Isomerization and hydroformylation of butenes under the catalysis of Rh-BIPHEPHOS
2021-09-10JIANGWeiliHELimeiHUANGBinCHENYaqiZHOUGuanglinZHOUHongjun
JIANG Wei-li,HE Li-mei,HUANG Bin,CHEN Ya-qi,ZHOU Guang-lin,*,ZHOU Hong-jun
(1.College of New Energy and Materials, Beijing Key Laboratory of Biogas Upgrading Utilization, China University of Petroleum (Beijing), Beijing 102249, China;2.BOE Technology Group Co., Ltd, Chengdu 610000, China)
Abstract:The isomerization and hydroformylation of butene under the catalysis of Rh-BIPHEPHOS were investigated by varying the butene feed and reaction temperature.The results indicate that in the presence of syngas,with 1-butene as feed,more isomerization product (i.e.2-butene) is detected in comparison with the hydroformylation products,whereas with 2-butene as the feed,the hydroformylation is superior to isomerization.Due to the equilibrium limit between 1-butene and 2-butene,the increase of temperature shows a great positive effect on the isomerization reaction.Furthermore,under the catalysis of Rh-BIPHEPHOS,more n-pentanal is always formed than i-pentanal,as the larger bite angle of BIPHEPHOS restricts the intermediate rearrangement in the formation of i-pentanal.
Key words:hydroformylation;rhodium;catalysis;butene;pentanal;isomerization
In spite of the increasing attention on the heterogeneous catalysis in chemical industry,homogeneous reactions still keep their special advantages in high reactivity,high product selectivity and well clarified mechanism[1-4].Hydroformylation is undoubtedly one of the most important homogeneous catalytic reactions in industry[5-8],which is considered as an atomically economic,high efficient and environmentally friendly route to produce aldehyde from olefins.The catalysts used in hydroformylation are in general based on rhodium and cobalt complexes.Shell reported the application of phosphine ligands in hydroformylation catalyzed by cobalt compound in 1960’s.Later,rhodium-phosphines complexes became more famous because of their higher reactivity under milder reaction conditions[8,9].In the rhodium-catalyzed hydroformylation,the P-ligands were used to control the geometry of the complex and thus regulate the catalysis process through the well-known electronic and steric effect[10-13].
n-pentanal is a useful intermediate in both pharmaceutical and chemical industry[8,14,15].A larger demand ofn-pentanal has been expected,because one of its derivatives,viz.,2-propyl-heptanol (2-PH),is considered as the new-generation plasticizer alcohol[16].n-Pentanal can be directly produced from hydroformylation of 1-butene,but mixed butenes (1-butene and 2-butene) as feedstock are more economical[14,15,17,18].It is easy to generate linear aldehyde from terminal alkenes by the catalysis of Rh-TPP (TPP=triphenylphosphine);however,the yield ratio of linear aldehyde to branched aldehyde (n/i) in the hydroformylation of internal alkenes is usually very low,since the internal alkene may undergo isomerization prior to hydroformylation to produce linear aldehyde[19-22].As the internal alkenes are usually more readily available than terminal alkenes,it is a big challenge to develop effective isomerizationhydroformylation catalysts[12,23-28].The important breakthroughs were made in the end of last century with bidentate diphosphines as ligands,such as BISBI[29],NAPHOS[30]and Xantphos[31,32].A high regioselectivity for linear aldehydes were obtained in the hydroformylation of internal alkenes with diphosphines due to their large bite angles[33-35].Recently,a brilliant ligand,viz.,BIPHEPHOS,was reported with excellent catalytic performance in the tandem isomerization-hydroformylation reactions towards linear aldehydes[25,36-41];it showed a superior efficiency together with a much highern-regioselectivity compared to diphosphines.
The present work aims to explore the general rule in the hydroformylation of mixed C4alkenes to producen-pentanal catalyzed by Rh-BIPHEPHOS[42].With different butene feedstocks,the relationship between isomerization and hydroformylation was investigated at different temperatures,to help determine the appropriate reaction conditions to getn-pentanal.
1 Experimental
1.1 Materials
1-butene (> 99.95%),2-butene (> 99.95%) and syngaswere purchased from Beijing Beiwen Qiti Company.Rh(acac)(CO)2(> 99%) was purchased from Beijing Persisted Technology Company Limited.BIPHEPHOS (≥ 97%) was purchased from Okeanos Technology Company Limited.The mixed butene feedstock was made by mixing 1-butene and 2-butene with a ratio of 1∶4.
1.2 Reaction test
In a typical reaction,0.009 mmol of Rh(acac)(CO)2,0.036 mmol of BIPHEPHOS,and 30 mL of toluene (as the solvent) were put rapidly into a 100 mL stainless steel autoclave with mechanical stirring.N2flow was then introduced into the autoclave continually for 10 min before the syngas,to replace the air.Subsequently,the reactor was filled with syngas to 0.5 MPa,and about 5.0 g of feedstock was pumped into the reactor.After that,the system was heated to the targeted reaction temperature (70-110 °C) under a stirring rate of 1000 r/min.Additional syngas was flowed into the autoclave to control the pressure at 1.0 MPa during the reaction.The products were analyzed every 0.5 or 1.0 h by a GC2000-II gas chromatograph with a flame ionization detector (FID),equipped with a DB-1 column (60 m×0.25 mm×0.25 μm).The composition of C4 mixture in the reaction solution was analyzed by a Fuli-9790II gas chromatograph with a FID detector and RT alumina bond/Na2SO4column of 50 m ×0.53 mm×10.0 μm.The conversion of feedstock (X),the yield of product (Y) and the ratio of 1-butene to 2-butene (R) were calculated using the following equations:
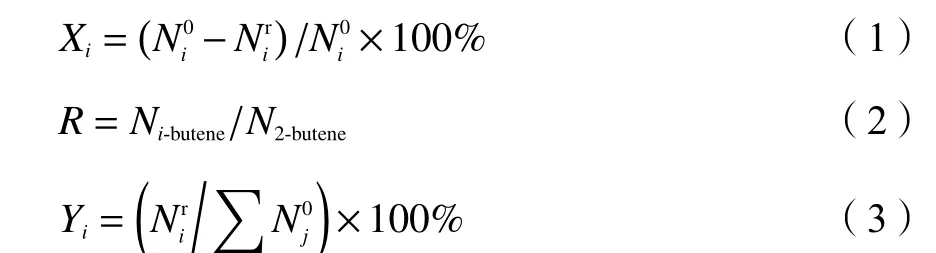
whereis the initial molar quantity of substancei,is the molar quantity of substanceiin the products,andN1-buteneandN2-butenerepresent the mole quantity of 1-butene and 2-butene respectively.
2 Results and discussion
Based on the widely accepted hydroformylation mechanism[43],Figure 1 depicts the reaction routes of butenes with syngas under the catalysis of Rh-BIPHEPHOS.Both 1-butene and 2-butene may undergo hydroformylation to given-pentanal andi-pentanal.It is worth noting that 1-butene can react with syngas to generaten-pentanal andi-pentanal through the same metal-alkene intermediate B .For 2-butene,after coordinating with the active Rh species A,the intermediate C must isomerize to B before yieldingnpentanal.Meanwhile,1-butene and 2-butene may also isomerize to each other reversibly (circle A-B-C-A).

Figure 1 Mechanism of hydroformylation and isomerization of 1-butene and 2-butene
2.1 Reactions from 1-butene
Figures 2-6 show the reaction results of 1-butene with syngas under Rh-BIPHEPHOS at different temperatures (70-110 °C).At a low temperature (70 °C),the conversion of 1-butene is rather low at the beginning,so are the yields of 2-butene and pentanals.After one hour later,the reaction proceeds faster,with a rapid increase of the 1-butene conversion andnpentanal yield.It means that the hydroformylation of 1-butene is enhanced as more and more active catalysis species are formed.The yield ofi-pentanal also increases,but more than one order of magnitude lower than that ofn-pentanal,indicating that the formation ofi-pentanal is inhibited under current conditions.At the same time,the 2-butene yield increases markedly and the final yield of 2-butene is even higher than that ofnpentanal,suggesting that isomerization was the dominant reaction.After about 4 h later,both 1-butene conversion and product yields level off,suggesting the approach of reaction equilibrium.

Figure 2 1-butene conversion for the hydroformylation with syngas under Rh-BIPHEPHOS at different temperatures

Figure 3 2-butene yield for the hydroformylation of 1-butene with syngas under Rh-BIPHEPHOS at different temperatures

Figure 4 n-pentanal yield for the hydroformylation of 1-butene with syngas under Rh-BIPHEPHOS at different temperatures

Figure 5 i-pentanal yield for the hydroformylation of 1-butene with syngas under Rh-BIPHEPHOS at different temperatures
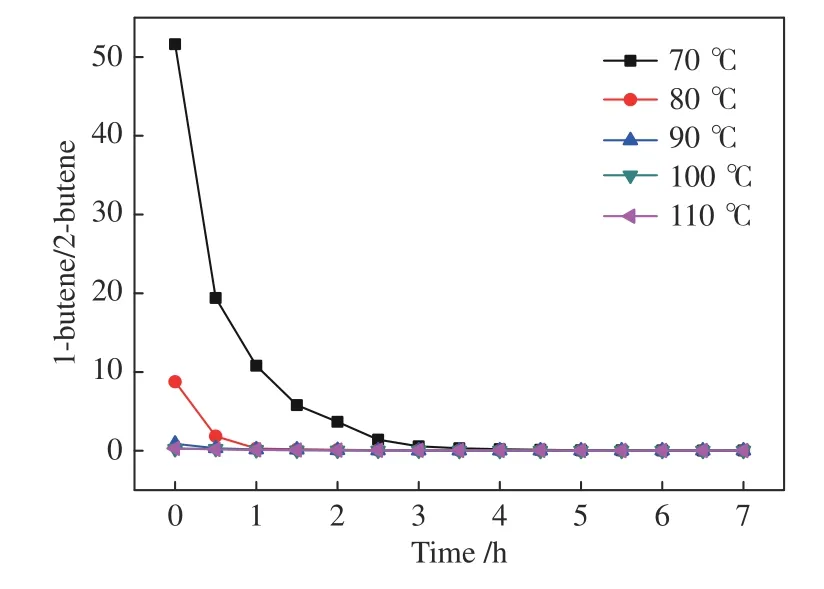
Figure 6 Ratio of 1-butene/2-butene in the products for the hydroformylation of 1-butene with syngas under Rh-BIPHEPHOS at different temperatures
With the increase of reaction temperature,the conversion of 1-butene increases rapidly and the 2-butene yield rises at a similar degree,indicating that the isomerization of 1-butene to 2-butene is extremely facilitated at higher temperature.Then-pentanal yield,however,declines slightly at higher temperature.Although thei-pentanal yield is enhanced slightly with increasing temperature,the 2-butene yield increases rapidly at first,then decreases slightly,and at last levels off;it can be concluded that the formation ofi-pentanal is mainly ascribed to the hydroformylation of the largely increased amount of 2-butene.
Furthermore,it is interesting to note that the conversion of 1-butene at different temperatures gets close to a similar value with the time,so does the 2-butene yield.The ratio of 1-butene content to 2-butene at different temperatures are then calculated (Figure 6);apparently,the final 1-butene/2-butene ratio at each temperature is about 0.02,which should be the equilibrium ratio of these two substrates.
2.2 Reactions from 2-butene
For the reactions of 2-butene with syngas,as demonstrated in Figures 7-11,it is strange that 2-butene conversion was very low at 70 °C,always below 5%,and so are the product yields.Since a considerable amount ofn-pentanal can be generated from 1-butene hydroformylation at 70 °C (Figure 4),the only explanation of the low product yields with 2-butene as feed at this temperature is that the isomerization of 2-butene to 1-butene was extremely restricted.That is,the suppressed steps should be among A-C-D-B.

Figure 7 2-butene conversion for the hydroformylation with syngas under Rh-BIPHEPHOS at different temperatures

Figure 8 1-butene yields for the hydroformylation of 2-butene with syngas under Rh-BIPHEPHOS at different temperatures
When the temperature is raised to 80-90 °C,all the indexes including 2-butene conversion and the yields ofn-pentanal,i-pentanal and 1-butene increase gradually.Further increasing the temperature to 100-110 °C,the 2-butene conversion and product yields become much higher,demonstrating a positive temperature effect.In the temperature range of 80-110 °C (Figures 8-9),the final 1-butene yield reaches a steady value,with the ratio of 1-butene/2-butene between 0.01-0.03,showing a similar equilibrium status to that in the 1-butene reaction system.The corresponding data at 70 °C are an exception because the isomerization is strongly suppressed.Therefore,the lower 1-butene yield in this system must be driven by the equilibrium between 1-butene and 2-butene.
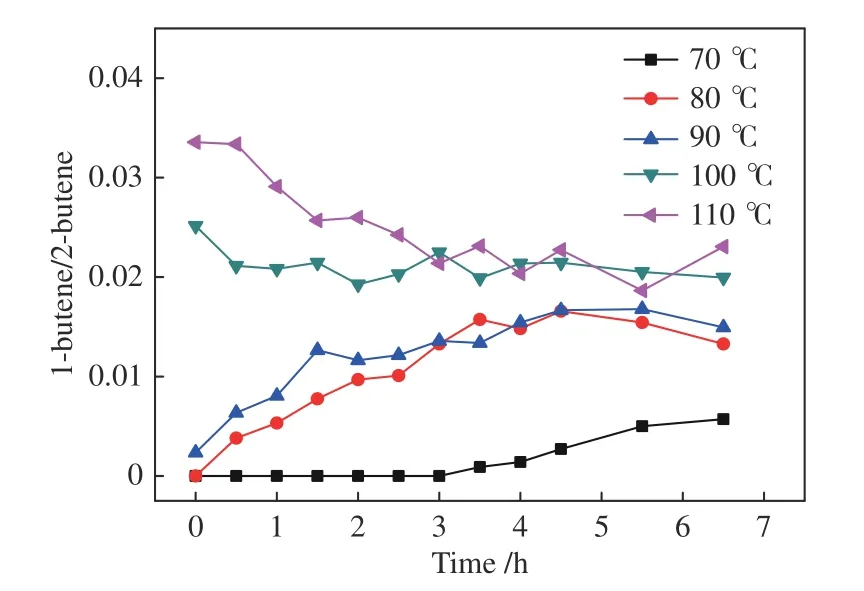
Figure 9 Ratio of 1-butene/2-butene in the product for the hydroformylation of 2-butene with syngas under Rh-BIPHEPHOS at different temperatures

Figure 10 n-pentanal yield for the hydroformylation of 2-butene with syngas under Rh-BIPHEPHOS at different temperatures

Figure 11 i-pentanal yield for the hydroformylation of 2-butene with syngas under Rh-BIPHEPHOS at different temperatures
Then-pentanal yield is here greatly improved at higher temperature (Figure 10),contrary to the observation for the hydroformylation of 1-butene.It is probably ascribed to the fact that the formation of 1-butene through isomerization is facilitated at higher temperature,which can promote then-pentanal yield.In detail,although the 1-butene yield is very low,the transformation of key intermediate D to B is favored at a higher temperature (Figure 1);B can yield either 1-butene orn-pentanal.Since the 1-butene content is restricted by the equilibrium between 1-butene and 2-butene,onlyn-pentanal yield is significantly improved.Moreover,at a higher temperature (100-110 °C),the initial 1-butene yield is improved considerably,but it declines slowly with the time,suggesting that the formation of 1-butene is restricted only by the equilibrium.
Thei-pentanal yield is also enhanced at higher temperature (Figure 11),though still very low (below 1%).The loweri-pentanal yield in both 1-butene and 2-butene reaction systems reveal that some steps in theipentanal circle (Figure 1,A-B-D-E-F-A and A-C-D-EF-A) are suppressed.The transformation of A-B-D and A-C-D should be relatively easy because the isomerization between 1-butene and 2-butene is easily achieved at a high temperature.Thus,the suppressed steps should be within D-E-F.It is known that under the catalysis of Rh-TPP,then/iratio with either 1-butene or 2-butene as the feed is always much lower than that under the catalysis of Rh-BIPHEPHOS[44],which was also confirmed by our previous studies(Supplementary Material).The most probable reason is that the larger bite angle of BIPHEPHOS makes the insertion and rearrangement of CO more difficult.
2.3 Reactions from mixture of 1-butene and 2-butene
A mixture of 1-butene and 2-butene (n1-butene/n1-butene=1/4) was prepared to simulate the industrial mixed C4feed.As expected,as shown in Figure 12,the conversion of 1-butene increases with the increase of temperature.The final 1-butene conversion at each temperature arrives at about 90%,showing a similar trend as illustrated in Figure 2.
The conversion of 2-butene,however,is below zero at the beginning in all the five tests in Figure 13.Because the initial ratio of 1-butene/2-butene was 1/4,higher than their equilibrium ratio (ca.0.03),the isomerization of 1-butene to 2-butene appears as the prior reaction in this system and a higher temperature(facilitated by high temperature) may leads to a larger initial content of 2-butene.Particularly,at 70 °C,2-butene conversion is always below zero,meaning that all the pentanals are produced from 1-butene,resulting in a lower pentanal yield.It is also worth noting that then-pentanal yield at 70 °C in Figure 4 is much higher than that in Figure 14,which is probably due to that in the 1-butene reaction system,the high 1-butene content results in a rapid irreversible increase of then-pentanal yield.With the increase of temperature,the accelerated isomerization may reduce the 1-butene content involved in hydroformylation,leading to a weak temperature effect.At a temperature above 80 °C,the 2-butene conversion rise above zero at the late stage of the reaction.The yield ofi-pentanal shown in Figure 15 is still relatively low,in the same order of magnitude as Figure 5 and Figure 11.

Figure 12 1-butene conversion for the hydroformylation of mixed-butenes (n1-butene/n1-butene=1/4) with syngas under Rh-BIPHEPHOS at different temperatures

Figure 13 2-butene conversion for the hydroformylation of mixed-butenes (n1-butene/n1-butene=1/4) with syngas under Rh-BIPHEPHOS at different temperatures
Figure 16 also shows that the initial ratio of 1-butene/2-butene declines with the increase of temperature,owing to a facilitated isomerization from 1-butene to 2-butene.After reaction for 3.5 h,all the five tests show an equilibrium 1-butene/2-butene ratio of about 0.03.

Figure 14 n-pentanal yield for the hydroformylation of mixedbutenes (n1-butene/n1-butene=1/4) with syngas under Rh-BIPHEPHOS at different temperatures

Figure 15 i-pentanal yield for the hydroformylation of mixedbutenes (n1-butene/n1-butene=1/4) with syngas under Rh-BIPHEPHOS at different temperatures

Figure 16 1-butene/2-butene ratio in the reaction mixture for the hydroformylation of mixed-butenes (n1-butene/n1-butene=1/4)with syngas under Rh-BIPHEPHOS at different temperatures
2.4 About the reaction mechanism
The mechanism of the isomerization and hydroformylation of 1-butene and 2-butene with syngas under the catalysis of Rh-BIPHEPHOS can thus be summarized as follows (Figure 1).The catalytically active species A reacts with butene molecule reversibly,forming intermediate B (for 1-butene) or C(for 2-butene);C is thermodynamically more stable than B.B and C can all be reversibly rearranged to intermediate D,through which the isomerization between 1-butene and 2-butene takes place.The intermediate D can give rise to the key species F through CO insertion and arrangement,which is the rate-determining step to producei-pentanal.As the larger bite angle of BIPHEPHOS makes it very difficult to form F from D,the yield ofi-pentanal was always very low.On contrary,the intermediate B can undergo H-transfer,CO-insertion and rearrangement to yield the key species I,i.e.the precursor ofn-pentanal.In this cycle,the linear alkyl chain on the complexes can reduce the steric hindrance in comparison to branched alkyl chain in thei-pentanal cycle,leading to an absolute superiority to formn-pentanal.
3 Conclusions
The isomerization and hydroformylation of butene under the catalysis of Rh-BIPHEPHOS were investigated by varying the butene feed and reaction temperature.The results demonstrate that no matter which feed (1-butene,2-butene or mixed butenes) is adopted,1-butene and 2-butene can transform into each other with an equilibrium 1-butene/2-butene ratio of about 0.03,except for the reaction of 2-butene at a very low temperature.
Both 1-butene and 2-butene can react with syngas to yieldn-pentanal andi-pentanal.Since the steric hindrance of branched intermediate is larger than that of linear intermediate,n-pentanal is the dominant product compared toi-pentanal.The isomerization is more facilitated at higher temperature,in comparison to the hydroformylation.In regard to 2-butene,it needs to isomerize before hydroformylation ton-pentanal;thus,as the isomerization was extremely inhibited at a low temperature,the isomerization and hydroformylation of 2-butene are all suppressed.A low temperature (70 °C)favors the hydroformylation of 1-butene ton-pentanal,whereas a high temperature (100-110 °C) is beneficial to the reaction of 2-butene ton-pentanal.
