Rapid Detection of Cronobacter sakazakii in Powdered Infant Formula by Immunomagnetic Separation Coupled with Visual Nanogold Hybridization Probe
2021-08-31JIANGYujunYANGTaoMANChaoxinZHOUWenqiWANGRuiCHENSihanYANGXinyanFUShiqianGAOPingpingRENYuweiZHANGWei
JIANG Yujun, YANG Tao, MAN Chaoxin, ZHOU Wenqi, WANG Rui, CHEN Sihan, YANG Xinyan,FU Shiqian, GAO Pingping, REN Yuwei, ZHANG Wei
(Key Lab of Dairy Science, Ministry of Education, College of Food Science, Northeast Agricultural University, Harbin 150030, China)
Abstract: A rapid and sensitive assay for detecting Cronobacter sakazakii in powdered infant formula (PIF) was developed by utilizing immunomagnetic separation (IMS) combined with visual gold nanoparticle (GNP) hybridization probe.C. sakazakii was captured via antibody-functionalized magnetic particles (MPs) and the polymerase chain reaction amplicon of the target bacteria was analyzed by GNP instead of traditionally used electrophoresis. Immunomagnetic particles (IMPs)were obtained by mixing together 100 µL of MPs, 120 µL of carbodiimide hydrochloride (EDC) and 80 µL of monoclonal antibody against C. sakazakii with 0.02% (V/V) added Tween-20. Specificity test showed positive results only for C. sakazakii. C. sakazakii could be detected at 102 CFU/mL and 103 CFU/g in pure culture and artificially contaminated PIF without pre-enrichment, respectively. After 3 h of pre-enrichment, this assay was able to detect as low as 4.5 × 101 CFU/g of C. sakazakii in PIF. The results of the visual assay were consistent with those of electrophoresis and ultraviolet scanning spectroscopy. Therefore, the IMS-GNP probe assay proved to be an alternative to gel electrophoresis for the rapid detection of C. sakazakii in PIF samples.
Keywords: Cronobacter sakazakii; immunomagnetic separation; gold nanoparticles; powdered infant formula; visual detection
Cronobacterspp. was defined as a new genus from the Enterobacteriaceae family in 2008[1]. Currently, seven species are comprised in theCronobactergenus,C. sakazakii,C. condimenti,C. dublinensis,C. malonaticus,C. muytjensii,C. turicensis, andC. universalis[2-3]. Special concern had been put forward on powdered infant formula (PIF) after two serious infectious diseases of infants were caused byC. sakazakii[4].C. sakazakiiis the important opportunistic pathogen for infants from PIF, which can cause severe meningitis and necrotizing enterocolitis[5-6]. Thus, a rapid,specific and sensitive assay is needed to be conducted for detectingC. sakazakii.
The conventional methods for detectingC. sakazakiiwere non-selective or selective medium, differential plating and phenotypic identification. These processes are always laborious and time-consuming[7]. Polymerase chain reaction(PCR) is a milestone for the detection of bacteria compared with the traditional detection methods. Nevertheless, this technique is always restricted by the PCR-mediated inhibition from complex food matrices, such as fat, protein and other components[8]. One of the methods for eliminating PCR assay inhibitors is to capture the target bacteria from sample with a separated procedure. Immunomagnetic separation (IMS) has attracted attention extensively for easy operation and high separating efficiency in recent years, which can eliminate PCR inhibitors in complex food matrices[9-10]. IMS has been used to capture bacteria from complex samples and analytes could be detected sensitively after PCR amplification[11-12].But so far, only a few studies have explored the detection ofCronobacterspp. by the IMS method. For example, the IMS method was used to detectEnterobacter sakazakiiin milk powder[13]. However, this research was quite timeconsuming for plate counting and ignored the classification ofC. sakazakii. A probe-magnetic separation and PCR method was reported to detectCronobacterspp.[14]. But the main species ofCronobacterspp. did not be tested for specificity and this method lacked verification in food samples.Although the immunoliposome-based separation was applied to the detection ofCronobacterspp. in food samples[15-16],these system could only detectCronobacterspp. rather thanC. sakazakii. And higher mortality rates ofCronobacterspp. seem to be especially associated withC. sakazakiifor neonates and infants[17]. In addition, electrophoresis is the most common method for the analysis of DNA amplicon.But specialized equipments, longer time and toxic dye of nucleic acid were required in electrophoresis[18]. The visual assay of gold nanoparticles (GNPs) probe takes advantage of the special surface effect under high salt concentration[19].The modified GNPs by DNA probe will accumulate within a certain high salt concentration and color of the solution will change from red to purple. If the GNPs probe hybridizes with the target DNA, the color change will be avoided by salt aggregation[20]. This colorimetry is fast and safe for alternative electrophoresis to analyze DNA amplicon. There is no colorimetric method of GNPs probe for detectingC. sakazakiiin PIF after IMS assay. Therefore, a valid IMS and visual assay is required for rapid and accurate detection ofC. sakazakii.
In this study, a rapid, sensitive and highly specific method was established by IMS coupled with GNPs colorimetry to detectC. sakazakiiin PIF. The immunomagnetic particles (IMPs) were acquired by optimizing the additive amount of carbodiimide hydrochloride(EDC), Tween-20 and monoclonal antibody ofC. sakazakii.C. sakazakiiwas captured by IMPs and DNA of cell was extracted and amplified. The DNA amplicon was analyzed by visual GNPs hybridization probe assay. The solution of GNPs would be red in the presence of DNA amplicon, conversely, it turned to purple. Specificity and sensitivity were both tested in pure culture. In order to verify the application in the actual sample,sensitivity of the developed assay was inspected in artificially contaminated PIF. The visual results were also verified by electrophoresis and ultraviolet scanning spectrum. We hope that this method has a greatly significance for detectingC. sakazakiiin PIF.
1 Materials and Methods
1.1 Materials and reagents
The magnetic particles (MPs) with a mean diameter of 538.8 nm were purchased from Huier Nano Technique Ltd.(Luoyang, China); The monoclonal antibody (1 mg/mL)againstC. sakazakiiwas obtained from Prajna Biology Technique Ltd. (Shanghai, China); The DNA extraction kit was purchased from TianGen biotech Co. Ltd. (Beijing,China); The EDC, 2-morpholine ethyl sulfonic acid (MES)and phosphate buffered saline (PBS) were purchased from Sigma-Aldrich (USA); The 2 × PCR Master Mix was obtained from Sangon Biotech Co. Ltd. (Shanghai, China);The trypticase soy agar (TSA) was purchased from Hope Bio-Technology Co. Ltd. (Qingdao, China); The GNPs were purchased from Spahui Biotechnology Co. Ltd. (Beijing,China). DNA probe was modified with sulfydryl and 10 adenine according to the report[18], which could bond GNPs and maintain functional activity of oligonucleotides. Then,it was synthesized from Sangon Biotech Co. Ltd. (Shanghai,China). All other reagents were used in this study with analytical grade and ultrapure water was employed in solution.
DNA probe: 5’-SH-AAA AAA AAA ATC GTG CTG CGA GTT TGA GAG ACT CTG ACA CAC CGC G-3’.
C. sakazakiiATCC 29544 was used as the target strain for subsequent experiments. In addition, a total of 13Cronobacterstrains and 9 non-Cronobacterstrains were used to determine the specificity test. All bacteria were incubated in nutrient broth (NB, Hope Bio-Technology Co. Ltd., China)with gently shaking (150 r/min) at 37 ℃ for overnight.
1.2 Instruments and equipments
The particle size analyzer was purchased from Malvern Instrument Co. Ltd. (Mastersizer 2000, UK); The ultraviolet scanner was obtained from Shimazu instrument Co. Ltd.(UV-2600, Japan).
1.3 Methods
1.3.1 Preparation of GNPs probe
The preparation of GNPs (15 nm) probe was conducted based on previous study with modification[21]. The 900 µL of GNPs solution and 0.5 nmol of DNA probe were blended with gently shaking (150 r/min) at 50 ℃ for 22 h. Then, the 100 μL of phosphate buffer solution A (0.1 mol/L Na2HPO4,0.1 mol/L NaH2PO4, 0.1 g/100 mL SDS, pH 8.0) was added to mixture. The 100 μL of phosphate buffer solution B(0.01 mol/L Na2HPO4, 0.01 mol/L NaH2PO4, 0.01 g/100 mL SDS, 11.688 g NaCl, pH 8.0) was added slowly for aging.The solution was centrifuged at 20 000 ×gfor 30 min and the supernatant was removed. The precipitate was resuspended in 100 μL of phosphate buffer solution C (0.01 mol/L Na2HPO4,0.01 mol/L NaH2PO4, 0.01 g/100 mL SDS, 0.585 g NaCl,pH 8.0) and stored at 4 ℃.
1.3.2 Fabrication of MPs-antibody conjugate
The 4000 titer of monoclonal antibodies againstC. sakazakiiwere selected and verified with traditional indirect enzyme-linked immunosorbent assay (iELISA)[22].The addition amount of EDC, Tween-20 and antibody were optimized for the preparation of IMPs. Briefly, 100 μL of carboxylic MPs (10 mg/mL) were transferred into centrifuge tube, then 400 μL of MES (0.015 mol/L, pH 6.5) buffer was added. The centrifuge tube was treated by ultrasound at 200 W for 10 minutes. The supernatant was discarded after magnetic separation, and the aggregation was re-suspended in 400 μL of MES buffer. The 10 mg/mL of EDC solution (0,50, 100, 120, 140, 150, 160, 200 μL) was added for activating surface carboxyl from MPs, and the tube was shaken gently at room temperature for 30 minutes. After the MPs were washed for thrice, the activated MPs were re-suspended in 400 μL of MES solution that containing various concentrations of Tween-20 (0%, 0.02%, 0.04%, 0.06%, 0.08%) and antibody(20, 40, 60, 80, 100 μL). The mixture was shaken gently at room temperature for overnight and magnetic separation.Then, the aggregation was re-suspended in PBS (0.01 mol/L,pH 7.4) after washed three times by PBST (0.01 mol/L PBS containing 0.05% Tween-20, pH 7.4) and stored at 4 ℃.The absorbance of the initial and free antibody solution were measured at 260 nm and 280 nm. The concentration of antibody was assessed by the Lowry-Kalokar formula(1). And the coupling efficiency of antibody was calculated according to the formula (2). The particle size analyzer was used to analyse the size distribution of MPs and IMPs.

WhereArepresents the concentration of antibody after coupling;Brepresents the concentration of antibody without coupling.
1.3.3 Principle of the IMS-GNPs probe assay
Fig. 1 illustrated the testing process of IMS-GNPs probe assay for detectingC. sakazakiiin PIF.C. sakazakiiwas captured by IMPs that formed with carboxylic MPs and monoclonal antibodies. The complexes were separated from PIF by magnet. Then, DNA ofC. sakazakiiwere extracted and amplified by PCR. The amplicon was analysed via GNPs probe instead of traditional electrophoresis. The detection probe was formed with single strand DNA onto the surface of GNPs that could hybridize DNA amplicon ofC. sakazakii.The sandwich complexes were structured by amplicon, probe and GNPs, which maintained the property of color under the high salt solution. Under the condition that DNA from the surface of GNPs was insufficient, GNPs would accumulate within high salt and the color of solution would change from red to purple. If the GNPs probe hybridized with target DNA,the color induced by high salt aggregation would be avoided.So the observation of the naked eye could be achieved according to the discrimination of color.

Fig. 1 Schematic diagram of the IMS-GNP probe assay for the detection of C. sakazakii
1.3.4 Implementation of IMS-PCR assay
Briefly,C. sakazakii(ATCC 29544) was grown in NB media at 37 ℃ for 12 h and they were serially diluted to approximately 103CFU/mL with physiological saline(0.85%). Then, bacterial culture (400 μL) was transferred into a centrifuge tube and IMPs suspension (75 μL) were added.Subsequently, the tube was mixed gently at 37 ℃ for 60 min.After magnetic separation, the aggregation was washed three times with PBST (0.01 mol/L PBS containing 0.05% Tween-20,pH 7.4) and re-suspended in 100 μL of PBS solution(0.01 mol/L, pH 7.4) for the following PCR detection.Otherwise, the supernatant with unbound bacteria and bacterial culture suspension were appropriate diluted and counted onto TSA plate. The capture efficiency of the IMPs was calculated according to the formula (3).

WhereAis the total number of bacteria in samples/(CFU/mL);Bis the number of unbound bacteria to IMPs in the supernatant/(CFU/mL).
The DNA from IMPs-bacteria was extracted by a commercial DNA extraction kit. The PCR primers (Forward primer: 5’-CGG GTT ACG CAG GGT TGA-3’; Reverse primer: 5’-GCG GTT GCC AGT GAA TAA GAT-3’) were used in this system. PCR was performed in a total volume of 25 μL containing the following reagents: 2 μL of genomic DNA, 1 μL of each primer (10 μmol/L), 12.5 μL of 2 × PCR Master Mix, and sterile distilled water. The thermal cycling conditions were 94 ℃ for 3 min, 30 cycles of 94 ℃ for 30 s,61 ℃ for 45 s, 72 ℃ for 45 s and a final extension at 72 ℃for 5 min. Finally, the amplicon was analyzed by agarose gel(1%) electrophoresis. It was performed at 100 V for 30 min.1.3.5 Colorimetric assay of GNPs probe
After amplification, the amplicon was pre-heated at 95 ℃ for 5 min in water bath and the hybridization of GNPs probe and amplicon was performed in a total volume of 10 µL. The GNPs probe was annealed for selective hybridization to target DNA and color changes were monitored by the naked eye after the addition of MgSO4.
The proportion of amplicon with GNPs probe and concentration of MgSO4were optimized in colorimetric strategy. Gradient proportion of volume was carried out between PCR amplicon and GNPs probe. The total volumes were 10 μL and 9 groups were conducted. Their volume ratios were 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, respectively.The concentration (50, 100, 150, 250, 300, 350, 400,500 mmol/L) of MgSO4with 10 μL was added to the mixture of amplicon and GNPs probe at room temperature. The negative group was conducted by deionized water. The optimal volume and concentration were determined according to the difference of color between the positive and negative groups.
1.3.6 Specific and sensitive detection in pure culture
The specificity and sensitivity of the developed assay forC. sakazakiiwere measured in pure culture. All bacteria were adjusted to 108CFU/mL with sterile 0.85 g/100 mL physiological saline. Then, the IMPs and bacteria were mixed and verified by IMS-PCR assay. For negative control, the sterile deionized water served as amplified templates instead of nucleic acid.C. sakazakii(ATCC 29544) was serially diluted (from 105to 101CFU/mL) and used to test sensitivity of the IMS-PCR assay in pure culture.
1.3.7 Detection of artificially contaminated PIF withC. sakazakii
The PIF was purchased from supermarket of Harbin in China and used to certify the availability of the IMS-PCR and colorimetric assay. The 25 g of PIF sample was aseptically mixed with 225 mL of ultrapure water.C. sakazakii(ATCC 29544) suspensions of various concentrations were separately spiked with diluted PIF to achieve a final concentration from 106to 101CFU/g. These samples were detected to obtain sensitivity of the developed assay forC. sakazakiiin contaminated PIF without pre-enrichment. The change of color was observed by naked eye and the accuracy of the results was verified by ultraviolet scanning test.
An overnight culture ofC. sakazakii(ATCC 29544) was serially diluted to achieve a final concentration of 101CFU/g in reconstituted PIF. 400 μL of contaminated PIF sample was shaken (150 r/min) at 37 ℃ for different time at intervals of 3 h. Then, the 75 μL of prepared IMPs suspensions were added and the tube was mixed gently at 37 ℃ for 60 min.After magnetic separation, the aggregation was washed three times with PBST (0.01 mol/L PBS containing 0.05%Tween-20, pH 7.4) and re-suspended in 100 μL of PBS solution (0.01 mol/L, pH 7.4). The developed assay was implemented for detectingC. sakazakiiin contaminated PIF after pre-enrichment. The change of color was observed by naked eye and the accuracy of the results was verified by ultraviolet scanning test.
2 Results and Analysis
2.1 Preparation of IMPs
The activation of carboxylated MPs was an important step in the preparation of IMPs[23]. The carboxyl of MPs could be activated by EDC and structured the intermediate product that binding to the amino group on the surface of antibodies[24]. The effect of the addition of EDC for antibody coupling was shown in Fig. 2A. The efficiency of antibody coupling was 81.32% when 120 μL of EDC was added. If the addition of EDC was insufficient, the carboxyl groups of MPs would not fully form intermediates. Contrarily, the surface modification of MPs could be destroyed and the amount of antibody coupling would be affected due to the excess addition of EDC[25-26]. Compared with other researches,the efficiency of antibody coupling was applicable in this process[27]. So the consequences showed that the additive ratio of carboxylated MPs and EDC was 5:6. The fluidity of reaction system could be increased greatly with the addition of Tween-20 that had interfacial activation. Tween-20 could be adsorbed on the surface of MPs by Van der Waals force,increasing the energy barrier of reaccumulation of MPs,reducing their aggregation and sedimentation in liquid, and improving the stability of dispersion system of MPs[28]. From the Fig. 2B, the coupling efficiency could reach 81.87%when the volume of Tween-20 was 0.02% (V/V). But the efficiency of antibody coupling was decreased gradually with the increase of Tween-20. Because excess Tween-20 would generate foams and micelles, causing the restricted mobility of solution[29]. The appropriate addition of Tween-20 could be adsorbed on the surface of MPs to reduce its aggregation and the liquid could obtain better dispersity and mobility[30].It also increased the probability of contact between MPs and antibodies in the system[13]. Thus, the addition of 0.02%(V/V) Tween-20 was selected as the optimal volume. The amount of conjugated antibody was an important factor for the capture of bacteria[31]. As shown in Fig. 2C, 80 μL of antibody was the most applicable. In theory, the probability of contact between MPs and antibody could become larger as the increased addition of antibody[16]. Nevertheless, the carried amount of antibody by MPs could reach a limit when the addition was over 80 μL, causing waste of antibody and decline of coupling efficiency[12]. The result showed that the additive ratio of carboxylated MPs and antibody was 5:4.

Fig. 2 The optimization of EDC addition (A), Tween-20 addition (B)and antibody addition (C)
The size distribution profile, as shown in Fig. 3,represented typical batch of carboxylated MPs and IMPs with mean diameter of 538.8 nm and 1 970 nm. The IMPs was obtained through monoclonal antibody againstC. sakazakiiwith MPs, which caused the inconformity of size distribution[15]. The polydispersity index of carboxylated MPs and IMPs were 0.05 and 0.091, respectively. The two particles had a narrow size distribution (polydispersity index < 1), which indicated that they were both having good monodispersity[12]. Thus, the IMPs was suitable for the application.

Fig. 3 Size distribution of carboxylated MPs (A) and IMPs (B)
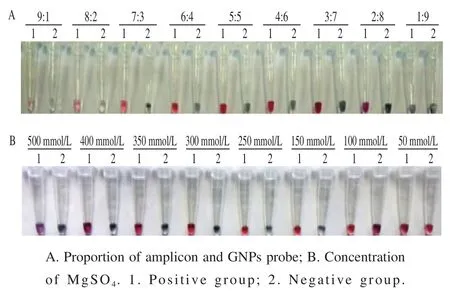
Fig. 4 Determination of optimal ratio between amplicon and GNPs probe and MgSO4 concentration
2.2 Determination of colorimetric assay of GNPs probe
As shown in Fig. 4A, there is a shallow red in the tube when PCR amplicon was added with a large proportion. The positive and negative groups were both close to colorless in high salt solution and it was not possible to accurately determine the presence of target amplicon. When the proportion of the GNPs probe was larger, there were a part of GNPs probe without hybridizing amplicon and the color of positive group was closer to the purple of negative group.The color difference of positive and negative group was the most obvious when the proportion of PCR amplicon and GNPs probe was 5:5. As shown in Fig. 4B, the bright red was revealed in positive group and the negative group was dark purple when the concentration of MgSO4was 300 mmol/L. Thus, the proportion of 5:5 between amplicon and GNPs probe and 300 mmol/L of MgSO4were selected as the optimal strategy in colorimetric assay.

Fig. 5 Sensitivity of the IMS-GNPs probe assay for C. sakazakii in contaminated PIF without pre-enrichment
2.3 Analytical specificity and sensitivity in pure culture
The specificity of IMS-PCR assay was tested with 22 bacterial strains. As shown in Table 1, the negative results were gotten from non-C. sakazakiistrains. The positive results were acquired only from theC. sakazakii,which indicated that this assay could be used to detectC. sakazakiirather thanCronobacterspp.. Compared with the chitosan-IMS method[13]and the probe-magnetic separation-PCR method[14], the more comprehensive species ofCronobacterspp. were covered in specificity test andC. sakazakiicould only be detected. The high specificity of this assay was attributed to monoclonal antibodies againstC. sakazakiifrom company.

Table 1 Specificity of IMS-PCR evaluated with selected bacterial strains

Table 2 Sensitivity of IMS-PCR for C. sakazakii in pure culture
The results of sensitivity were shown in Table 2. The capture efficiency of the IMPs was decreased significantly with the concentration ofC. sakazakiiincreasing from 103CFU/mL to 105CFU/mL. These indicated that bacteria and IMPs had a maximum binding rate[32]. The lowest concentration of capture could reach 102CFU/mL and the binding rate was 103CFU/mL at most in this system.The results of PCR were in correspond with the plate counting. Therefore, the developed assay was able to detect 102CFU/mL ofC. sakazakiiin pure culture. The detection limit of this assay was lower than that of the immunoliposomebased method in pure culture[16].
2.4 Validation of the IMS-GNPs probe assay with artificially contaminated PIF
The efficiency of the IMS-GNPs probe assay was needed to confirm in artificially contaminated PIF because PCR inhibitors were existed in food samples[15]. The results were shown in Fig. 5, the positive results of red were 103-106CFU/g through visual observation (Fig. 5A), because the hybridization of target DNA amplicon and GNPs probe formed a three-dimensional cross-linked complexes that avoided the aggregation of GNPs under high salt solution[18].Conversely, the color of solution showed purple due to the aggregation of GNPs[33]. As shown in Fig. 5B, the absorption peak of ultraviolet scanning was obvious when the concentration of bacteria was 103-106CFU/g in PIF.The specific absorption peak was disappeared due to the aggregation of GNPs under the less hybridization of target DNA[34]. The sample yielded PCR amplicon band when the cell concentration was as low as 103CFU/g without preenrichment (Fig. 5C). The colorimetric assay of GNPs probe was consistent with those of electrophoresis and ultraviolet scanning. Thus, the detection limit was as low as 103CFU/g in artificially contaminated PIF without pre-enrichment.A previous IMS report showed a lower detection limit(102CFU/g) in PIF[15], but they used only complex electrophoresis to verify the results of the DNA amplicon.

Fig. 6 Sensitivity of the IMS-GNPs probe assay for C. sakazakii of 101 CFU/g in contaminated PIF after pre-enrichment
Although the IMS method could reduce the inhibitory effect of complex food matrices, the IMS-PCR process could not achievement under the low concentration of bacteria. Hence, the pre-enrichment was crucial step for the improvement of sensitivity, which had been verified in some researches[16]. For example, a previous IMS-PCR reported that the LOD ofSalmonellain raw milk was less than 101CFU/mL after 5 h of pre-enrichment[12]. In this system,the positive results of GNPs probe could be observed after 3 h of pre-enrichment (Fig. 6A), which was consistent with the ultraviolet scanning spectrum (Fig. 6B) and the amplified band of electrophoresis (Fig. 6C). These results showed that the colorimetry was a valid assay to analyze DNA amplicon for alternative electrophoresis. So the detection limit of the developed assay was 101CFU/g forC. sakazakiiin PIF after 3 h of pre-enrichment. A previous IMS report showed a longer time of pre-enrichment (8 h) to reach 101CFU/g in artificially contaminated PIF[15]. Although the detection limit of the developed assay for PIF without pre-enrichment was higher than that of the reported IMS method, the time of preenrichment of the developed assay was shorter. Meanwhile,visual GNPs probe was used to replace electrophoresis in the developed assay of this research. The assay could get a benign LOD and shorter time of pre-enrichment compared with other previous researches[35]. Thus, this rapid and sensitive method could be applied to diagnoseC. sakazakiiin PIF.
3 Conclusions
In this study, a sensitive, rapid and specific assay was developed for the detection ofC. sakazakiiin artificially contaminated PIF. To our knowledge, this is the first report of IMS and visual GNPs hybridization probe assay for detectingC. sakazakiiin PIF. The IMPs were obtained by the MPs, EDC and monoclonal antibody againstC. sakazakiiwith additive ratio of 5:6:4 and 0.02% (V/V) Tween-20 was used as surfactant. This assay showed a strong specificity forC. sakazakii. The detection limits ofC. sakazakiiwere 102CFU/mL and 103CFU/g in pure culture and contaminated PIF without pre-enrichment, respectively. In addition,C. sakazakiicould be detected at 4.5 × 101CFU/g in contaminated PIF after 3 h of pre-enrichment. The visual results were consistent with electrophoresis and ultraviolet scanning spectrum. These results implied that the IMS-PCR assay was no effect to PCR inhibitors in actual samples and the colorimetric assay of GNPs probe was alternative for complicated electrophoresis. In the future, the developed assay will be helpful for the detection ofC. sakazakiiin PIF samples. And DNA amplicon of other foodborne pathogens will be confirmed by simple and visual GNPs hybridization probe assay.
