慢羽鸡成纤维细胞内源性白血病病毒ev21基因敲除
2021-12-15徐天鹏吴思乐温健韦义荣李思佳谢龙虞霖田宋中宝陆阳清
徐天鹏 吴思乐 温健 韦义荣 李思佳 谢龙 虞霖田 宋中宝 陆阳清
摘要:【目的】探索在慢羽鸡成纤维细胞中敲除ev21基因的可行性,净化鸡群内源性逆转录病毒,同时为快速培育缺失ev21基因的慢羽鸡配套系打下基础。【方法】根据ev21基因序列(KY235336)特点,分别在其5'和3'端各设计2个sgRNA,用于構建4种不同sgRNA的打靶质粒,筛选出在5'和3'端打靶效率较高的sgRNA。然后基于CRISPR/Cas9基因编辑技术对ev21基因进行剪切,并通过同源重组方式以红色荧光蛋白(mCherry)的DNA片段(CAG-mCherry)替换ev21基因,实现对慢羽鸡成纤维细胞内源性白血病病毒ev21基因定点敲除。【结果】在慢羽鸡成纤维细胞中能检测到ev21基因,构建的4种sgRNA(sgRNA1~sgRNA4)均能成功插入对应的打靶质粒中,经嘌呤霉素筛选及T7E1酶切检测,发现转染4种不同sgRNA打靶质粒后慢羽鸡成纤维细胞均有不同程度的死亡,其中又以sgRNA1和sgRNA3的基因敲除效率较高。同时针对同源位点左右同源臂构建表达mCherry的供体质粒,以其转染293T细胞12 h后均能表达出mCherry。以sgRNA1和sgRNA3打靶质粒及供体质粒共同转染慢羽鸡成纤维细胞,观察发现成纤维细胞内的mCherry持续表达,至转染后第30 d通过流式细胞仪分选收集红色荧光阳性成纤维细胞,并提取其总DNA进行PCR鉴定与基因测序,结果显示红色荧光阳性成纤维细胞中有目的片段(CAG-mCherry)插入,即以插入替换方式能实现对ev21基因的敲除。【结论】基于crispr/cas9基因编辑技术的基因敲除方法能成功敲除慢羽鸡成纤维细胞内源性白血病病毒ev21基因,为培育缺失ev21基因的慢羽鸡品系提供技术支持。
关键词: 慢羽鸡;禽白血病病毒(ALV);ev21基因;CRISPR/Cas9;基因敲除
中图分类号: S831.2 文献标志码: A 文章编号:2095-1191(2021)08-2259-08
Knockout of endogenous leukemia gene ev21 in slow
feathering chicken
XU Tian-peng1, WU Si-le1, WEN Jian1,WEI Yi-rong1, LI Si-jia1, XIE Long1,
YU Lin-tian1, SONG Zhong-bao2, LU Yang-qing1*
(1College of Animal Science and Technology, Guangxi University/State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources, Nanning 530005,China; 2Nanning Liangfeng Agriculture and Animal
Husbandry Co., Ltd., Nanning 530005,China)
Abstract:【Objective】The objective was to explore the feasibility of knockout of ev21 gene in slow feathering chicken fibroblasts for the endogenous retroviruses purification of chicken flocks, and lay a foundation for rapid breeding of slow feathering chicken lines without ev21 gene. 【Method】 Based on the characteristics of ev21 gene sequence(KY235336), two sgRNAs were designed at each of its 5' and 3' ends, respectively, for the construction of four different sgRNA targeting plasmids, and the sgRNAs with higher efficiency at the 5' and 3' ends were screened. Then the ev21 gene was cut based on CRISPR/Cas9 gene editing technology and the ev21 gene was replaced by a DNA fragment of red fluorescent protein(mCherry) (CAG-mCherry) by homologous recombination to achieve targeted knockout of the ev21 gene of endogenous leukemia virus in slow feathering chicken fibroblasts. 【Result】The results showed that the ev21 gene could be detected in slow feathering chicken fibroblasts, and the four constructed sgRNAs(sgRNA1-sgRNA4) could be successfully inserted into the corresponding targeting plasmids. After puromycin screening and T7E1 digestion assay, it was found that slow feathering chicken fibroblasts had different degrees of death after transfection with the four different sgRNAs, among which the knockdown efficiency of sgRNA1 and sgRNA3 was higher. Donor plasmids containing the left and right homology arms and expressing mCherry were also constructed with reference to the homologous site. All 293T cells were able to express mCherry after 12 h transfection with the donor plasmid. Cells were found to consistently express mCherry when slow feathering chicken fibroblasts were co-transfected with sgRNA1 and sgRNA3 targeting plasmids and donor plasmids. Red fluorescent positive fibroblasts were collected by flow cytometry at day 30 after transfection, and their total DNA was extracted for PCR identification and gene sequencing, which showed that the target fragment (CAG-mCherry) was inserted in the red fluorescent positive fibroblasts, and therefore the knockdown of ev21 gene could be achieved by insertion substitution. 【Conclusion】The gene knockout method based on CRISPR/Cas9 gene editing technology can successfully knock out the ev21 gene of endogenous leukemia virus in slow feathering chicken fibroblasts, and provide technical support for breeding slow feathering chicken strains without ev21 gene.
Key words: slow feathering chicken; avian leukemia virus(ALV); ev21 gene; CRISPR/Cas9; gene knockout
Foundation item: National Natural Science Foundation of China(31960157); Guangxi University Student Innovation and Entrepreneurship Training Project(201910593306)
0 引言
【研究意义】禽白血病病毒(Avian leukosis virus,ALV)是一种在进化过程中整合到基因组中并随宿主基因组遗传复制的内源性反转录病毒(Endogenous retroviruses,ERVs)(曹利利等,2020;Mason et al.,2020)。根据寄主、抗体反应和受体的不同,ALV被分为A~J等10个亚群,其中A、B、C、D、E和J亚群的宿主都是鸡(刘健等,2019)。A、B、C、D和J亚群为致病性强的外源性病毒,而E亚群(Avian leukosis virus subgroup E,ALVE)是致病性弱的内源性病毒(崔治中,2010)。ALVE的表达除了影响外源病毒感染和疾病发生外,还对家禽的重要经济性状产生影响(廖卓锋等,2019),主要影响其产蛋性能及母源抗体水平(Smith and Fadly,1988)。ALVE不仅可在家禽个体间传播,还能通过配子将其传播给下一代,给传统的净化选择手段带来严峻挑战(Payne and Nair,2012)。ev21基因是ALVE的受体基因,且与羽速基因K紧密连锁(王麟等,2017)。因此,利用分子生物学的基因编辑技术开展ev21基因敲除研究,对净化ALVE感染及通过羽速基因鉴别雌雄个体具有重要意义。【前人研究进展】1988年,Bacon等在所有的慢羽鸡中发现ev21基因,但在快羽鸡中未发现,故推断ev21基因和慢羽基因K紧密连锁或慢羽基因K是由野生型快羽基因k+插入ev21基因突变所导致。随后,Levin和Simth(1990)研究发现,在鸡基因组中至少有1个额外的DNA区域与ev21基因整合位点的DNA序列高度同源,且这个同源区域存在ev21插入(OR)和无ev21插入(UR)2种情况。Elferink等(2008)的研究首次从分子水平上解释ev21基因和慢羽基因K及快羽基因k+间的关系。K基因是由2个与k+基因相同的串联重复序列构成,且这个重复序列导致K基因上催乳素受体基因(PRLR)及编码精子鞭毛蛋白2基因(SPEF2)部分重复。K基因上有ev21基因插入,而在k+相同的位置并无ev21基因插入(Elferink et al.,2008)。PRLR和SPEF2基因是控制羽速性状的良好候选基因(Bu et al.,2013;Zhao et al.,2016)。Takenouchi等(2018)对52个品种1994羽鸡进行全面检测,结果发现几乎所有的慢羽鸡中同时含有ev21基因与PRLR和SPEF2基因不完全重复的片段(ID),并发现几乎所有的快羽鸡都缺少ev21基因和ID,表明ev21基因与慢羽基因的表达无必然联系。由于ev21基因与K基因紧密连锁,因此种鸡生产上应用快慢羽自别雌雄选淘公鸡时无法严格净化ev21基因。【本研究切入点】相对于传统的翻肛鉴别法,通过羽速基因鉴别雏鸡性别具有简便易行、快速准确及节省劳力的优点,且能避免翻肛鉴别对雏鸡产生应激反应和减少疾病传播。羽速基因与ALVE的ev21基因紧密连锁,ev21基因的表达会干扰对禽白血病的检测,虽然自然界存在极少量缺少ev21基因的慢羽个体,但通过筛选育种需要花费大量的人力、物力和时间。【拟解决的关键问题】通过CRISPR/Cas9基因编辑技术对慢羽鸡成纤维细胞中ALVE的ev21基因进行定点敲除,探索在鸡慢羽鸡成纤维细胞中敲除ev21基因的可行性,净化鸡群的ERVs,同时为快速培育缺失ev21基因的慢羽雞配套系打下基础。
1 材料与方法
1. 1 试验材料
供试种蛋为广西东兰乌鸡(慢羽鸡)和广西麻鸡(快羽鸡)的种蛋,TIANamp Genomic DNA Kit(DP304)、0.25%胰酶/EDTA(Gibco/25200-056)、DMEM/F12培养液(Gibco/11320033)、嘌呤霉素(Invitrogen)及Xfect? Transfection Reagent等购自TaKaRa公司;倒置显微镜和免疫荧光系统购自日本Olympus公司。
1. 2 鸡胚胎成纤维细胞分离及培养
在37 ℃和65%相对湿度条件下将种蛋孵化至第7 d,取出鸡胚放入无菌培养皿中,显微镜下去除四肢、头部、内脏和性腺;以无菌PBS洗涤3遍后,用灭菌手术剪剪碎鸡胚;加入0.25%胰酶,在37 ℃水浴中消化15 min,每隔5 min振荡摇晃1次,待其消化完毕加入等量含血清的DMEM/F12培养液终止消化;然后15000 r/min离心10 min,弃上清液,将细胞重悬并接种至细胞培养瓶中,置于37 ℃、5% CO2培养箱进行培养,待细胞融合达90%~95%时进行传代和冻存处理。
1. 3 引物设计与合成
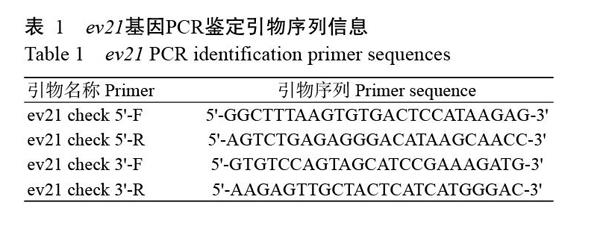
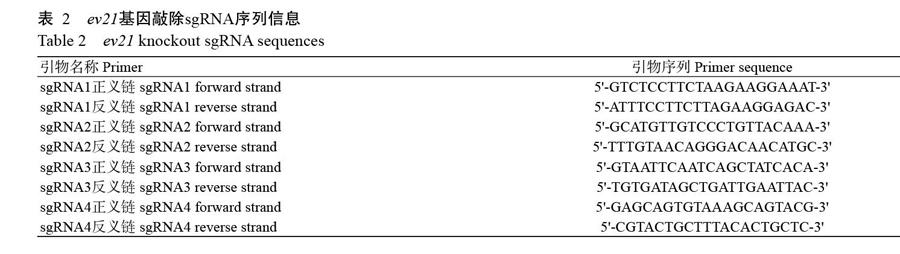
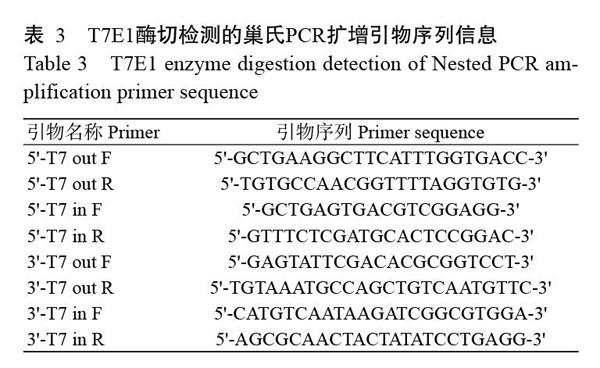
根据NCBI已公布的ev21基因序列(KY235336),分别在ev21基因的5'和3'端各设计1对引物(表1)。同时,在ev21基因5'端设计2个sgRNA(sgRNA1和sgRNA2),在3'端设计2个sgRNA(sgRNA3和sgRNA4),具体序列信息见表2。此外,在5'和3'端打靶位点左右两侧400 bp左右的位置分别设计2对相互包含的巢氏PCR扩增引物(表3),用于sgRNA效率检测(T7E1酶切检测);并设计1对引物用于鉴定目的片段(CAG-mCherry)的插入情况,具体序列信息见表4。所有引物合成和测序均委托北京六合华大基因科技公司完成。
1. 4 DNA提取及PCR鉴定
收集快羽鸡和慢羽鸡的成纤维细胞,使用DNA提取试剂盒(TIANamp Genomic DNA Kit)提取总DNA。为精确检测阳性细胞基因组DNA编辑情况,采用高保真酶(PrimerSTAR Max DNA Polymerase)在BioRad C1000 PCR仪中进行扩增,PCR反应体系20.0 μL:2×Enzyme Mixed(PrimerSTAR/Tiangene Taq)10.0 μL,DNA模板100 ng,正、反义引物(10 μmol/L)各0.5 μL,ddH2O补足至20.0 μL。扩增程序:98 ℃预变性10 s;98 ℃ 10 s,55~60 ℃ 15 s,72 ℃ 15 s(1000 bp/15 s),进行35个循环;4 ℃结束反应。PCR扩增产物采用2.0%琼脂糖凝胶电泳进行鉴定。
1. 5 细胞转染
细胞转染流程严格按照XfectTM Transfection Rea-gent说明进行操作。在6孔板中,每孔质粒用量为8 μg,打靶质粒和供体质粒的用量为1∶1;转染12 h后将转染液更换为新鲜的培养液。
1. 6 流式细胞分选
以打靶质粒和供体质粒共转染慢羽鸡成纤维细胞,待对照组(单转供体质粒)成纤维细胞的红光完全消失后,通过流式细胞仪分选出携带红色荧光的阳性细胞,接种至培养皿上继续培养。
2 结果与分析
2. 1 ev21基因插入慢羽鸡成纤维细胞基因组的鉴定结果
为鉴定慢羽鸡基因组中是否成功插入ev21基因,分别提取慢羽鸡和快羽鸡的成纤维细胞总DNA进行PCR检测,结果(图1)显示,慢羽鸡成纤维细胞基因组DNA在5'和3'端均有1条清晰且单一的条带,而快羽鸡成纤维细胞基因组DNA无任何条带,说明慢羽鸡成纤维细胞基因组中有ev21基因插入。
2. 2 打靶质粒构建情况及sgRNA打靶效率检测结果
以U6启动子驱动sgRNA表达,CAG启动子驱动Cas9蛋白表达,分别构建4种不同sgRNA的打靶质粒,其测序结果显示4种sgRNA均成功插入对应的打靶质粒中(图2-A)。为进一步筛选出打靶效率较高的sgRNA,将慢羽鸡成纤维细胞分为4组,分别转染4种不同的sgRNA打靶质粒,经嘌呤霉素筛选48 h后,与转染前相比,发现各组成纤维细胞均有不同程度的死亡(图2-B),收集细胞并提取总DNA。采用巢氏PCR扩增目的片段,纯化回收的目的片段以T7E1进行酶切,酶切产物经2.0%琼脂糖凝胶电泳鉴定,结果显示sgRNA1和sgRNA3的目的条带灰度较低(图2-C),说明其基因敲除效率较高。
2. 3 供体质粒构建情况
ev21基因全长7524 bp,包括上、下游序列共9679 bp(图3-A)。其中,1~970 bp为鸡Z染色体序列,970~8500 bp为ev21基因序列(王麒等,2017)。为敲除慢羽鸡成纤维细胞中的ev21基因,采取同源重组修复(Homologous recombination repair,HDR)策略,以红色荧光蛋白(mCherry)的DNA片段(CAG-mCherry)替换整个ev21基因(图3-B)。针对同源位点左右同源臂构建表达mCherry的供体质粒,供体质粒主要由基本骨架(PUC19)、左端同源臂(HAL)、右端同源臂(HA)和mCherry部分组成,其中mCherry是质粒(XP61)经EcoR V和Age I双酶切获得。对供体质粒的左右同源臂进行测序,测序结果显示左右同源臂无突变(图3-C),即成功构建获得供体质粒。
2. 4 ev21基因敲除及鉴定结果
为探索在慢羽鸡成纤维细胞敲除内源性ev21基因的可能性,将慢羽鸡成纤维细胞分为试验组和对照组,试验组成纤维细胞共同转染sgRNA1和sgRNA3打靶质粒及供体质粒,对照组成纤维细胞仅转染供体质粒。转染后48 h观察发现,对照组和试验组均有部分成纤维细胞表达出mCherry(图4-A);转染后第7 d,对照组成纤维细胞的红色荧光基本消失,而试验组中红色荧光比例的成纤维细胞越来越多(图4-B);至转染后第10 d,对试验组的成纤维细胞进行流式细胞分选,收集携带红色荧光的阳性细胞;转染后第30 d,试验组成纤维细胞内的mCherry持续表达(图4-C),说明CAG-mCherry已成功整合到慢羽鸡成纤维细胞基因组中,即成功建立了稳定表达mCherry的细胞系。
为鉴定红色荧光阳性成纤维细胞中ev21基因的敲除情况,分别在跨基因组和插入片段(CAG-mCherry)的5'和3'端设计特异性扩增引物,通过流式细胞仪分选收集转染后第30 d的红色荧光阳性成纤维细胞,提取其总DNA进行PCR鉴定,结果(图4-D)显示红色荧光阳性成纤维细胞中有CAG-mCherry插入。同时对sgRNA的靶位点进行测序,结果(图4-E)显示靶位点附近的序列与预测结果一致,说明基于CRISPR/Cas9基因编辑技术通过插入替换方式能成功实现对ev21基因的敲除。
3 讨论
鸡是从红色原鸡进化而来,为最常见的家养动物之一。在长期进化的过程中,鸡一直面临着病原微生物的威胁与浸染。病原侵染不仅引发动物疾病,还会导致动物机体的基因組发生改变,包括基因(碱基或片段)的插入、缺失及突变等(Wildschutte et al.,2016)。基因组改变进而造成表型性状发生改变,导致疾病发生、生长性能和繁殖性能下降及适应性降低等。Wang等(2016)研究发现,在ZNF132基因中插入16 bp碱基会显著影响海南黑山羊的体长;Zang等(2016)研究证实,DGAT2基因3'-UTR发生13 bp插入突变后,会影响猪的背膘厚度及瘦肉率;Wei等(2018)研究表明,将19 bp碱基插入PLAGI基因会导致牛的生长性能下降。有关鸡的研究表明,CDKN3基因启动子区域多等位基因插入后会影响其生长性能和屠宰性状(Li et al.,2018);ZNF764L基因发生22 bp插入与鸡的初生重、胸宽及体斜长密切相关(Han et al.,2019);鸡PTH1R基因第1个内含子中51 bp碱基插入突变与其生长和屠宰性能密切相关(Ren et al.,2020)。可见,动物基因组中发生插入或缺失突变会影响其生产性能。
病毒是导致鸡基因组发生改变的重要因素之一,特别是逆转录病毒。内源性逆转录病毒EAV-HP插入并整合到鸡SLCO1B3基因5'-UTR,会导致SLCO1B3基因在鸡输卵管蛋壳腺中高表达,致使鸡生产绿壳蛋(Wang et al.,2013)。Park等(2013)研究表明,ALV插入并整合到TYR基因第4个内含子会导致TYR基因逆转录本功能失活,从而形成隐形白突变。ALV是一种典型的逆转录病毒,自1908年首次分离获得以来,全球许多国家均有暴发流行,其垂直和水平传播引起的临床和亚临床感染给养禽业造成巨大经济损失(刘公平等,2000;余河玲等,2020)。经过几十年的探索与研究,虽然已取得一定成绩,但禽白血病尚未得到有效遏制,仍需耗费大量人力和物力去筛选及淘汰携带病毒的鸡群。本研究首次尝试以基因编辑技术探索解决禽白血病对家禽养殖产业的危害,在分离培养的慢羽鸡成纤维细胞中检测出ev21基因,并成功构建获得4种sgRNA的打靶质粒和供体质粒;以基因敲除效率较高的sgRNA1和sgRNA3打靶质粒和供体质粒共同转染慢羽成纤维细胞,转染后第30 d采用流式细胞仪分选收集携带红色荧光的阳性细胞,即得到稳定表达mCherry的细胞系;随后通过PCR扩增与基因测序相结合的方法在sgRNA靶位点检测到目的片段(CAG-mCherry)精准插入,且以插入替换方式成功实现对ev21基因的敲除。可见,在体外培养的慢羽鸡成纤维细胞中敲除内源性白血病病毒ev21基因具有可行性,可为培育缺失ev21基因的慢羽鸡品系提供技术支持。
4 结论
基于CRISPR/Cas9基因编辑技术的基因敲除方法能成功敲除慢羽鸡成纤维细胞内源性白血病病毒ev21基因,为培育缺失ev21基因的慢羽鸡品系提供技术支持。
参考文献:
曹利利,董航,郭衍冰,姜旭,姚新华,苑淑贤,邵洪泽,宋建臣,贾立军. 2020. 禽白血病病毒ELISA检测方法的建立与初步应用[J]. 中国动物传染病学报,28(1):16-21. [Cao L L,Dong H,Guo Y B,Jiang X,Yao X H,Yuan S X,Shao H Z,Song J C,Jia L J. 2020. Development and preliminary application of ELISA method for detection of avian leukosis virus[J]. Chinese Journal of Animal Infectious Diseases,28(1):16-21.]
崔治中. 2010. 鸡白血病及其鉴别诊断和预防控制[J]. 中国家禽,32(8):1-12. [Cui Z Z. 2010. Differential diagnosis,prevention and control of avian leukosis[J]. China Poultry,32(8):1-12.]
廖卓鋒,刘永,谢强明,杨柳平,杨润. 2019. 禽白血病研究进展[J]. 当代畜牧,(8):65-67. [Liao Z F,Liu Y,Xie Q M,Yang L P,Yang R. 2019. Development in avian leucosis research[J]. Contemporary Animal Husbandry,(8):65-67.]
刘公平,赵振芬,刘福安. 2000. 禽白血病病毒研究进展[J]. 中国兽医学报,20(6):621-623. [Liu G P,Zhao Z F,Liu F A. 2000. Advance of research on avian lekosis virus[J]. Chinese Journal of Veterinary Science,20(6):621-623.] doi:10.3969/j.issn.1005- 4545.2000.06.030.
刘健,李凯航,鞠厚斌,李鑫,葛菲菲,杨德全,杨显超,葛杰,邓波,周锦萍. 2019. 七彩山鸡内源性禽白血病病毒的检测与env基因序列分析[J/OL]. 中国动物传染病学报. http://kns.cnki.net/kcms/detail/31.2031.S.20190917.1522. 051.html. [Liu J,Li K H,Ju H B,Li X,Ge F F,Yang D Q,Yang X C,Ge J,Deng B,Zhou J P. 2019. Detection and env gene analysis of endogenous avian leukosis virus from phasianus colchicas[J/OL]. Chinese Journal of Animal Infectious Diseases. http://kns.cnki.net/kcms/detail/31.2031.S.20190917.1522.051.html.]
王麒,王晗,张秀玲,刘春杨,张乐超,李兰会,李祥龙. 2017. 鸡内源白血病病毒ev21与慢羽非连锁分析和LTR区启动子活性分析[J]. 畜牧兽医学报,48(5):930-937. [Wang Q,Wang H,Zhang X L,Liu C Y,Zhang L C,Li L H,Li X L. 2017. Analysis of non-linkage between endogenous ALV-ev21 and late feathering of chickens and its promo-ter activity of LTR[J]. Acta Veterinaria et Zootechnica Sinica,48(5):930-937.] doi:10.11843/j.issn.0366-6964. 2017.05.018.
余河玲,朱師良,何启牮,王彦. 2020. PDPK1 3'UTR双荧光素酶报告载体的构建及与gga-miR-148a-5p的靶向验证[J]. 江苏农业学报,36(1):147-151. [Yu H L,Zhu S L,He Q J,Wang Y. 2020. Construction of PDPK1 3'UTR dual luciferase reporter vector and targeting verification with gga-miR-148a-5p[J]. Jiangsu Journal of Agricultural Sciences,36(1):147-151.] doi:10.3969/j.issn.1000-4440. 2020.01.020.
Bacon L D,Smith E,Crittenden L B,Havenstein G B. 1988. Association of the slow feathering(K) and an endogenous viral(ev21) gene on the Z chromosome of chickens[J]. Poultry Science,67(2):191-197. doi:10.3382/ps.0670 191.
Bu G X,Huang G,Fu H,Li J,Huang S M,Wang Y J. 2013. Characterization of the novel duplicated PRLR gene at the late-feathering K locus in Lohmann chickens[J]. Journal of Molecular Endocrinology,51(2):261-76. doi:10. 1530/JME-13-0068.
Elferink M G,Vallée A A A,Jungerius A P,Crooijmans R P M A,Groenen M A M. 2008. Partial duplication of the PRLR and SPEF2 genes at the late feathering locus in chicken[J]. BMC Genomics,9(1):391. doi: 10.1186/1471-2164-9-391.
Han R L,Wang X N,Wang X L,Guo Y P,Li D H,Li G X,Wang Y B,Kang X T,Li Z J. 2019. Chicken ZNF764L gene:mRNA expression profile,alternative splicing analysis and association analysis between first exon indel mutation and economic traits[J]. Gene,695:92-98. doi:10. 1016/j.gene.2019.02.010.
Levin I,Smith E J. 1990. Molecular analysis of endogenous virus ev21-slow feathering complex of chickens. 1. Cloning of proviral-cell junction fragment and unoccupied integration site[J]. Poultry Science,69(11):2017-2026. doi: 10. 3382/ps.0692017.
Li W Y,Liu D L,Tang S Q,Li D H,Han R L,Tian Y D,Li H,Li G X,Li W T,Liu X J,Kang X T,Li Z J. 2018. A multiallelic indel in the promoter region of the Cyclin-dependent kinase inhibitor 3 gene is significantly associated with body weight and carcass traits in chickens[J]. Poultry Science,98(2):556-565. doi:10.3382/ps/pey404.
Mason A S,Lund A R,Hocking P M,Fulton J E,Burt D W. 2020. Identification and characterisation of endogenous avian leukosis virus subgroup E(ALVE) insertions in chi-cken whole genome sequencing data[J]. Mobile DNA,11: 22. doi:10.1186/s13100-020-00216-w.
Park M N,Kim T H,Lee J H,Choi J A,Heo K N,Kim C D,Choo H J,Han J Y,Lee T,Lee J H,Lee K T. 2013. Genetic variations of chicken TYR gene and associations with feather colorof Korean Native Chicken(KNC)[J]. Korean Journal of Poultry Science,40(2):139-145. doi:10.5536/KJPS.2013.40.2.139.
Payne L N,Nair V. 2012. The long view:40 years of avian leukosis research[J]. Avian Pathology,41(1):11-19. doi:10.1080/03079457.2011.646237.
Ren T,Zhang Z,Fu R,Yang Y,Li W,Liang J,Mo G,Luo W,Zhang X. 2020. A 51 bp indel polymorphism within the PTH1R gene is significantly associated with chicken growth and carcass traits[J]. Animal Genetics,51(4):568-578. doi:10.1111/age.12942.
Smith E J,Fadly A M. 1988. Influence of congenital transmission of endogenous virus-21 on the immune response to avian leukosis virus infection and the incidence of tumors in chickens[J]. Poultry Science,67(12):1674-1679. doi:10.3382/ps.0671674.
Takenouchi A,Toshishige M,Ito N,Tsudzuki M. 2018. Endogenous viral gene ev21 is not responsible for the expression of late feathering in chickens[J]. Poultry Scien-ce,97(2):403-411. doi:10.3382/ps/pex345.
Wang X,Yang Q,Luo J,Wang Y,Feng Q,Zhang Z H,Lei C Z,Chen H,Lan Y F. 2016. Novel 16-bp insertion/deletion variant of ZNF132 gene and its influence on growth traits in goats[J]. Journal of Animal & Plant Sciences,26:1813-1818.
Wang Z P,Qu L J,Yao J F,Yang X L,Li G,Zhang Y Y,Li J Y,Wang X T,Bai J R,Xu G Y,Deng X M,Yang N,Wu C X. 2013. An EAV-HP insertion in 5' flanking region of SLCO1B3 causes blue eggshell in the chicken[J]. PLoS Genetics,9(1):e1003183. doi:10.1371/journal.pgen.100 3183.
Wei X,Hua H,Li Z,Xu J W,Lei C Z,Zhang G M,Dang R H,Niu H,Qi X L,Chen H,Huang Y Z. 2018. Detection of 19-bp deletion within PLAG1 gene and its effect on growth traits in cattle[J]. Gene,675:144-149. doi:10.1016/ j.gene.2018.06.041.
Wildschutte J H,Williams Z H,Montesion M,Subramanian R P,Kidd J M,Coffin J M. 2016. Discovery of unfixed endogenous retrovirus insertions in diverse human populations[J]. Proceedings of the National Academy of Scien-ces of the United States of America,113(16):E2326- E2334. doi:10.1073/pnas.1602336113.
Zang L,Wang Y D,Sun B X,Zhang X,Yang C H,Kang L,Zhao Z H,Jiang Y L. 2016. Identification of a 13 bp indel polymorphism in the 3'-UTR of DGAT2 gene associa-ted with backfat thickness and lean percentage in pigs[J]. Gene,576(2):729-733. doi:10.1016/j.gene.2015.09. 047.
Zhao J,Yao J,Li F,Yang Z,Sun Z,Qu L,Wang K,Su Y,Zhang A,Montgomery S A,Geng T,Cui H. 2016. Identification of candidate genes for chicken early- and late-feathering[J]. Poultry Science,95(7):1498-1503. doi:10.3382/ps/pew131.
(责任编辑 兰宗宝)
收稿日期:2020-07-20
基金项目:国家自然科学基金项目(31960157);广西大学大学生创新创业训练计划项目(201910593306)
通讯作者:陆阳清(1976-),https://orcid.org/0000-0003-1641-6142,博士,研究員,主要从事干细胞及动物繁殖生物技术研究工作,E-mail:lyq@gxu.edu.cn
第一作者:徐天鹏(1995-),https://orcid.org/0000-0003-0175-0295,研究方向为动物遗传育种与繁殖,E-mail:1491159801@qq.com
