血浆标本病毒灭活处理对伏立康唑、利奈唑胺、万古霉素和替考拉宁血药浓度检测的影响
2021-10-15李红莲周琼张峻黄桦王晶晶姚勤
李红莲 周琼 张峻 黄桦 王晶晶 姚勤
中图分类号 R917 文献标志码 A 文章编号 1001-0408(2021)19-2394-06
DOI 10.6039/j.issn.1001-0408.2021.19.15
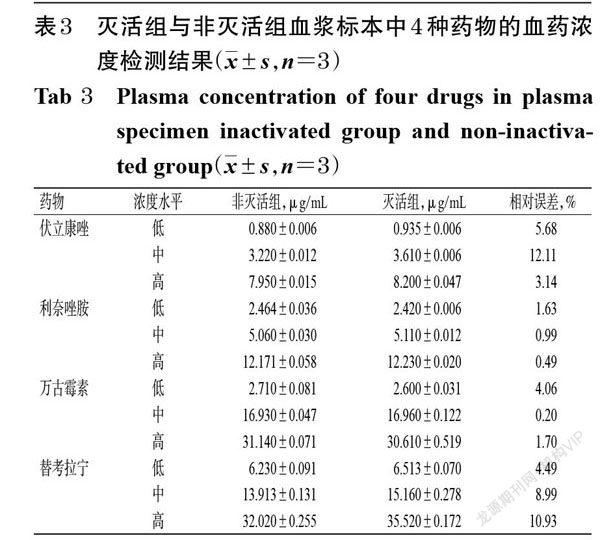
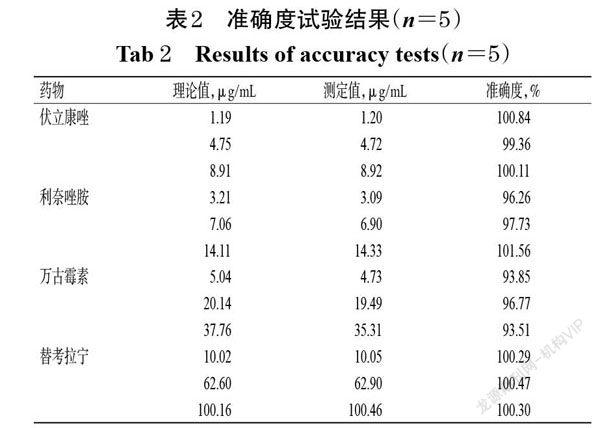
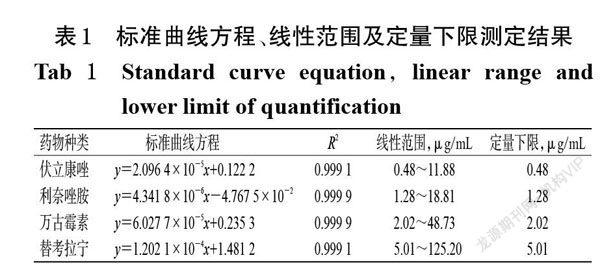
摘 要 目的:研究血浆标本病毒灭活处理对伏立康唑、利奈唑胺、万古霉素和替考拉宁血药浓度检测的影响。方法:以我院36例住院患者分别行常规伏立康唑、利奈唑胺、万古霉素和替考拉宁血药浓度检查后剩余的血浆为检测标本(每种药物的含药血浆均为9份),根据各药物的常规血药浓度检测结果按3个一组将其分别分为低、中、高3个浓度水平的混合标本;然后将不同浓度水平的混合标本分别分为灭活组和非灭活组,每组3份。灭活组标本采用56 ℃干式恒温金属浴加热30 min进行灭活处理,非灭活组标本不作处理。两组血浆样本分别进行前处理后,采用二维液相色谱法分别测定4种药物的血药浓度,比较其检测结果差异。结果:含伏立康唑、利奈唑胺、万古霉素和替考拉宁的血浆标本经56 ℃干式恒温金属浴加热30 min灭活后依然较稳定;相較于非灭活组,灭活组低、中、高浓度水平混合血浆样本中上述4种药物的血药浓度检测结果的相对误差均小于15%。结论:采用二维液相色谱法测定血浆标本中伏立康唑、利奈唑胺、万古霉素和替考拉宁的血药浓度时,可采用56 ℃干式恒温金属浴加热30 min的方法对血浆标本进行灭活。
关键词 伏立康唑;利奈唑胺;万古霉素;替考拉宁;血药浓度;血浆标本;病毒灭活
Effects of Virus Inactivation Treatment of Plasma Specimen on Plasma Concentration Determination of Voriconazole,Linezolid,Vancomycin and Teicoplanin
LI Honglian,ZHOU Qiong,ZHANG Jun,HUANG Hua,WANG Jingjing,YAO Qin(Dept. of Clinical Pharmacy, the First Affiliated Hospital of Kunming Medical University, Kunming 650000, China)
ABSTRACT OBJECTIVE: To study the effects of virus inactivation treatment of plasma specimen on plasma concentration determination of voriconzole,linezolid,vancomycin and teicoplanin. METHODS: The remaining plasma of 36 inpatients in our hospital after routine blood concentration examination of voriconazole, linezolid, vancomycin and teicoplanin were collected as specimen (9 drug-contained plasma specimens for each drug), and merged into three different concentration levels (low, medium, high) of mixed samples according the results of routine blood test. Then the mixed samples with different concentration levels were divided into inactivated group and non-inactivated group, with 3 samples in each group. The inactivated plasma samples were heated at 56 ℃ for 30 min in metal bath with constant temperature. Non-inactivated group were not treated. After pretreating plasma sample of 2 groups, 2-dimensional liquid chromatography was used to detect plasma concentration of the four drugs; the difference of detection result between inactivated group and non-inactivated group were analyzed. RESULTS: Plasma samples containing voriconazole, linezolid, vancomycin and teicoplanin were still stable after heating at 56 ℃ for 30 min in metal bath with constant temperature. Compared with non-inactivated group, relative error of plasma concentration detection result of above 4 drugs were all lower than 15% in low, medium, high concentration mixed samples of inactivated group. CONCLUSIONS: Plasma samples can be inactivated by heating at 56 ℃ for 30 min in metal bath with constant temperature, when the plasma concentration of voriconazole,linezolid,vancomycin and teicoplanin are determined by 2-dimensional liquid chromatography.
