induced N2O emission reductions after field incorporation in a loam soil[J]. Geoderma, 2016, 267: 10-16.
[2]GRIGGS D J, NOGUER M. Climate change 2001: the scientific basis. contribution of working group I to the third assessment report of the intergovernmental panel on climate change[J]. Weather, 2002, 57: 267-269.
[3]王成己,唐莉娜,胡忠良,等. 生物炭和炭基肥在烟草农业的应用及展望[J]. 核农学报,2021, 35(4): 997-1007.
WANG C J, TANG L N, HU Z L, et al. The application and prospect of biochar and carbon-based fertilizer in tobacco agriculture[J]. Journal of Nuclear Agriculture, 2021, 35(4): 997-1007.
[4]赵永超,李振杰,刘志华,等. 基于循环经济理念指导下的现代烟草农业发展分析[J]. 山西农经,2020(9):20-21.
ZHAO Y C, LI Z J, LIU Z H, et al. Analysis of the development of modern tobacco agriculture based on the concept of circular economy[J]. Shanxi Agricultural Economics, 2020(9): 20-21.
[5]MIAO Y, STEWART B A, ZHANG F. Long-term experiments for sustainable nutrient management in China. A review[J]. Agronomy for Sustainable Development, 2011, 31(2): 397-414.
[6]ZHU X, BURGER M, DOANE T A, et al. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability[J]. Proceedings of the National Academy of Sciences, 2013, 110(16): 6328-6333.
[7]符春敏,尹黎燕,邓燕,等. 施肥模式对菠萝产量及农田氧化亚氮排放的影响[J]. 热带生物学报,2020,11(3):331-340.
FU C M, YIN L Y, DENG Y, et al. Effects of fertilization patterns on pineapple yield and farmland nitrous oxide emissions[J]. Chinese Journal of Tropical Biology, 2020, 11(3): 331-340.
[8]SHAABAN M, PENG Q, BASHIR S, et al. Restoring effect of soil acidity and Cu on N2O emissions from an acidic soil[J]. Journal of Environmental Management, 2019, 250: 109535.
[9]CHEN Z, TU X, MENG H, et al. Microbial process-oriented understanding of stimulation of soil N2O emission following the input of organic materials[J]. Environmental Pollution, 2021, 284: 117176.
[10]彭艳,朱健,杨成,等. 酸性土壤中的碳氮耦合作用与N2O流失研究[J]. 环境科学与技术,2019,42(6):57-63.
PENG Y, ZHU J, YANG C, et al. Carbon and nitrogen coupling and N2O emission in acidic soils among different vegetation types[J]. Environmental Science & Technology, 2019, 42(6): 57-63.
[11]JIA Z J, CONRAD R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil[J]. Environmental Microbiology, 2009, 11: 1658-1671.
[12]LU L, JUA Z J. Urease gene-containing archaea dominate autotrophic ammonia oxidation in two acid soils[J]. Environmental Microbiology, 2013, 15: 1795-1809.
[13]QU Z, WANG J, ALMOY T, et al. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils[J]. Global change biology, 2014, 20(5): 1685-1698.
[14]GUO J H, LIU X J, ZHANG Y, et al. Significant acidification in major Chinese croplands[J]. Science, 2010, 327: 1008-1010.
[15]曹文超,宋贺,王娅静,等. 农田土壤N2O排放的关键过程及影响因素[J]. 植物营养与肥料学报,2019,25(10):1781-1798.
CAO W C, SONG H, WANG Y J, et al. The key process and influencing factors of N2O emission from farmland soil[J]. Journal of Plant Nutrition and Fertilizer, 2019, 25(10): 1781-1798.
[16]刘永卓. 重金属污染稻田土壤温室气体产生相关的微生物群落结构及活性变化[D]. 南京:南京农业大学,2012.
LIU Y Z. Changes in soil microbial community structure and activity with special reference to greenhouse gases production from rice paddies with heavy metal pollution across south China[D]. Nanjing: Nanjing Agricultural University, 2012.
[17]MAGALHAES C M, MACHADO A, MATOS R, et al. Impact of copper on the diversity, abundance and transcription of nitrte and nitrous oxide reductase genes in an urban European estuary[J]. FEMS Microbiology ecology, 2011, 77: 274-284.
[18]周通. 重金屬污染对稻田土壤有机碳矿化、秸秆分解及温室气体排放的影响[D]. 南京:南京农业大学,2013.
ZHOU T. The impact of heavy metal pollution on rice soil organic carbon mineralization, straw decomposition and greenhouse gas emissions[D]. Nanjing: Nanjing Agricultural University, 2013.
[19]赵迪. 重金属胁迫对潮滩沉积物反硝化作用影响机制的初步研究[D]. 上海:华东师范大学,2013.
ZHAO D. A preliminary study on the mechanism of heavy metal stress on the denitrification of tidal flat sediments[D]. Shanghai: East China Normal University, 2013.
[20]GUI M, CHEN Q, MA T, et al. Effects of heavy metals on aerobic denitrification by strain Pseudomonas stutzeri PCN-1[J]. Applied Microbiology and Biotechnology, 2017, 101(4): 1717-1727.
[21]孔祥方,魏树和,赵继蓉,等. 旺盛期烟草对镉富集敏感性研究[J]. 中国环境科学,2021,41(10):4872-4877.
KONG X F, WEI S H, ZHAO J R, et al. Study on the susceptibility of tobacco to cadmium enrichment in the vigorous period[J]. China Environmental Science, 2021, 41(10): 4872-4877.
[22]宋波,楊子杰,张云霞. 广西西江流域土壤镉含量特征及风险评估[J]. 环境科学,2018,39(4):1889-1900.
SONG B, YANG Z J, ZHANG Y X. Characteristics and risk assessment of soil cadmium in the Xijiang River Basin, Guangxi[J]. Environmental Science, 2018, 39(4): 1889-1900.
[23]鲍士旦. 土壤农化分析[M]. 北京:中国农业出版社,2000.
BAO S D. Soil agrochemical analysis[M]. Beijing: China Agriculture Press, 2000.
[24]CAI X, LIN Z, PENTTINEN P, et al. Effects of conversion from a natural evergreen broadleaf forest to a Moso bamboo plantation on the soil nutrient pools, microbial biomass and enzyme activities in a subtropical area[J]. Forest Ecology and Management, 2018, 422: 161-171.
[25]KHANOM A, AZAD M A K, ALI M M, et al. Plants and microbes' responses to the net nitrification rates of chemical fertilizers in vegetable soils[J]. Applied Soil Ecology, 2021, 158: 103783.
[26]刘杏认,赵光昕,张晴雯,等. 生物炭对华北农田土壤N2O通量及相关功能基因丰度的影响[J]. 环境科学,2018,39(8):3816-3825.
LIU X R, ZHAO G X, ZHANG Q W, et al. The effect of biochar on soil N2O flux and related functional gene abundance in North China farmland[J]. Environmental Science, 2018, 39(8): 3816-3825.
[27]ZHANG J B, MÜLLER C, CAI Z C. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils[J]. Soil Biology and Biochemistry, 2015, 84: 199-209.
[28]BERGAUST L, MAO Y J, BAKKEN L R, et al. Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrogen oxide reductase in Paracoccus denitrificans[J]. Applied and Environmental Microbiology, 2010, 76: 6387-6396.
[29]LIU B, FROSTEGÅRD Å, BAKKEN L R. Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ[J]. Mbio, 2014, 5(3): e01383-14.
[30]STRICKLAND M S, ROUSK J. Considering fungal: bacterial dominance in soils-methods, controls, and ecosystem implications[J]. Soil Biology & Biochemistry, 2010, 42: 1385-1395.
[31]RÜTTING T, HUYGENS D, BOECKXP P, et al. Increased fungal dominance in N2O emission hotspots along a natural pH gradient in organic forest soil[J]. Biology and Fertility of Soils, 2013, 49: 715-721.
[32]HUANG Y, XIAO X, LONG X. Fungal denitrification contributes significantly to N2O production in a highly acidic tea soil[J]. Journal of Soils & Sediments, 2017, 17(6): 1599-1606.
[33]KHALIQ M A, TARIN M W K, JING X G, et al. Soil liming effects on CH4, N2O emission and Cd, Pb accumulation in upland and paddy rice[J]. Environmental Pollution, 2019, 248: 408-420.
[34]SENBAYRAM M, CHEN R, BUDAIA A, et al. N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations[J]. Agriculture, Ecosystems and Environment, 2012, 147: 4-12.
[35]BADAGLIACCA G, BENÍTEZ E, AMATO G, et al. Long-term effects of contrasting tillage on soil organic carbon, nitrous oxide and ammonia emissions in a Mediterranean Vertisol under different crop sequences[J]. Science of The Total Environment, 2018, 619: 18-27.
[36]CHEN Y X, WANG K X, LIN Q, et al. Effects of heavy metals on ammonification, nitrification and denitrification in maize rhizosphere[J]. Pedosphere, 2001, 11(2): 115-122.
[37]HOLTAN-HARTWIG L, BECHMANN M, HØYǺS T R, et al. Heavy metals tolerance of soil denitrifying communities: N2O dynamics[J]. Soil Biology & Biochemistry, 2002, 34(8): 1181-1190.
[38]ANDERSSON A, NILSSON K O. Influence of lime and soil pH on Cd availability to plants[J]. Ambio, 1974: 198-200.
[39]SHAABAN M, PENG Q A, HU R G, et al. Dolomite application to acidic soils: a promising option for mitigating N2O emissions[J]. Environmental Science and Pollution Research, 2015, 22(24): 19961-19970.
2761501186297
