Perspectives and challenges of hydrogen storage in solid-state hydrides
2021-04-13ZhenChenZhongliangMaJieZhengXingguoLiEtsuoAkibaHaiWenLi
Zhen Chen,Zhongliang Ma,Jie Zheng,Xingguo Li,Etsuo Akiba,Hai-Wen Li,6,
1 International Research Center for Hydrogen Energy,Kyushu University,744 Motooka Nishi-ku,Fukuoka 819-0395,Japan
2 School of Chemistry and Chemical Engineering,Qilu University of Technology(Shandong Academy of Sciences),Jinan 250353,China
3 College of Materials Science and Engineering,Nanjing Tech University,Nanjing 211816,China
4 Beijing National Laboratory for Molecular Science,College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,China
5 International Institute for Carbon-Neutral Energy Research,Kyushu University,744 Motooka Nishi-ku,Fukuoka 819-0395,Japan
6 Platform of Inter/Transdisciplinary Energy Research,Kyushu University,744 Motooka Nishi-ku,Fukuoka 819-0395,Japan
Keywords:Hydrogen Hydrogen storage Hydride Hydrogen energy Renewable energy Environment
ABSTRACT Hydrogen has been widely considered as a clean energy carrier that bridges the energy producers and energy consumers in an efficient and safe way for a sustainable society.Hydrogen can be stored in a gas,liquid and solid states and each method has its unique advantage.Though compressed hydrogen and liquefied hydrogen are mature technologies for industrial applications,appropriate measures are necessary to deal with the issues at high pressure up to around 100 MPa and low temperature at around 20 K.Distinct from those technologies,storing hydrogen in solid-state hydrides can realize a more compact and much safer approach that does not require high hydrogen pressure and cryogenic temperature.In this review,we will provide an overview of the major material groups that are capable of absorbing and desorbing hydrogen reversibly.The main features on hydrogen storage properties of each material group are summarized,together with the discussion of the key issues and the guidance of materials design,aiming at providing insights for new material development as well as industrial applications.
1.Hydrogen as Clean Energy Carrier for Sustainable Development
Sustainable development of human being relies heavily on the stable supply of clean energies that are not harmful to the environment.In this regard,renewable energies(REs)such as solar and wind power have been considered as a promising solution and the installation of REs has been growing rapidly in the past decades [1].By 2017,the total amount of electricity generated by solar and wind reached 443,554 and 1,127,319 GW·h−1,respectively [2].The fluctuated nature with time of solar and wind energies as well as the limited acceptance capacity of the current power grid aggravate the curtailment of RE electricity,leading to the demand of effective energy storage and transport technologies.
Hydrogen,the ninth most abundant element on Earth's crust(1.4 g·kg−1) and the second most abundant element in Earth's sea (109 g·L−1)[3]has been widely accepted as clean energy carrier since hydrogen can be produced from water and water will be re-produced after power generation via hydrogen combustion or fuel cells[4].Compared to the known energy storage technologies such as battery,capacitor,flywheel,compressed air,and so on,hydrogen storage by means of pure hydrogen or hydrogen carriers(i.e.chemicals have a high hydrogen capacity)with high hydrogen density shows advantages at long term and large-scale energy storage[5–9].More importantly,due to the chemical nature and the high energy density of hydrogen or hydrogen carriers,hydrogen storage possesses huge potential for energy transport from energy producers to energy consumers,which is not possible by most of other ways[10–16].Moreover,hydrogen can be important chemical stocks like hydrogen reduction of iron ore[17]and syngas or hydrocarbon fuel production by hydrogen reduction of CO2[18].In brief,development of advanced hydrogen storage technologies is in great need for the transition to a sustainable low-carbon society.
2.Diverse Hydrogen Storage Technologies
Hydrogen can be stored in gaseous(compressed hydrogen),liquid(liquefied hydrogen,liquid hydrogen carriers)and solid(solid hydrides and nanoporous materials)states,as summarized in Fig.1.

Fig.1.Typical hydrogen storage methods.
Compressed high-pressure hydrogen is the most mature and convenient technology.Compression helps to improve the hydrogen density,but suffers from the non-ideal PVT properties of high-pressure hydrogen,resulting in much larger volume than that of ideal gas.The compressed hydrogen can be stored in high-pressure cylinders,which are classified as Types I,II III and IV[19].To withstand the same pressure,Type IV with polymeric liner fully wrapped with a fiber-resin composite is the lightest one among all the types.This offers the opportunity of type IV for onboard hydrogen storage(hydrogen density:5.7 wt%,40 kg·m−3) [20]for fuel cell vehicles (FCVs) such as MIRAI.Although Type IV cylinder has been used successfully in FCVs,further reduction of storage volume is still a big technological challenge for vehicle design.Furthermore,safety measure and cost reduction are also important factors for widespread use of high-pressure hydrogen in the coming hydrogen economy.
Hydrogen can also be stored in cryogenic temperature(21.2 K)as liquefied hydrogen in cryogenic tanks.The volume can be reduced to 1/800 compared to hydrogen gas at the standard condition.Due to the extremely low critical temperature(33 K),around 30%of the energy in the stored hydrogen is consumed for cooling and compression at the current Joule-Thompson cycle(Linde cycle)[4],which finally increases the supply cost of hydrogen.Integration of hydrogen liquefaction process with liquefied natural gas (LNG) [21],in which cold energy from the vaporization of LNG can be used to precool hydrogen,offers opportunity to improve the liquefaction efficiency.Moreover,development of magnetic cooling is another main stream in order to improve the liquefaction efficiency [22].Though liquid hydrogen containers include sophisticated thermal insulation systems,boil-off losses cannot be avoided due to the heat flow from the surroundings.Liquid vehicle storage for onboard application can be feasible in the case of a fairly continuous driving,in which boil-off loss can be neglected.Since the boil-off losses are proportional to the ratio of surface area to volume,liquid hydrogen possesses big advantage in largescale hydrogen storage/transportation over long distances.Aiming at transport liquid hydrogen in bulk from Australia to Japan,Kawasaki Heavy Industries has been promoting the project[23].
Liquid-state hydrogen carriers include liquid organic hydrides,ammonia,formic acid,methanol,and so on.Among them,liquid organic hydrides and ammonia does not release CO2during dehydrogenation,whereas formic acid and methanol possess high potential in the carbon recycling systems such as carbon dioxide capture,utilization and storage(CCUS)systems.Liquid organic hydrogen carrier(LOHC)[24–27]uses unsaturated organic compounds such as cycloalkanes and heterocycles to store hydrogen at room temperature.For example,toluene absorbs hydrogen to form methylcyclohaxane[28,29],which can return to toluene after releasing hydrogen for recycle use.LOHC like toluene is a component of gasoline,therefore,it can be transported using present chemical tankers and infrastructures,implying the advantage for large scale,long term and long distance hydrogen storage/transport under ambient temperature and pressure.There are several key issues expected to overcome for practical applications,such as tuning the de-/re-hydrogenation thermodynamics,designing efficient,low cost and robust catalyst for enhancing the de-/re-hydrogenation kinetics,conversion ratio and selectivity and separation of hydrogen from LOHC vapor[30].Ammonia(NH3)has been widely used as a precursor for fertilizer and nitrogenous compounds.The concept of using NH3as a hydrogen carrier has been discussed for more than 70 years because of high hydrogen density of 17.65 wt% and easy liquefaction under 8.88 bar(1 bar=105Pa)at 21°C[31].The globally massive production(approximately 170 million t⋅a–1)based on the famous Haber–Bosch process and distribution of NH3offers the advantage to use the current facilities to store/transport hydrogen in large scale.Since the current process consumes 2% of global energy and emits 3% of global CO2,new and green ammonia synthesis process is highly expected[32].Furthermore,due to the endothermic nature of dehydrogenation from NH3,the decomposition temperature is expected to decrease by designing effective catalysts.For instance,the most active metal catalyst Ru still requires a temperature above 873 K[33].In addition to the high dehydrogenation temperature,separation of hydrogen from NH3is also necessary because even 1 ppm NH3can significantly degrade the performance of polymer electrolyte fuel cell (PEFC) [34].Alternatively,NH3can be used directly as a fuel for direct combustion or ammonia fuel cell[35,36].
Hydrogen can be also stored in solid-state materials,which can be classified into two groups,i.e.physisorption materials with high surface area as well as interstitial and non-interstitial hydrides.Physisorption materials adsorb molecular hydrogen via van der Waals force,which is usually below 10 kJ·mol−1H2[37].Due to such small adsorption energy,hydrogen adsorption measurement is generally carried out at 77 K.An empirical rule(Chahine's rule)has been proposed [38],i.e.maximum 1.0 wt% of excess hydrogen can be adsorbed at liquid nitrogen temperature for every 500 m2·g−1of the BET surface area,which suggests that higher surface area would have larger hydrogen adsorption capacity.Thus far,a large number of high surface nanoporous materials have been investigated,mainly divided into carbon-based and metal organic frameworks(MOFs)-based groups[39].Among them,the excess hydrogen capacity can exceed 14 wt%maximum in NU-1501-Al with surface area 7310 m2·g−1,although only at 77 K[40].The volumetric capacity at 77 K and both gravimetric and volumetric capacities at room temperature need to be improved for practical applications.In contrast to the physical adsorption of molecular hydrogen on material surface,hydrogen forms metallic,covalent or ionic chemical bonding with neighbor elements in solid-state interstitial and non-interstitial hydrides.The hydrogen-related chemical bonding in interstitial and non-interstitial hydrides helps reduce the mean distance between hydrogen atoms,improving largely the volumetric capacity.Fig.2 shows the comparison of volumetric capacity in various hydrogen storage methods.For instance,1 kg of hydrogen has a volume of approximately 11,200 L at standard condition.The volume can be reduced to 76.9 L,43.5 L or 26.3 L when compressed up to 15 MPa,35 MPa or 70 MPa,and can be further reduced to 14.1 L at cryogenic temperature of 20 K with a state of liquefied hydrogen.In comparison with those of compressed hydrogen at high pressure and liquefied hydrogen at cryogenic temperature,hydrogen can be stored in much smaller volume in solidstate hydrides,i.e.9.6 L at LaNi5H6,9.1 L at MgH2and 8.2 L at Mg(BH4)2.More interestingly,the volume can be reduced to 6.4 L at NH4BH4though it is too unstable for reversible storage[41].Moreover,hydrogen storage in solid-state hydrides does not require high pressure and cryogenic temperature like compressed hydrogen and liquefied hydrogen,providing a safe and compact approach for hydrogen storage.This review,therefore,focuses on the state-of-the-art progresses of solidstate hydrides,in which the main features on hydrogen storage properties of each material group is depicted in detail,ac companied with the discussion on the key issues and the guidance of materials design,aiming at providing insights for new material development as well as industrial applications.
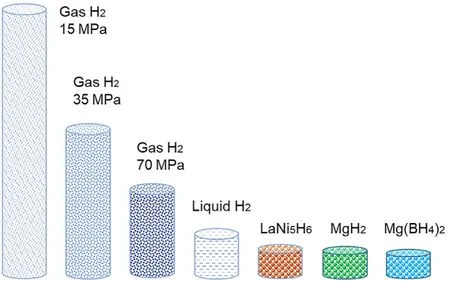
Fig.2.Comparison on the volumes for 1 kg H2 in various methods and materials.
3.Fundamental Principle and Requirements of Solid-State Hydrides for Hydrogen Storage
Hydrogen storage in solid-state hydrides provides a safe and compact method for hydrogen storage.In general,hydrogen absorption and desorption in metal/alloys proceeds via the following steps,as shown in Fig.3(a)and(c).Hydrogen molecules in the gas phase adsorb physically on the surface of metal/alloys and then dissociate into two hydrogen atoms.Subsequently,the hydrogen atoms on the surface diffuse into the bulk and occupy interstitial sites of the metal sublattice to form hydride.During the hydrogen desorption process,the hydrogen atoms in the interstitial sites diffuse out of the bulk to the surface of the metal/alloy,and then bond together to form hydrogen molecules.Although hydrogen has the smallest atomic radius of 25 pm,hydrogen absorption to form intestinal hydrides generally induces the sublattice expansion by 20%–30%.
The hydrogen storage properties are widely characterized by Pressure-Composition-Isotherm(PCI)curve,which includes three regions,as shown in Fig.3(b).The hydrogen absorption property with hydrogen pressure increment is measured at a given temperature.The hydrogen concentration CHincreases with hydrogen pressure increases up to the plateau pressure,appearing as a slope at the left side of plateau.At this region,hydrogen concentration is very small and hydrogen atoms dissolve into sublattice of metal/alloy to form solid solution phase(α-phase).At higher hydrogen pressure,the hydride phase(β-phase)starts to form.Hydrogen concentration increases at the constant pressure region(plateau region),in which the saturated α-phase converts to β-phase with the concentration increment.At this region,the Gibbs free energy is a function of temperature,as expressed below:

where P0=1.01325×105Pa.Based on this equation,the frequently used Van't Hoff equation can be obtained,as plotted in Fig.3(b).

The units of ΔH and ΔS based on Eq.(2) are kJ·mol−1and J·mol−1·K−1,respectively.The entropy change ΔS is assumed to be a constant of 130.858 J·mol−1·K−1at 298 K,which is equal to the loss of the standard entropy of hydrogen gas.It should be noticed that the assumed value of ΔS may change in some hydrides,e.g.ΔS is only 82 J·mol−1·K−1for the high-entropy alloy HfNbTiVZr[42].The temperature-dependent plateau pressure is the equilibrium dissociation pressure of the hydride(β-phase),thus being a measure of the stability of the hydride[43,44].In other words,thermodynamic stability of the hydride can be reduced by increasing the plateau pressure at a given temperature.Moreover,the width of the plateau region determines the maximum amount of reversibly absorbed and desorbed hydrogen.Hysteresis of absorption and desorption processes can be observed in many systems.After complete conversion to the hydride(β-phase),further dissolution of hydrogen occurs with the pressure increment,as shown in Fig.3(c).
Solid-state hydrides can be classified into two groups of interstitial hydrides and non-interstitial hydrides(also be denoted stoichiometric compounds) based on the nature of chemical bonding[45–47].Hydrogen forms metallic bond with metal atoms by occupying the interstitial spaces of tetrahedral and/or octahedral sites in sublattice of interstitial hydrides,whereas hydrogen forms covalent and/or ionic bond with neighbor elements in non-interstitial hydrides.Fig.4(a)shows the typical examples,such as interstitial hydrides of LaNi5H6,TiCr2H4,TiFeH2,Ti-V-Cr-H,and non-interstitial hydrides of NaAlH4,Mg(NH2)2,LiBH4,α-MgH2.Each type of solidstate hydrides has its advantage from the viewpoints of hydrogen density and reactivity of hydrogen absorption and desorption,as illustrated in Fig.4(b).Since interstitial hydrides mainly comprise transition metals and non-interstitial hydrides mainly comprise light elements,most of non-interstitial hydrides has higher hydrogen capacity,especially per mass,than that of interstitial hydrides.On the other hand,the metallic bond in the interstitial hydrides is much weaker than the covalent and/or ionic bond in non-interstitial hydrides,contributing to lower hydrogen release temperature for interstitial hydrides.Moreover,the hydrogen absorption and desorption reactions in the interstitial hydrides mainly depend on the dissociation and recombination on the surface and the diffusion in the bulk of hydrogen atoms,whereas the metallic structure does not change significantly.In contrast,hydrogen desorption proceeds via the decomposition of non-interstitial hydrides,and hydrogen absorption via recombination of decomposed product,requiring the diffusion of not only hydrogen atoms,but also the other constituent elements.Interstitial hydrides,therefore,generally show better reactivity,i.e.,lower reaction temperature,faster reaction kinetics and better cycling behavior,than that of non-interstitial hydrides.

Fig.3.Schematic illustration of(a)solid solution phase(α-phase),(b)Pressure-Composition-Isotherm(PCI)curve and the deduced van't Hoff plot,and(c)hydride phase(β-phase)in a typical metal[43].
Onboard hydrogen storage of FCVs is one of the most challenging applications for solid-state hydrides,because all the following requirements need to be satisfied at the same time:1) gravimetric and volumetric hydrogen density,2)working temperature and pressure,3) de-hydrogenation/re-hydrogenation kinetics,and 4) reversibility and durability.Toward the development of advanced hydrogen storage materials,significant progress has been achieved in the past half century,especially on the aspects of materials design and preparation,elucidation of mechanism on hydrogen absorption and desorption,and hydrogen storage property improvement for interstitial and non-interstitial hydrides [48].We will particularly focus on the representative achievements and design guidelines of interstitial and non-interstitial hydrides in the following.
4.Interstitial Hydrides
Intermetallic hydrides represent the hydrides of metals or alloys(also called hydrogen storage/absorbing alloys)comprised of transition metals,in which hydrogen occupies interstitial tetrahedral and/or octahedral sites via metallic bond.The pure metals,for example,Ti,tend to form stable element hydride such as TiH2,which releases hydrogen above 673 K.In contrast,the alloy that contains a non-hydride forming transition metal at ambient conditions shows reduced thermodynamic stability.For example,TiFeH2can release hydrogen at room temperature.Table 1 shows the typical interstitial hydrides,which are thehydrides of various kind of alloys comprised of a hydride easily forming metal A(ex.Ti,V,Zr,Y)and a non-hydride forming metal B(ex.Cr,Mn,Fe).There are several types of alloys for hydrogen storage,such as intermetallic compounds of AB5,AB3,A2B7,AB2,AB,A2B and solid solution alloys with body centered cubic(BCC)structure.

Table 1 Typical interstitial hydrides

Fig.4.(a)Typical examples and(b)comparison on main features of interstitial and non-interstitial hydrides.
4.1.AB5 -type alloys
AB5-type alloys(A:rare earth metal,B:d-transition metal)usually have a CaCu5-type structure(P6/mmm).The birth of hydrogen storage field is considered to be based on the investigation of magnetic properties of SmCo5in hydrogen in 1969[55].LaNi5is a typical example of AB5-type alloy.It forms the hydride of LaNi5H6via absorption of 1.4 wt%of hydrogen(hydrogen over metal ratio H/M=1).The hydrogen absorption and desorption occur easily at room temperature without activation treatment,attributed to the formation of metallic Niprecipitates with catalytic function by spontaneous surface segregation[56].The chemical composition of LaNi5has been modified as MmNi5-based alloys,in which La is replaced by Mm(Mischmetal as a mixture of rare earth metals)and Ni substituted by Mn,Co,Al,etc.,in order to improve the hydrogen storage properties for practical applications[57].Thanks for the good hydrogen storage performance at ambient conditions,abundant research achievements and mass production of MmNi5-based alloys,demonstration projects for stationary storage of renewable energies have been carrying out in many countries[15].In addition,MmNi5-based alloys have been successfully used as a negative electrode material of Nickel-Metal Hydride(Ni-MH)battery[58–60],which is the first commercialized example of solid-state hydrides and nowadays widely used in hybrid vehicles like Prius.
4.2.AB2 -type alloys
AB2-type alloys(A:Ti and Zr,B:Cr,Mn and V),are usually called Laves phase alloys,forming crystal structures of hexagonal C14,cubic C15 and hexagonal C36.In AB2alloys,Ti and Zr at the A side and Cr,Mn and V at the B side,can substitute each other,respectively,leading to several typical alloys,such as TiCr2,TiMn1.5,ZrCr2,ZrMn2and ZrV2.AB2alloys absorb more hydrogen than LaNi5,and the maximum hydrogen capacity reaches H/M=1.5,i.e.1.9 wt%of hydrogen in TiMn1.5.Compared to AB5alloys,AB2alloys have wider temperature range of hydrogen absorption and desorption.For instance,TiCr2-based alloy can absorb and desorb hydrogen at 200 K,suggesting the potential for heat pump applications[61].Ti-Mn alloy has a wide solid solution range,i.e.from TiMn2to TiMn1.5.The hydrogen absorption depends significantly on the chemical composition:the stoichiometric TiMn2does not absorb hydrogen,whereas TiMn1.5was found to have the maximum hydrogen capacity of 1.9 wt%among different composition ratios of Ti and Mn[62].
4.3.AB-type alloys
TiFe,as the representative example of AB-type alloys,was found in 1974[63].It absorbs and desorbs hydrogen at room temperature and the maximum hydrogen capacity reaches 1.9 wt%,similar as that of TiMn1.5.The rich abundance in the earth crust and the relatively low price of Ti and Fe are also important support for the extensive investigation of TiFe as hydrogen storage material.The hydrogen absorption reaction,however,suffers from the difficult activation,i.e.at 673 K above 3 MPa of hydrogen.The two-step absorption(two-step plateau),large hysteresis between the absorption and desorption plateaus,and the degradation with hydrogen absorption/desorption cycling are also key issues for practical applications.Great efforts including doping and substitution by other elements as well as severe plastic deformation have been devoted to tackle those issues[64,65].
4.4.AB3 -type alloys
AB3-type alloys RMg2Ni9(R=La,Ce,Pr,Nd,Sm and Gd)with(La,Mg)Ni3phase were firstly reported in 1997[66].The crystal structure is a stacking of a [RNi5(AB5)]subunit and a [MgNi2(AB2)]subunit along c axis.The hydrogen storage properties of the ternary alloys,La2MgNi9,La5Mg2Ni23and La3MgNi14,were reported in 2000.The maximum hydrogen capacity(H/M)of the La0.7Mg0.3Ni2.8Co0.5reaches 1.1,whereas 0.8 for MmNi4.0Mn0.3Al0.3Co0.4,at 333 K in the pressure range of 10−3MPa to 1.0 MPa.More importantly,a large discharge capacity of 410 mA·h·g−1was found in La0.7Mg0.3Ni2.8Co0.5,1.3 times larger than that of AB5-type alloys,proving the high potential as the negative electrode of Ni-MH battery [67].The new type Ni-MH battery using AB3-type alloys was commercialized in 2005.
4.5.BCC solid solution alloys
Distinct from the abovementioned ABxtype intermetallic compounds,solid solution alloys with body centered cubic(BCC)structures have been attracting great interest due to its high hydrogen capacity of H/M=2.V-based BCC solid solution alloys,such as V-Ti-Fe,V-Ti-Mn and V-Ti-Cr found in 1980s,absorb and desorb hydrogen via two steps(two-step plateau).Hydrogen absorbed in the first plateau is hard to release because of very low plateau pressure at room temperature.The maximum reversible hydrogen storage capacity can reach close to 3 wt%at room temperature.Many efforts has been devoted to solve the issues of difficult activation,sluggish kinetics,sloped plateau and degraded cycling capacity[68].Increasing the V content seems to be one of the effective ways to accelerate hydrogen absorption,improve the hydrogen storage capacity,and flatten the hydrogen desorption plateau[69].A concept of Laves phase related BCC solid solution was proposed to improve the hydrogen storage performance,as proved in the multiphase V-Ti-Mn alloy.Heat treatments to enhance the homogeneity of alloy composition are also important approach to improve hydrogen storage properties of BCC alloys.The plateau of V-Ti-Cr BCC phase depends largely on the lattice parameter and the appropriate lattice parameter for room temperature hydrogen storage was found in the range of 0.3020–0.3040 nm[70].
4.6.Rule of reversed stability
Miedema's semi-empirical rule of reversed stability is a well-known approach to predict the stability of a given intermetallic compoundhydrogen system[71].In this rule,the intermetallic compound ABnis supposed to form ABnH2mvia hydrogen absorption,and the formation of A-H and B-H bond is assumed in ABnH2m.Here,A denotes a hydride easily forming metal,and B indicates a non-hydride forming metal.The Miedema's rule can be expressed as below:

Generally,ΔH(AHm)is negative and has the largest absolute value,whereas ΔH(BnHm)is small and may be positive.For a given system,therefore,ΔH(AHm)+ΔH(BnHm) is an almost constant value.If ΔH(ABn)becomes more negative(i.e.more stable ABn),ΔH(ABnH2m)becomes more positive(i.e.more unstable ABnH2m).This means that the more stable an intermetallic compound(ABn),the less stable the corresponding hydride(ABn),and vice versa.In other words,stabilization of the intermetallic compound can help to reduce the thermodynamic stability of the corresponding hydride.
4.7.Unit cell volume of alloy as an indicator of thermodynamic stability of hydride

Fig.5.Correlation between the plateau pressure and the unit cell volume at(a)AB5 -type alloys[72,73]and(b)Ti-V-Mn BCC alloys[74].
Hydrogen atoms occupy the interstitial tetrahedral and/or octahedral sites in the lattice of interstitial hydrides.The thermodynamic stability is known to be mainly dominated by the crystal structure of the alloys.In this regard,unit cell volume of the alloys can serve as an indicator to tune the thermodynamic stability of the corresponding hydrides,based on the close relationship between the plateau pressure and the unit cell volume,as shown in Fig.5.In both the AB5-type and the Ti-V-Mn alloys,the plateau pressure of dehydrogenation decreases significantly with increasing the unit cell volume,though the slopes are different with the chemical compositions of the alloys [72–74].This means the larger a unit cell volume of the alloy,the lower a dehydrogenation plateau,and thus the more thermodynamically stable hydride.In other words,substitution with element with smaller radius can be a feasible approach to destabilize the thermodynamics of the hydride.
4.8.New interstitial hydrides with high entropy alloys
To date,large number of interstitial hydrides have been developed,whereas most of them are derivatives of the intermetallic compounds found in 1970s.Most of the interstitial hydrides are capable to absorb and desorb hydrogen at room temperature,but with limited hydrogen capacity of H/M ratio ≤2.Distinct from the intermetallic compounds collected in the phase diagram handbook,high entropy alloys(HEAs)comprised of five or more elements with atomic ratio of 5%–35%,emerged recently as a new class material for hydrogen storage because of the flexible chemical composition beyond the equilibrium phase diagrams.TiVZrNbHf with BCC structure,for example,can absorb hydrogen via a single step reaction with a maximum capacity of H/M=2.5,exceeding the value for the dihydride MH2of the constituting transition metals.Such high hydrogen capacity suggests hydrogen occupies not only tetrahedral but also the octahedral sites in the pseudo-fcc lattice[75].Another example of TiZrCrMnFeNi with C14 structure was recently found to absorb hydrogen with very fast kinetics,i.e.1.7 wt% of hydrogen can be absorbed within 90 s at room temperature[76].
5.Non-interstitial Hydrides
In the non-interstitial hydrides,hydrogen forms a covalent or ionic chemical bonding.It includes a number of materials groups,such as complex hydrides of metal alanates (M(AlH4)n),metal amides (M(NH2)n)and metal borohydrides(M(BH4)n),Mg-based hydrides,Al hydride (AlH3),ammonia borane (NH3BH3),and so on,as shown in Table 2.Most of the non-interstitial hydrides are comprised of light elements and thus possess high hydrogen capacity above 5 wt%and some reaches close to 20 wt%,which is several times large than that of the interstitial hydrides.Non-interstitial hydrides,therefore,are expected as high density hydrogen storage materials and great efforts have been devoted to the relevant research works.Nevertheless AlH3and NH3BH3possessing very high hydrogen capacity above 10 wt%,it is hard to be re-hydrogenated after dehydrogenation due to their very low thermodynamic stability [4,77,78],and thus considered to be used for oneway irreversible hydrogen source via hydrolysis.Here we will focus on the non-interstitial hydrides that hold the capability of reversible hydrogen storage,such as metal alanates,metal amides,metal borohydrides and Mg-based hydrides.
5.1.Metal alanate M(AlH4 )n
Metal alanates are generally expressed as M(AlH4)n.Here,n refers to the valence of a metal M(mainly from alkali and alkaline-earth metals)and H binds with Al via a covalent bond.NaAlH4,as a typical complex hydride,releases totally 5.5 wt%of hydrogen via the following steps at 483 K and 523 K,respectively[87].Different with interstitial hydrides,an intermediate phase Na3AlH6forms during the dehydrogenation and rehydrogenation process.

Table 2 Typical non-interstitial hydrides

The complex hydride was not considered as a hydrogen storage material because it cannot be rehydrogenated under moderate conditions.The reversible hydrogen storage of NaAlH4was firstly realized by the addition of Ti-based catalyst as reported in 1997[87].It triggered extensive investigations on the non-interstitial hydrides,especially for complex hydrides of metal alanates,metal amides and metal borohydrides.While Ti-based catalysts like TiCl3,are the most common choice in the reported works for NaAlH4,Sc-or Ce-based catalysts have been found to be more advantageous,especially at lower hydrogenation pressures[88,89].In practice,the achievable,long-term,reversible hydrogen storage capacity of Ti-doped NaAlH4is 3.5 wt%-4 wt%[90],2 to 3 times higher than that of the intermetallic hydrides.Although other types of alanates like LiAlH4,KAlH4,Mg(AlH4)2,Ca(AlH4)2and Y(AlH4)3have been extensively investigated,to the best of our knowledge,NaAlH4holds the most potential among the reported alanates from the viewpoint of practical application for hydrogen storage at close to room temperature[91,92].
5.2.Metal amide M(NH2 )n
Metal amide has the general form of M(NH2)n,where n is the valence of a metal M(mainly from alkali and alkaline-earth metals)and H forms covalent bond with N.M(NH2)nitself does not release hydrogen,it releases ammonia gas upon heating.Hydrogen absorption and desorption occur when making a composite with metal hydride M'Hm.Therefore,metal amide systems for hydrogen storage are always referred to the amide-hydride composite systems,i.e.M(NH2)n-M'Hm.Here M and M' can be the same metal or different metals.In 2002,LiNH2-LiH composite was firstly reported as a hydrogen storage material[93].It releases large amount of hydrogen as high as 10.4 wt%in total upon heating,i.e.5.2 wt%below 473 K and 5.2 wt%below 700 K,according to the following equations.
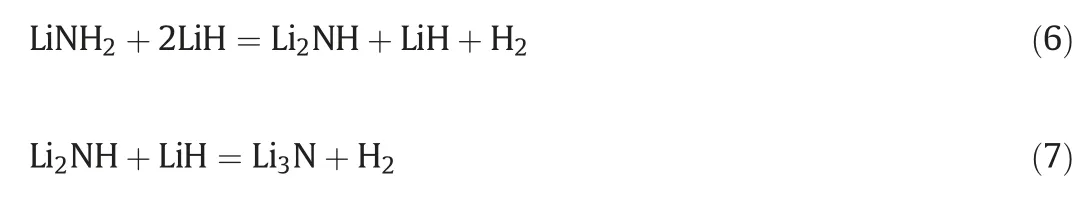
Similar to that of NaAlH4,an intermediate phase of Li2NH is formed during the dehydrogenation of LiNH2.Changing the chemical composition of the amide-hydride system is a feasible approach to tune the thermodynamics.In line with this,a number of composite systems have been investigated in order to reduce the dehydrogenation/rehydrogenation temperature to close to ambient temperature [94].Among the reported amide-hydride systems,Mg(NH2)2-2LiH two-component system possesses relatively high reversible hydrogen capacity of 5.6 wt%at around 423 K.2 Mg(NH2)2-3LiH-4LiBH4threecomponent system is capable to dehydrogenate and rehydrogenate at temperature below 373 K and the reversible hydrogen capacity is close to 6 wt%[95].Furthermore,a lot of efforts have been devoted to enhance the dehydrogenation/rehydrogenation kinetics,which are influenced by the interface reaction,nucleation/nuclei growth,and/or diffusion processes in the solid–solid reactions[94,96,97].A remarkable improvements were achieved by the addition of small amount of KH to the Mg(NH2)2-2LiH system[98,99].It is worth noting that the suppression of ammonia release during hydrogenation needs to be solved in order to avoid the catalyst poisoning of PEM fuel cells.
5.3.Metal borohydride M(BH4 )n
Metal borohydrides M(BH4)n(n is the valence of M)are comprised of a metal cation Mn+and a[BH4]-polyatomic anion,in which H forms a covalent with B.Large variation of M(BH4)n,like LiBH4,Mg(BH4)2and Ca(BH4)2,possessing hydrogen capacity higher than 10 wt%,have been widely investigated as potential candidates for onboard hydrogen storage[100].The dehydrogenation process of M(BH4)nis more complicated than those of M(AlH4)nand M(NH2)n-M'Hmsystems.Take the extensively investigated Mg(BH4)2as an example.Mg(BH4)2releases approximately 14.9 wt%of hydrogen via several endothermic steps accompanied with the formation of several intermediate compounds including MgB3H8,MgB5H9and MgB12H12,formation of which largely depends on the dehydrogenation conditions[101–103].The formation of[B12H12]2−with an icosahedral boron cage has been widely regarded as the main reason for the degradation of rehydrogenation.Rehydrogenation of[B12H12]2−to form[BH4]−requires high temperature to break the strong B-B bond in the icosahedral boron cage and high pressure hydrogen to stabilize[BH4]−at such high temperature[104].For instance,rehydrogenation for LiBH4was conducted at 873 K under 35 MPa of hydrogen[105].There are two approaches to suppress the formation of[B12H12]2−∶1)reducing dehydrogenation temperature to form smaller anion like[B3H8]2−[106,107],and 2)making a composite to alter the dehydrogenation process such as the well-known LiBH4-MgH2system[108,109].The high thermodynamic stability of M(BH4)ncan be reduced by two approaches:destabilization of M(BH4)nby a)choosing M with large electronegativity,and b)stabilization of dehydrogenation products by making reactive hydride composites,as shown in Fig.6(a).
5.4.Electronegativity as an indicator of thermodynamic stability
A good correlation between the dehydrogenation temperature Tdof M(BH4)nand the electronegativity of M has been found in 2006,as shown in Fig.6(b).Despite the Tdobtained from the temperature programmed desorption spectra,including both thermodynamic and kinetic parameters,the correlation is true that the thermodynamics of M(BH4)ninversely depend on the number of the electronegativity of M[110].This suggests that choosing the M with larger electronegativity can help to reduce the thermodynamics.Moreover,the combination of metals with different electronegativity can further tune the thermodynamics,as the example of Li-Zr-BH4system shown in Fig.6(b)[111].Note that some exception can be found in rare earth borohydrides RE(BH4)3,in which the decomposition temperature increases with the increased Pauling electronegativity[112].
5.5.Reactive hydride composite to tune thermodynamics
Reactive hydride composites,i.e.combining M(BH4)nwith elements,metal hydrides or complex hydrides,is another approach to reduce the thermodynamics through the formation of more stable dehydrogenation products.The representative example is the LiBH4-MgH2composite,as shown in Fig.6(c).A single phase of LiBH4releases 13.9 wt%of hydrogen up heating to 873 K and the dehydrogenation enthalpy is 74 kJ·mol−1H2.The dehydrogenation enthalpy can be significantly reduced to 40.5 kJ·mol−1H2by making a 2LiBH4+MgH2composite,through which the dehydrogenation product of B in LiBH4was stabilized as MgB2in the composite.It is worth emphasizing that the 2LiBH4+MgH2composite does not only show a reduced thermodynamics,but also exhibits outstanding reversibility at 673 K compared to the single phase of LiBH4[108,109].
5.6.Magnesium hydride MgH2

Fig.6.(a)Strategies for the reduction of dehydrogenation enthalpy of metal borohydrides M(BH4 )n ,(b)dehydrogenation temperature of M(BH4 )n as a function of electronegativity of M,and(c)comparison of dehydrogenation enthalpy between 2LiBH4 +MgH2 composite and the single phase of LiBH4 .
Magnesium is the eighth most abundant element in the earth's crust,and its hydride MgH2can absorb 7.6 wt%of hydrogen.These advantages have been attracting great interest on the investigation of MgH2for hydrogen storage[14,113,114].In principal,MgH2can be prepared by hydrogenation of Mg metal,whereas the hydrogenation suffers from the sluggish kinetics resulted from the oxide layer on the surface of Mg and the sluggish diffusion of hydrogen in MgH2.Therefore,hydrogenation of bulk Mg generally needs high temperature of approximately 673 K under hydrogen with the pressure above 3 MPa [115,116].MgH2can be expressed as Mg1.91+H0.26−,which is much weaker ionicity than the theoretical expectations[117].The high dehydrogenation enthalpy(75 kJ·mol−1H2)of MgH2requires a temperature above 573 K,which hinders the applications at ambient temperature.Several approaches have been reported for the destabilization of MgH2,including 1) forming an alloy with a hydride non-forming element,such as the well-known Mg2Ni[118];2)changing the reaction pathway by making composite like the typical MgH2/Si system[119];3)nano-engineering to reduce the crystal grain size below 2 nm,as predicted by theoretical calculation[120];4)Preparation of metastable alloys by high-pressure torsion or ball milling to weaken the local hydrogen related chemical bonding[121–123]Furthermore,great efforts have been devoted to enhance the hydrogenation/dehydrogenation kinetics.In addition to the nanosize effect[124–126],large varieties of additives have been extensively investigated [127–129].For instance,Nb2O5was reported to show significant catalytic effect,by which 4.5 wt%of hydrogen can be absorbed within 15 s at room temperature under 1 MPa of hydrogen[130,131].
6.Perspectives and Challenges
Solid-state interstitial and non-interstitial hydrides are important candidates for storing hydrogen in a compact and safe way.Most of the efforts,so far,have been devoted to the most challenging application of onboard hydrogen storage for light weight fuel cell vehicles.Although significantly progresses have been achieved in solid-state hydrides,including materials synthesis,mechanistic understanding and performance improvement,there is still no material that can meet all the requirements for the practical onboard application.On the other hand,some solid-state hydrides have been proven the feasibility on the applications for heavy vehicles like fuel cell buses,trucks or fork lifts,and for stationary energy storage,by the recent demonstration activities.Continuous and strong efforts on the further improvement of hydrogen storage performances of solid-state hydrides are necessary in order to meet with the fast growing demand for hydrogen storage.In this regard,the acceleration of the development of new materials and the breakthrough in solid-state hydrides are expected by the organic integration with machine learning and artificial intelligence technologies.In parallel,the production technologies of solid-state hydrides in the industrial scale need to be established,especially for the interstitial hydrides proven being feasible for practical applications.Moreover,appropriate combinations of different methods are also reasonable approaches to improve the efficiency,safety and economy of hydrogen storage technologies.In addition to the superior hydrogen storage function,solidstate hydrides can server other energy related functions,including a)mechanical function for quiet hydrogen compression and actuator[15,132–134],b)electrochemical function as electrode materials and electrolytesinall-solid-statebatteries[6,10,59,135–141],c)superconducting material[142,143],and 4)heat pump of heat storage and refrigeration[144–147],as summarized in Fig.7.

Fig.7.Multiple functions of solid-state hydrides.
Significant progresses have been achieved within the short history of half century in the field of solid-state hydrogen storage and the progresses have brought deep impact not only on the advancement of hydrogen storage technologies,but also on the widespread of Ni-MH batteries and the high efficiency hybrid vehicles.No doubt that the continuous efforts on the development of solid-state hydrides will bring further positive impact on the advancement of hydrogen storage technologies and the widespread use of hydrogen in global energy transitions.In the long run,the efforts will definitely contribute to enforce the energy security,improve the utility efficiency of renewable energies,enhance the environmental quality and finally realize a truly sustainable society.
Acknowledgements
The authors gratefully acknowledge the support from the JSPS KAKENHI(Grant Number 18H01738)and the Progress 100 program of Kyushu University.ZC is grateful to Qilu University of Technology(Shandong Academy of Sciences)for the financial support by the program of studying/visiting abroad(No.450404).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Wet flue gas desulfurization performance of 330 MW coal-fired power unit based on computational fluid dynamics region identification of flow pattern and transfer process
- EMMS-based modeling of gas–solid generalized fluidization:Towards a unified phase diagram
- Using expansion units to improve CO2 absorption for natural gas purification-A study on the hydrodynamics and mass transfer
- Simulation and experimental study on the surface morphology and energy lost of the target material under non-overlapping impact of angular particles
- Numerical optimization for blades of Intermig impeller in solid–liquid stirred tank
