Displacement of shale gas confined in illite shale by flue gas:A molecular simulation study
2021-04-13TongTaoShitaoWangYixinQuDapengCao
Tong Tao,Shitao Wang,Yixin Qu,Dapeng Cao
State Key Laboratory of Organic-Inorganic Composites,Beijing University of Chemical Technology,Beijing 100029,China
Keywords:Displacement of shale gas Flue gas Illite shale Organic matter Molecular simulation
ABSTRACT The shale gas is an unconventional supplementary energy to traditional fossil energy,and is stored in layered rocks with low permeability and porosity,which leads to the difficulty for exploration of shale gas.Therefore,using CO2 gas to displace shale gas has become an important topic.In this work,we use molecular simulations to study the displacement of shale gas by flue gas rather than CO2 ,in which flue gas is modeled as a binary mixture of CO2 and N2 and the shale model is represented by inorganic Illite and organic methylnaphthalene.CH4 is used as a shale gas model.Compared to the pure CO2 ,flue gas is easily available and the cost of displacement by flue gas would become lower.Results indicate that the pore size of shale is an important factor in the process of displacing shale gas and simultaneously sequestrating flue gas,while the flue gas N2 -CO2 ratio shows a small effect on the process of CH4 displacement,because the high partial pressure of flue gas is the main driving force for displacement of shale gas.Moreover,the geological condition also has a significant effect on the process of CH4 displacement by flue gas.Therefore,we suggest that the burial depth of 1 km is suitable operation condition for shale gas displacement.It is expected that this work provides a useful guidance for exploitation of shale gas and sequestration of greenhouse gas.
1.Introduction
The demand for fossil fuels is increasing,due to the rapid development of modern industries and economies around the world[1,2].Simultaneously,the environmental issue and climate change caused by increased CO2emissions are another important concerns[3,4].Therefore,in the past few decades,the exploration of shale oil/gas has been increasingly implemented due to its advantages of huge reserves and environment friendliness [5,6].Previous reports indicate that shale gas has contributed more than 40 percent of natural gas production in the United States,and it is predicted that shale gas will reach a half in 15 years [7].As for China,the great efforts have been made toward the exploration of shale gas in past decades and a great progress has been achieved[8–10].
The exploitation of shale gas remains a major challenge because of the complex geological and geomorphological conditions of shales [11,12].Common methods for shale gas extraction include the fracturing and horizontal drilling techniques [11,13,14].However,these technologies are accompanied by threats to the environment and human health [15–20],which is a development dilemma.Recent studies show that using CO2to displace shale gas is a promising method,which can enhance the production of shale gas and sequestrate CO2simultaneously [21–23].Enhancing shale gas recovery by sequestrating CO2can not only break through the bottleneck of shale gas production but also can achieve the purpose of capturing and sequestrating greenhouse gases,which has aroused extensive interests from scientists [24–26].Generally,the geological conditions of shale gas reservoirs are at high temperature and high pressure,so it is very expensive to study adsorption and displacement of shale gas through experimental measurements.As a result,it is a good alternative to use computer simulation to study the process of CH4displacement by CO2in nanoporous media [27–30].Wang [31]used grand canonical Monte Carlo simulations(GCMC)and molecular dynamics(MD)to investigate the effects of pore size and water density on the competitive adsorption of CH4-CO2mixtures in the nanoscale pores modeled by Na-montmorillonite,and found that Namontmorillonite clay prefers to adsorb CO2than CH4,and the adsorption selectivity of CO2/CH4in shale decreases slightly with the increase of water content and then increases.Chong [32]explored the competitive adsorption and density distribution of CO2-CH4mixtures on the surface of illite clay under dry conditions and found that the illite has higher CO2adsorption selectivity over CH4.Pathak [26]performed MD simulations to study the interactions between kerogen with shale gas and CO2,and revealed that CO2displacement can enhance shale gas production.Liu [33]investigated the process of CH4displacement by injecting CO2in graphite slit pores under various conditions.All these investigations indicate that CO2displacement would significantly enhance production of shale gas due to its larger affinity to adsorbents than methane.
In fact,in the process of shale gas displacement,obtaining pure carbon dioxide source is quite expensive.Generally,before CO2is injected into the ground,it is necessary to purify or separate carbon dioxide from the flue gas and nearby power plants,or even to purchase carbon dioxide[34].Moreover,in the process of transporting CO2by pipeline,the surface of pipeline is easily corroded in moisture condition,which may also cause extra costs [35].Previous investigations indicate that the injecting pressure is an important factor for displacement of shale gas,and it is easier to displace shale gas under higher injecting pressure [36–38].Therefore,we believe that it is possible to use other gases to displace shale gas at high injection pressure.The best choice is to use flue gas,because the flue gas (which is a mixture of N2,CO2and a small amount of other gases) has the advantages of easily accessible,cheap and wide sources distribution [39–42].In fact,N2injected into shale can reduce the partial pressure and promote desorption of shale gas,and maintaining the high pressure of N2possibly increases the permeability by swelling the shale reservoirs [43].In theory,both nitrogen and carbon dioxide can improve the recovery of shale gas,so the flue gas (i.e.the mixture of the two gases)can be also considered as a candidate for the shale gas displacement.

Fig.1.Illustration of molecular model of the illite shale pore,which contains inorganic illite and organic matter of methylnaphthalene.
In this work,we use molecular simulation to systematically investigate the displacement behavior of shale gas by flue gas.Considering the composition of shale,a shale model consisting of inorganic and organic parts is first proposed.Then,the effects of pore size of shale and CO2–N2ratios in flue gas on the process of shale gas displacement by flue gas in shale model are explored,and the effect of different geological conditions on displacement of shale gas by flue gas is also discussed.Finally,some conclusions are drawn.
2.Computational Section
2.1.Models
Shale is typically consisted of clay mineral,organic matter,quartz,etc.Among them,organic matter is mainly complex polymer such as kerogen (about 10%).Quartz and clay minerals (including montmorillonite,illite and kaolinite) make up about 80%of shale content.Also,the proportion of quartz and clay minerals varies for different shales[44–46].Therefore,it is required to build a comprehensive shale model containing organic and inorganic matter to study the behavior of shale gas in shale reservoirs.By analyzing the samples in the Sichuan Basin of China,it is found that the illite is the main clay mineral in the shales[47,48].So the inorganic part of the shale model was represented by two pieces of illites,which is a common 2:1 clay mineral consisting of T-O-T three layers (T represents a tetrahedron composed of Si-O and O represents a octahedron composed of Al-O).In this study,the comp osition of the illite model is[49–51].In order to verify the reasonability of the illite model,we simulated adsorption isotherms of CH4in the modeled illite with 2 nm pore at T=313 and 328 K(Figs.S1 and S2 in Supporting Information).The results indicated that the methane adsorption isotherms were consistent with literature from Zhang et al.[50],which confirmed the accuracy of the pure illite model.The details on simulations were presented in Supporting Information.Generally,polycyclic aromatic hydrocarbons are considered to be a major organic component,particularly in shales[36].So,we used methylnaphthalene(C11H10)molecules to represent the organic matter in shale.In order to be consistent with the experimental shale sample organic content of 5.08%[52],fourteen C11H10molecules-adsorbed the illite slit pore was used as the shale model,as shown in Fig.1.
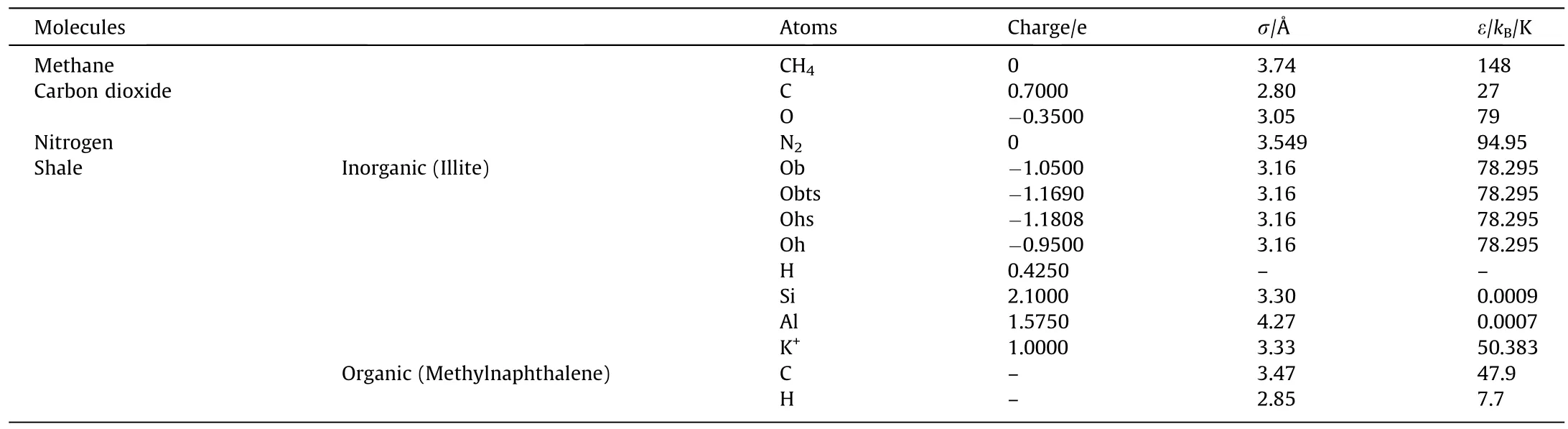
Table 1 Atomic charges and force field parameters for shale model and adsorbates of CH4 ,CO2 ,and N2 [46–52](1Å=0.1nm)

Fig.2.Adsorption isotherm of(a)Methane and(b)the flue gas in the shale pores of shale model at various pore sizes,where the temperature is 310 K and =15 MPa are fixed (i.e.the geological condition of 1 km).The injection pressure of flue gas increased from 0 to 75 MPa,and the ratio of N2 and CO2 in the flue gas is set to 5:1.(c) The amount of displaced methane,and the insert of (c) is the recovery rate of methane at Pflue gas =75 MPa.(d) The SAFG where the sequestration amounts of N2 and CO2 are separately indicated.

Fig.3.(a)The process of CH4 displacement by flue gas.The micro-scale snapshots of N2 -CO2 -CH4 ternary mixture within the shale pores,where the temperature is 310 K and the injection pressure of flue gas is(b)0 MPa,(c)15 MPa,(d)30 MPa,(e)50 MPa,(f)75 MPa. =15 MPa is fixed,the ratio of N2 and CO2 in the flue gas is set to 5:1 and the pore size is 25 Å.(g) The color scheme.
In this study,a simulation box (32.00Å × 35.81Å × c Å(1 Å=0.1 nm)),where the c is the distance in Z direction depending on the pore size)consisting of two layers of shale model was established,and the layer of shale model is divided into the organic and inorganic parts (Fig.1).And the inorganic part consists of 24 clay unit cells(6×4×1 supercell).Two illite sheets form a slit pore with an adjustable aperture,and then the adsorbed methylnaphthalene(C11H10) molecules constitute the organic part of the model.In the process,fourteen C11H10molecules were dispersed in the slitlike illite model by using molecular dynamics simulation with the canonical ensemble.Once the equilibrium state was reached,then we used the quenched structure to perform the following GCMC simulation,i.e.,during GCMC simulations,the two pieces of illites and the 14 methylnaphthalene molecules in the shale model were rigid and fixed.
The CLAYFF force field [53]was used to describe Illite and the Dreiding force field [54]was used to depict methylnaphthalene.Methane and nitrogen are represented by a united atom model(TraPPE,Koneshan) with a single site [55–58],while carbon dioxide was modeled as an rigid liner molecule,consisting of two 1.16 Å C-O bonds and a 180° O-C-O bond angle,and all three atoms are charged[59].All these force filed parameters and atomic charges are shown in Table 1.Since methane and nitrogen are nonpolar molecules and both single-site models are not charged,we only considered the Lennard-Jones(LJ)potential to calculate intermolecular interaction.For CO2molecules,we also considered the Coulombic potentials.The Lorentz-Berthelot mixing ruleswere used to calculate the LJ cross interactions.
2.2.Simulation methods
We adopted grand canonical Monte Carlo (GCMC) simulations to investigate the process of CH4displacement by flue gas in shale gas reservoirs.During the simulations,the μ(chemical potential),V(system volume) and T (temperature) are fixed while the number of adsorbed molecules and the energy of system are changeable.At the start of each simulation,the slit pore of the shale model was full of the pre-adsorbed CH4molecules.According to the pressure conditions at different geological depths,the CH4fractional pressure in mixtures of CO2-N2-CH4was determined.Then,the flue gas with a certain CO2-N2ratio was injected into the shale model.The amount of N2and CO2were determined by their respective partial pressures in the injected flue gas.When the system reaches equilibrium,we analyzed the composition of the mixed gas to calculate the amount of CO2,N2and CH4in the slit shale model.The flue gas injection pressure increased gradually (i.e.the flue gas fractional pressure) in the mixtures of CH4and flue gas,up to 75 MPa.In the computational packages MuSiC,the acceptanceprobability of movement is related to the component fugacity of gas bulk phase,thereby avoiding the transformation of chemical potential into pressure.In the simulation,the fugacity was calculated by using the Peng-Robinson equation of state (EOS) [60].In each GCMC simulation case,we used the first 10 million steps to reach the equilibration of system and second 10 million for sampling.

Fig.4.The density profiles along the z-direction,where the temperature is 310 K and the injection pressure of flue gas is(a)0 MPa,(b)30 MPa,(c)75 MPa. =15 MPa is fixed,and the ratio of N2 and CO2 in the flue gas is set to 5:1 and the pore size is 25 Å.
In the process of CH4displacement by flue gas,we defined the amount of displaced CH4as

where Ninitialand NPfluegasare the number of adsorbed methane in the slit pore when the flue gas fractional pressure in the mixed gas equals to 0 and Pfluegas,respectively.
To be consistent with the geological conditions of the shale reservoir,the T/P conditions are determined by a specific geological depth[61,62],which are presented in the Supporting Information.In this study,we used the computational packages Multipurpose Simulation Code (MuSiC) to perform GCMC simulations [63].
3.Results and Discussion
3.1.The effect of pore size on displacement of shale gas by flue gas
Considering the real case of flue gas,we first simulated the flue gas displacement with N2:CO2of 5:1,which is the common concentration of flue gas produced in industry.First,we study the process of CH4displacement by flue gas under 1 km geological conditions,which corresponds to temperature of 310 K and pressure of 15 MPa,and the temperature and partial pressure of CH4were set accordingly.Then,the flue gas was injected into the shale model and injection pressure of flue gas was gradually increased to 75 MPa.When the system reaches equilibrium,we calculated and analyzed the adsorption amount and the composition of the adsorbed mixed gas.
Considering the influence of pore size,we conducted a set of simulations in different shale models with varying pore size.The loading amount of methane (LAM) and the sequestration amount of flue gas (SAFG) in slit pores under varying flue gas injection pressure were shown in Fig.2a and b.The LAM is inversely related to flue gas injection pressure,which shows that CH4adsorbed in the slit pores are displaced by Flue Gas with simultaneously sequestrating N2– CO2in the shale model.In the adsorbed phase,CO2has stronger affinity toward the illite shale than CH4,and the partial CH4in the shale model is displaced by the higher pressure N2/CO2flue gas,and pulled out of the shale pores.

Fig.5.Adsorption isotherm of (a) methane and (b) the flue gas in the shale pores of shale model at various N2 -CO2 ratios of flue gas,where the temperature is 310 K and =15 MPa are fixed(i.e.the geological condition of 1 km).(c)The recovery rate of CH4 at Pflue gas =75 MPa.(d)The SAFG where the sequestration amounts of N2 and CO2
It is found that the pore size in the shale model is critical for the CH4displacement and the SAFG.Fig.2c and d show the amount of displaced CH4and the SAFG at the flue gas injection pressure of 75 MPa,in which the sequestration amounts of nitrogen and carbon dioxide are calculated separately.The pore sizes of shale gas reservoirs are divided into micropores (the diameter ≤20 Å),mesopores (20 Å
The inset of Fig.2c shows that the recovery rate of CH4decreases with an increase in pore size.The reason may be that the adsorption selectivity of CO2/CH4in shale is inversely proportional to the pore size.This is consistent with the findings of previous publications [24,31,64].Therefore,an increase in pore size will result in a slight decrease in the recovery rate of shale gas.However,for shale gas production,it is more important to consider the amount of displaced CH4rather than the recovery rate of shale gas.
Fig.3a shows the LAM and the SAFG in the shale pore under different flue gas injection pressures,where the sequestration of N2and CO2are shown separately,while Fig.3b–f show the snapshots of CO2-N2-CH4ternary mixture within the shale pores when flue gas injection pressure is 0,15,30,50 and 75 MPa,respectively,where the CH4partial pressure is fixed at 15 MPa and T=310 K corresponding to the condition of 1 km geological depth,and the pore size is 25 Å.In the initial state,when the injection pressure of the flue gas is 0 MPa,the shale pores are filled completely with CH4molecules(Fig.3b).With the injection of flue gas,CH4is displaced and the flue gas is adsorbed into the shale pore simultaneously(Fig.3c–f).These snapshots in Fig.3b–f show that the carbon dioxide prefers to accumulate on the surfaces of shale pores while nitrogen is distributed throughout the shale pore.
The snapshot provides a good qualitative description on the distribution of gases in adsorbed phase.To better understand the microstructure of adsorbed gases,Fig.4 shows the density profiles of gases in the shale pore of 25 Å at three pressures p=0,30,and 75 MPa.Interestingly,all the density profiles present a asymmetric distribution,which is attributed to the asymmetric distribution of K+ions covered on the different opposite surfaces in Fig.1.The K+ions near the surface occupy some adsorption sites,and lead to the asymmetric distribution of gas,especially for methane at p=0 MPa in Fig.4a.Similarly,the local density profiles of N2and CO2at p=30 and 75 MPa also present the asymmetric distribution due to the K+ion asymmetric presence.As shown in Fig.3,the local density of CO2near the surface is significantly higher than the one at the pore center,indicating that CO2prefers to be adsorbed on the surface,while N2is distributed in the whole pore owing to its high partial pressure.

Fig.6.Adsorption isotherm of (a) methane and (b) the flue gas in the shale pores of shale model at various N2 -CO2 ratios of flue gas,where the temperature is 310 K and =15 MPa are fixed(i.e.the geological condition of 1 km).(c)The recovery rate of CH4 at Pflue gas =75 MPa.(d)The SAFG where the sequestration amounts of N2 and CO2 are indicated separately.The pore size is 50 Å (mesopore).
3.2.The effect of flue gas N2 -CO2 ratio on displacement of shale gas by flue gas
To explore the effect of flue gas N2-CO2ratio on displacement of shale gas,we simulated the SAFG and the displacement of CH4in shale pores with varying N2-CO2ratios at 310 K and the CH4partial pressure of 15 MPa.The N2-CO2radios used in the simulations are 5:1,2:1,1:1,1:2 and 1:5.Since pores are mainly distributed in micro-scale and meso-scale,we considered two types of shale models,a micropore with pore size of 20 Å and a mesopore with pore size of 50 Å.The process of CH4displacement by flue gas in micropore (20 Å) is shown in Fig.5.Fig.5a shows the change of LAM with flue gas injection pressure at different flue gas N2-CO2ratios.Interestingly,the ratio of N2-CO2has little effect on displacement of CH4.However,as shown in the Fig.5b,the SAFG increases slightly when the N2-CO2ratio in the flue gas changes from 5:1 to 1:5.With the increase of CO2ratio in flue gas,the amount of sequestrated N2decreases while the amount of sequestrated CO2increases under the flue gas injection pressure of 75 MPa(Fig.5d),because of the high partial pressure of CO2.Moreover,the increment of adsorbed CO2is more than the decrement of N2,so the SAFG is increased in the case of N2:CO2=1:5 (Fig.5b).Almost no change happens in the percentage of displacement of methane (Fig.5c).
We also studied the effect of the N2-CO2ratio in mesopore of 50 Å on the displacement of shale gas.Fig.6 shows the displacement behavior in the mesopore shale and almost similar phenomenon with Fig.5 appears.In the mesopore,when the N2-CO2ratio in the flue gas varies from 5:1 to 1:5,the SAFG increases more significantly,because of the multi-layer adsorption of CO2.When the N2-CO2ratio in the flue gas is 1:1,that is,the concentration of N2and CO2in the injected flue gas is equal or very close.However,the sequestration amounts will be affected by the adsorption selectivity of the shale model.The shale has a stronger affinity for CO2than N2,so the sequestration amount of CO2is larger than N2(see Fig.5d)when the N2-CO2ratio equals to 1.On the contrary,the pore size has a negative effect on the selectivity of gas,therefore the sequestration amounts of N2and CO2in Fig.6d showed the opposite situation when the N2-CO2ratio equals to 1.
It is noted that the N2-CO2ratio in the flue gas has no significant effect on the displacement of shale gas (Figs.5a,c,6a,c),which is the solid evidence why we can use flue gas to displace shale gas.It is found that the high partial pressure of flue gas is the driving force for displacement process of shale gas.In fact,if we used other gases to displace shale gas,it still works well at high injection pressure.

Fig.7.Adsorption isotherm of(a)methane and(b)the flue gas in the shale pores of shale model at various geological conditions,where N2 -CO2 ratio of flue gas is 5:1 and the pore size is 25 Å.(c) The recovery rate of CH4 at Pflue gas =75 MPa.(d) The SAFG where the sequestration amounts of N2 and CO2 are separately indicated.
3.3.The effect of geological depth on displacement of shale gas by flue gas
In this section,the effects of T/P conditions corresponding to particular geological conditions (i.e.the burial depth) on the process of CH4displacement were also investigated.Fig.7a shows the relationship between LAM and flue gas injection pressures at different burial conditions,where the ratio of N2-CO2in flue gas is set to 5:1 and the pore size is 20 Å.It can be seen that burial conditions have an important impact on displacement of shale gas.As the flue gas was injected into the shale pore,the LAM decreases with the increase of flue gas injection pressure,where the decrement of LAM is the displaced shale gas.
Fig.7b shows the variation of SAFG with flue gas injection pressure at different burial depths.Apparently,the SAFG increases with the increase of the flue gas injection pressure,while the sequestration amount decreases as the geological depth increases at the same injection pressure.Moreover,the displacement of CH4decreases slightly as the geological depth increases,when the flue gas injection pressure is kept at 75 MPa,as shown in Fig.7c.Furthermore,the T/P conditions also show a larger influence on the sequestration amount of N2than the sequestration amount of CO2,as shown in Fig.7d.At the flue gas injection pressure p=75 MPa,both the SAFG and percentage of CH4displacement are the maximum at the 1 km geological depth (see Figs.7c and 7d).That is to say,the displacement efficiency of CH4by flue gas is the highest at the 1 km geological depth.Importantly,it is found that the flue gas partial pressure is the key factor for the displacement of CH4.The higher the flue gas partial pressure,the larger is the recovery rate of CH4and the SAFG.
4.Conclusions
In this study,we have performed molecular simulations to systematically investigate the displacement process of shale gas by flue gas in a shale model containing organic matter and clay mineral,and explore the effects of various factors (such as the pore size,the N2-CO2ratio of flue gas,and geological condition) on the displacement process.The main conclusions are drawn as follows.(1)The CH4can be displaced by flue gas efficiently and the flue gas can be sequestrated simultaneously.Both displacement amount of methane and SAFG increase significantly with the increase of pore sizes.(2)The flue gas N2-CO2ratio shows a small effect on process of methane displacement,because the high partial pressure of flue gas is the main driving force for displacement of shale gas.(3)The geological condition also has a significant effect on the process of CH4displacement by flue gas.With the increase of the burial depth,the recovery rate of CH4,the amount of displaced CH4and the SAFG decrease obviously.The above findings and the conclusions indicate that shale gas displacement by flue gas at a burial depth of 1 km is more suitable operation condition.It is expected that this work provides a useful guidance for exploitation of shale gas and sequestration of greenhouse gas.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2020.09.015.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Perspectives and challenges of hydrogen storage in solid-state hydrides
- Wet flue gas desulfurization performance of 330 MW coal-fired power unit based on computational fluid dynamics region identification of flow pattern and transfer process
- EMMS-based modeling of gas–solid generalized fluidization:Towards a unified phase diagram
- Using expansion units to improve CO2 absorption for natural gas purification-A study on the hydrodynamics and mass transfer
- Simulation and experimental study on the surface morphology and energy lost of the target material under non-overlapping impact of angular particles
