Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
2021-04-13ChaoboSongJiapengZhangShuangLiShengbinYangEryiLuZhenhaoXiLianCenLingZhaoWeikangYuan
Chaobo Song,Jiapeng Zhang,Shuang Li,Shengbin Yang,Eryi Lu,*,Zhenhao Xi,*,Lian Cen,Ling Zhao,Weikang Yuan
1 State Key Laboratory of Chemical Engineering,School of Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
2 Department of Stomatology of Renji Hospital,School of Medicine,Shanghai Jiao Tong University,Shanghai 200127,China
3 Shanghai Key Laboratory of Orthopaedic Implant,Department of Orthopaedics Surgery,Shanghai Ninth People's Hospital,Shanghai Jiao Tong University School of Medicine,No.639,Zhizaoju Road,Shanghai 200011,China
Keywords:Scaffold Mesoporous bioactive glass Supercritical carbon dioxide Foaming Poly(lactic-co-glycolic acid)
ABSTRACT In this study,mesoporous bioactive glass particles(MBGs)are incorporated into poly(lactic-co-glycolic acid)(PLGA)to fabricate highly interconnected macroporous composite scaffolds with enhanced mechanical and biological properties via a developed supercritical carbon dioxide(scCO2 )foaming method.Scaffolds show favorable highly interconnected and macroporous structure through a high foaming pressure and long venting time foaming strategy.Specifically,scaffolds with porosity from 73%to 85%,pore size from 120 μm to 320 μm and interconnectivity of over 95%are controllably fabricated at MBG content from 0 wt%to 20 wt%.In comparison with neat PLGA scaffolds,composite scaffolds perform improved strength(up to 1.5 folds)and Young's modulus(up to 3 folds).The interconnected macroporous structure is beneficial to the ingrowth of cells.More importantly,composite scaffolds also provide a more promising microenvironment for cellular proliferation and adhesion with the release of bioactive ions.Hopefully,MBG/PLGA scaffolds developed by the green foaming strategy in this work show promising morphological,mechanical and biological features for tissue regeneration.
1.Introduction
Artificial scaffolds have attracted increasing attention as a promising alternative for tissue repair in clinic treatment,since the traditional autogenous grafts have disadvantages of the limited supply and extra severe pain at the donor site[1].To achieve desired tissue repair results,scaffolds are suggested to possess interconnected macroporous structure and excellent bioactivity for providing favorable microenvironment for the adhesion,proliferation and ingrowth of cell [2,3].Meanwhile,scaffolds as the temporary replacements of aimed tissue are also required to provide sufficient mechanical support[4].Herein,developing highly interconnected macroporous scaffolds with enhanced mechanical and biological properties is very meaningful and imperative,especially via a green and efficient fabrication method.
As scaffold materials,synthetic biodegradable polyesters,including poly(lactic-co-glycolic acid)(PLGA),polylactide(PLA),polyglycolide(PGA)and poly(ε-caprolactone)(PCL),have been approved by the US Food and Drug Administration (FDA) for clinical applications,and have lower price and steadier physicochemical properties in comparison with natural biodegradable polymers[5–8].Among these polyesters,PLGA possesses tunable mechanical properties,degradation period and hydrophilicity,owing to their controllable segment composition and molecular weight[9,10].Nonetheless,neat PLGA scaffolds are perceived to be lack of biological responses to promote the proliferation and adhesion of seed cells,which is believed to be vital to gain optimized tissue regeneration[11–14].To enhance the bioactivity of scaffolds,inorganic fillers (tricalcium phosphate (TCP),hydroxyaptite(HA)and bioactive glass(BG)[8–10,15]and organic filler(polysaccharide and protein)[16,17]have been incorporated into polyester matrix.More importantly,the addition of inorganic particles also promotes the mechanical properties of scaffolds[18,19].Meanwhile,compared with HA and TCP,BG has the potential to improve the regeneration of both hard and soft tissue,while HA and TCP are usually limited to hard tissue like bone and teeth[20,21].The release of bioactive ions from BG provides desirable microenvironment for a series of cellular activity including proliferation and adhesion [18,22,23].Especially,mesoporous bioactive glass(MBG)is a special type of BG with highly ordered mesoporous texture,large surface area and pore volume,leading to improved bioactivity compared with normal BG[2,19,24].
Besides the composite of MBG/PLGA is believed to be an outstanding material for tissue engineering,highly interconnected macroporous structure is also necessary for optimal tissue integration into the scaffolds after implantation[25,26].Numerous methods have been developed to fabricate porous structure,such as particle leaching[27,28],phase separation [29,30]and electrospinning [22,31–33].However,these methods have some limitations,such as the use of organic solvents,which may impose a post-processing step of purification to avoid cytotoxic events,and/or elevated temperature,which always results in accelerating polymer degradation[34].Supercritical carbon dioxide (scCO2) foaming,as a pore-forming method,has its unique advantages,including environmental friendliness,nonorganic reagents and mild processing conditions,since CO2is approved to be used in clinical applications,and possesses moderate supercritical points(critical temperature,31.1°C,and critical pressure,7.38 MPa)as well as plasticizing effects on polymer matrix to create mild processing conditions[35].Previously,a two-step method of foaming and particle leaching has been developed for the fabrication of inorganic filler modified PLGA scaffolds with favorable interconnected macropores,but the two-step processing and possible residual porogens always result in low efficiency and negative biological response,like inflammation[27,36].Research also has reported that plasticizers(ethanol and acetone)are able to increase the interconnectivity of polymer scaffolds,but this method might require a post-processing step of purification to avoid cytotoxic events [37,38].Recently Aurelio Salerno et al.have used eugenol as a bioactive plasticizer to fabricate interconnected PCL scaffolds[39],but the toxicity of eugenol is still unknown[40].Herein,developing a pure foaming strategy is still in need for the efficient and green fabrication of MBG/PLGA scaffolds with favorable interconnected macropores.
Regarding scCO2foaming,operating parameters(temperature,pressure and venting time)have significant effects on the pore structure of foams by adjusting the foaming process including pore nucleation,growth and solidification[35,41].Shakesheff,K.M.et al.have systemically studied the effects of foaming parameters on structure of PLGA foams,and reported that sufficient foaming pressure and venting time are vital for the formation of highly interconnected macroporous PLGA foams at mild foaming temperature [42–44].However,their studies are limited to neat PLGA.Studies have demonstrated that the addition of inorganic particles could act as nucleation agents and shift the pore nucleation mechanism from homogeneous nucleation to heterogeneous nucleation,as a result,altering morphology of scaffolds[45,46].Hence,in order to develop proper foaming strategy to form interconnected macroporous structure of MBG/PLGA scaffolds for tissue engineering,the effects of operating parameters and the addition of MBGs should be investigated in detail.
In this work,we proposed a green foaming method aided by scCO2to fabricate favorable highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties.First,to fabricate favorable scaffold morphology,foaming strategy was established and optimized by studying the effects of operation parameters and the content of MBGs on scaffolds morphology.Then,the potential of MBG/PLGA scaffolds for tissue engineering was elucidated through the measurement of mechanical properties and in vitro cellular responses.
2.Materials and Methods
2.1.Materials
PLGA(mole ratio of LA:GA=85:15,Mw=50 000,Tg=56°C)was purchased from Jinan Daigang Biomateiral Co.,Ltd.(China).Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Japan).PEOPPO-PEO (P123,Mw=5800) was purchased from Sigma-Aldrich(USA).Phosphate buffered saline(PBS),fetal bovine serum(FBS)and α-modified eagle medium (α-MEM) were purchased from HyClone(USA).Sprague–Dawley rats were supplied by Medical Center of Shanghai Jiaotong University (China).All other reagents were purchased from Sinopharm Chemical Reagent Co.,Ltd.(China).
2.2.Preparation of mesoporous bioactive glass particles(MBGs)
According to the research of Zhu and his coworkers,MBGs(molar ratio of Si/Ca/P=80/15/5)were synthesized as follows[47].Briefly,in a typical synthesis,4.0 g of non-ionic block copolymers EO20PO70EO20(P123,where EO is poly(ethylene oxide) and PO is poly(propylene oxide),6.7 g of tetraethyl orthosilicate,1.4 g of Ca(NO3)2·4H2O,0.73 g of triethyl phosphate and 1.0 ml of 0.5 mol·L−1HCl were dissolved in 60 ml of ethanol and stirred at about 25°C for 1 day.Then,the mixture was poured into a petri dish for an evaporation-induced self-assembly process at 60°C for over 2 days.Finally,the resulting dry gel was calcined at 700°C for 5 h to obtain MBGs.
2.3.Preparation of MBG/PLGA composite scaffolds
Prior to foaming,the composites of MBG/PLGA with different content of MBGs (0,5 wt%,10 wt%,15 wt% and 20 wt%) were obtained via a combination of ultrasonic shake and mechanical stir.A specific amount of MBGs (0.53,1.11,1.77,2.50 g) was added into 100 ml of CH2Cl2solutions and the resulting thin suspensions were ultrasonic shaken at 200 Hz for 15 min to disperse these particles homogeneously.After that,10 g of PLGA was introduced to the suspensions and the thick suspensions were stirred for 12 h to fully mix MBGs and PLGA.Then,the remaining CH2Cl2in the mixtures was removed to obtain composites by a two-day vacuum drying at 30°C.The batch foaming process was performed to prepare porous MBG/PLGA composite scaffolds,according to our previous research[48].Briefly,200 mg of composites were put into a custom-made Teflon mold,which possesses six wells with 12 mm in diameter and 15 mm in height.After that,the mold was placed horizontally at the bottom of a high-pressure vessel with a valid height of 80 mm and diameter of 50 mm.The vessel then was thread sealed and immersed into a super thermostat to reach set temperature(from 38°C to 50°C).After sweeping the high-pressure vessel with low-pressure CO2for three times,high pressure CO2was pumped into the vessel to reach set pressure(from 150 bar to 300 bar,1 bar=105Pa)by using a syringe pump(DZB-1A,Beijing Satellite Instrument Co.,China).The samples were saturated in CO2atmosphere at given pressure and temperature for 2 h.Thereafter,CO2was depressurized at a given venting time(from 2 min to 80 min)by controlling the opening of four needle valves(SS-4SKPS8MM,Swagelok,USA)for achieving a favorable depressurization control.Finally,after the vessel was carefully opened,the resulting porous MBG/PLGA scaffolds were obtained with 12 mm in diameter.
2.4.Morphology analysis
The morphology of MBGs was tested by transmission electron microscopy(TEM;JEM-3010,JEOL Ltd.,Japan).Furthermore,the mesoporous texture of MBGs was quantitatively measured by Adsorption-Micro Calorimetry Apparatus(Micromeritics Instrument,USA)at −195.7°C.Prior to measurements,MBGs were outgassed at 200°C in vacuum for 12 h.The Surface area was calculated by the Barrett–Emmett–Teller(BET)method.The pore volume and the pore diameter were calculated from the adsorption branches of the isotherms by using the Barrett–Joyner–Halanda(BJH)method.The distribution of MBGs within PLGA matrix was tested by an energy dispersive spectrometer (EDS;TEAMEDS,EDAX Inc.USA) using silicon and carbon element as the markers of MBGs and PLGA,respectively.The porous morphology of prepared scaffolds was examined by a scanning electron microscopy(SEM;JSM-6360LV,JEOL Ltd.,Japan)according to our previous research[48].Moreover,the pore size and porosity of prepared scaffolds were analyzed as described previously[49].In addition,the interconnectivity of scaffolds was analyzed by using a true volume and density measurement instrument(1200e,Quantachrome,USA)[15].The interconnectivity was calculated with following equations:

where m and ρfrepresent the mass and density of scaffold,which were obtained from porosity measurement[49].Vcrepresents the closed volume of scaffolds,which is given by the true volume and density measurement instrument.
2.5.Compressive properties
Prior to measurement,samples were cut into cylinders with 12 mm in diameter and 6 mm in height.The compressive tests were carried out on a universal testing machine(AG-2000A,Shimadzu,Japan)at a crosshead speed of 1 mm·min−1in the air under ambient conditions.The stress was calculated based on the apparent cross-sectional area of the samples and determined as a function of the strain.The yield compressive stress was recorded based on the stress–strain curves,and Young's modulus was calculated at 5%strains.Five samples were tested for each foaming trial.
2.6.In vitro release of bioactive ions
The in vitro ion release properties of the MBG/PLGA scaffolds were tested through immersing samples into phosphate buffer solutions(PBS,pH=7.4) at 37 °C.Samples were cut into thin plates with 12 mm in diameter and 2 mm in height,and then were immersed in 5 ml of PBS solution.At predetermined immersion times,samples were taken and the concentrations of calcium and silicon ions in the remaining solutions were simultaneously analyzed by inductively coupled plasma atomic emission spectrometry(725 ICP-OES,Agilent,USA).
2.7.Isolation and culture of rat bone marrow MSCs
Mesenchymal stem cells(MSCs)were isolated from rat bone marrow according to a normalized protocol.All experiments were approvedby the Animal Care and Use Committee of School of Medicine at Shanghai Jiaotong University.The proximal and distal epiphyses femora and tibiae of male adult Sprague–Dawley rats (180–200 g) were cut off and bone marrow tissue was flushed out with a normal culture medium(a-MEM supplemented with 10%FBS).Then,the resulting tissue was then placed in a culture dish in an incubator under a humidified atmosphere of 5%CO2in air at 37°C.Non-adherent hematopoietic cells were removed from the medium during medium changes,which were performed 7 days for the first time and every 3 days for the following ones.The cells underwent three passages and then were used for further experiments.

Table 1 Mesoporous texture of MBGs based on BET and BJH analyses
2.8.In vitro cellular response
A normalized operation was performed to seed MSCs onto foamed MBG/PLGA scaffolds[15].Prior to seeding,scaffold samples were also cut into thin plates with 12 mm in diameter and 2 mm in height to expose the internal porous structure,and then γ-sterilized at 25 k rad for 8 min.The sterilized samples were placed at the bottom of the 24-well plates,and then aliquots of cell suspensions were evenly seeded onto the surface of the scaffolds at a density of 2 × 104cells per sample.The medium was refreshed every 2 days.
2.8.1.CCK-8 testing
The proliferation of cells onto the samples was evaluated by typical CCK-8 assays on the 1st,3rd,and 5th days.On the predetermined days,the samples were incubated in a fresh cell culture medium containing 10 vol% CCK-8 solutions at 37 °C in 5% CO2for another 2 h.Then 200 μl of the reacted medium was measured at 450 nm by a microplate reader(SPECTRAmax 384,Molecular Devices,USA),and 5 samples were set for each group.
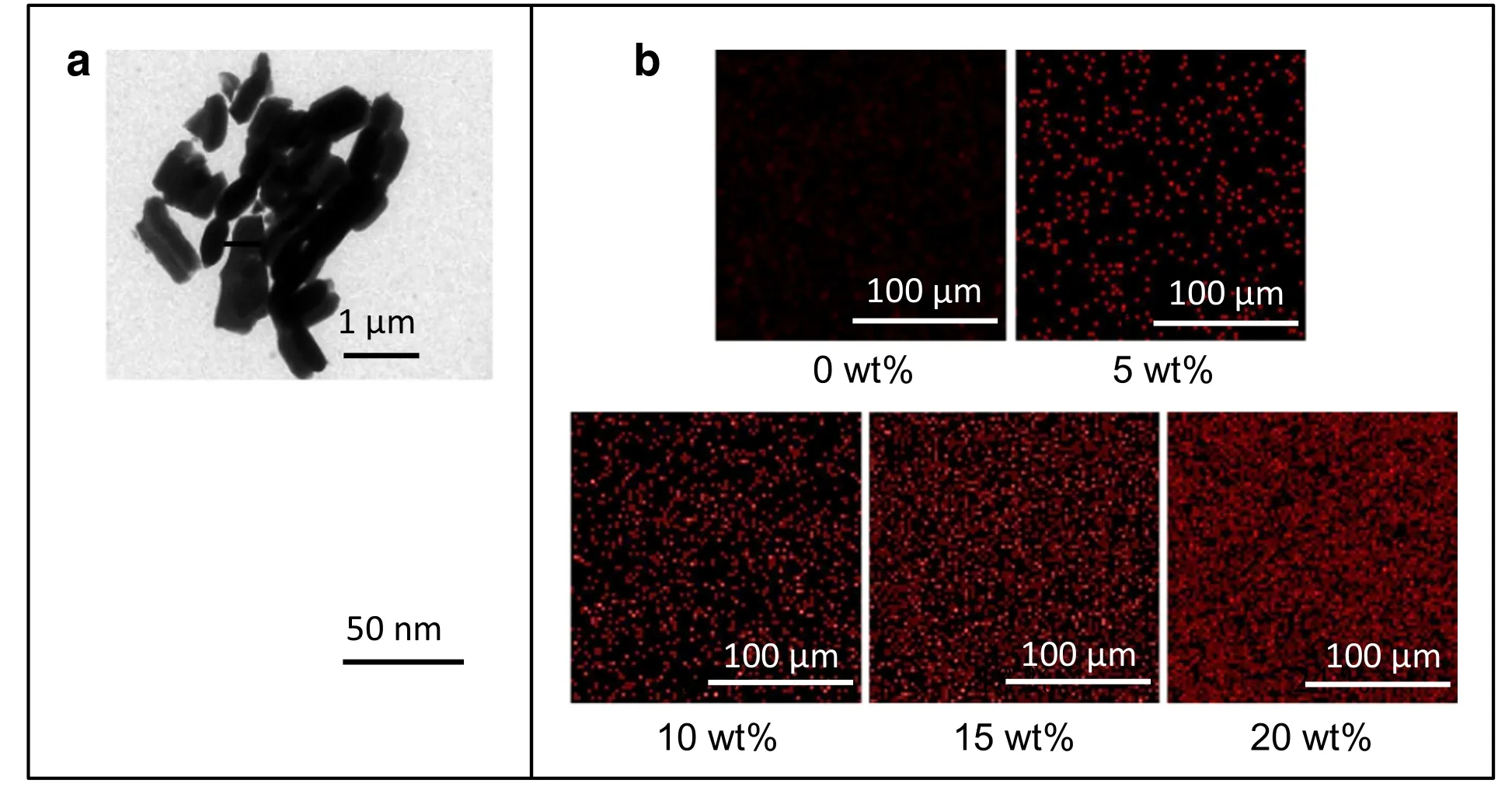
Fig.1.(a)TEM images of MBGs at low and high magnification.(b)EDS mapping of the silicon element distribution in the composites with content of MBGs increasing from 0 wt%to 20 wt%.(Red dots represent silicon atoms).

Fig.2.(a)SEM images,(b)interconnectivity,(c)pore size and(d)porosity of scaffolds with 5 wt%MBGs prepared at 45°C,venting time of 15 min under different pressures.
2.8.2.CLSM testing
The adhesion and distribution of MSCs onto the scaffolds was investigated by confocal laser scanning microscope (CLSM,Nikon A1R,Japan).In brief,the cell-scaffold samples were removed from the culture medium carefully and washed twice with PBS.Then the samples were fixed with 4%paraformaldehyde for 30 min at 4°C,following washed for 3 times with PBS and incubated within Phalloidine solution (5 μg·ml−1) for another 30 min.Hereafter,the samples were washed with PBS for 3 times again and incubated within DAPI(2 μg·ml−1)for 5 min.Finally,the samples were washed with PBS thoroughly and CLSM was used to further visualize the adhesion and 3D distribution of MSCs.
2.9.Statistical analysis
Data were expressed in the form of mean ± standard deviation(mean±SD).The statistical significance analysis between the groups was performed by the method of one-way student's t-test using SPSS13.0 software.The differences were considered significant at the level of p<0.05.
3.Results and Discussion
3.1.Composites of MBG/PLGA
First,the texture characteristics of prepared MBGs have been examined by TEM,BET and BJH analyses.As shown in Fig.1a,the fabricated MBGs show similar shape to short rods,and their length and width are mainly within 1 μm.In addition,the higher-magnification image demonstrates that the obvious mesoporous structure is well developed within the particles.Quantitatively,the BET and BJH analyses indicate that the mesopores inside MBGs is 4.10 nm in pore diameter,499 m2·g−1in surface area,and 0.359 cm3·g−1in pore volume,as listed in Table 1.After MBGs were incorporated into the PLGA matrix,EDS assays have been employed to discern the distribution of MBGs in MBG/PLGA composites by probing silicon element.Fig.1b shows that apparently more red dots(silicon element) can be observed in samples with higher content of MBGs,and basically,in all samples silicon element exhibits welldispersion,reflecting the uniform composites of MBGs and PLGA have been developed.
3.2.Formation of highly interconnected macroporous structure

Fig.3.(a)SEM images,(b)interconnectivity,(c)pore size and(d)porosity of scaffolds with 5 wt%MBGs prepared at 45°C,pressures of 250 bar using different venting time.
Then,the foaming parameters of supercritical carbon dioxide(scCO2) foaming method have been studied to optimize the pore structure for tissue engineering.Previously,Shakesheff and his coworkers have reported that foaming pressure and venting time have vital effects on the pore size and interconnectivity of PLGA scaffolds,by influencing the gas solubility and the degree of CO2supersaturation[42–44].Therefore,the effects of pressure (150 bar to 300 bar) and venting time (2,10,40 and 80 min) on the morphology of scaffolds with 5 wt%MBGs have been firstly tested at 45°C,and the corresponding SEM images and the quantitative analyses are shown in Figs.2&3.
As shown in Fig.2b,it is delightful that MBG/PLGA scaffolds exhibit highly interconnected structure (interconnectivity of over 95%) at high pressures of 250 bar and 300 bar.The high interconnectivity is believed to be a beneficial aspect for the applications as tissue scaffolds by providing essential translation channel for nutrition,wastes and cellular ingrowth.The solubility of CO2in foaming matrix is increased with the increase of pressure.On the one hand,the enhanced CO2solubility can enhance the plasticizing effects to reduce the melt strength of polymer matrix.On the other hand,the enhanced CO2solubility can also increase the degree of supersaturation during depressurization to enhance nucleation density,and the enhanced nucleation density can further reduce the thickness of pore-walls.Hence,the concerted effects of reduced pore-wall thickness and decreased melt strength ought to contribute to the high interconnectivity of samples prepared at the high pressure.Meanwhile,as shown in Fig.2d,an observable decrease of porosity with pressure increasing might be attributed to the increase of interconnectivity which enhances the diffusion of the CO2out the foaming matrix[50].In addition,scaffolds prepared at 200 bar obtain the smallest pore size,as shown in Fig.2c.Usually,the increase of foaming pressure can suppress the growth of pores to decrease the pore size by enhancing nucleation of pores [48].However,for scaffolds prepared at 250 bar and 300 bar,the strong rupture of porewalls by enhanced plasticizing effects and reduced pore-wall thickness might not only increase the interconnectivity to almost 100%,but also improve the coalescence of pores to increase the pore size.Previously,Zhang et al.have prepared PGA-TPP scaffolds by supercritical carbon dioxide foaming method,and the SEM images of interconnected samples with cracked and irregular pore-walls also show increased pore size[51].
The effects of venting time (2,10,40 and 80 min) on MBG/PLGA scaffolds morphology have been studied at the fixed foaming pressure 250 bar for maintaining sufficient interconnectivity.It can be seen from Fig.3b that all scaffolds at different venting time show high interconnectivity(about 95%).Notably,pore size observably increases with the venting time increasing,and the pore size of samples prepared at 40 min and 80 min is over 200 μm,which is favorable for cellular adhesion and proliferation by providing proper space(Fig.3c).With the increase of venting time,the depressurization gradient is reduced to decrease the CO2supersaturation degree,and consequently the pore nucleation is weakened[42,43].Therefore,at longer venting time,the amount of gas for pore growth is increased to obtain bigger pore size,since less CO2is consumed in nucleation [48].Furthermore,the enhanced pore growth should also contribute to the increasing porosity under longer venting time,as Fig.3d presents.It is delightful that,in our MBG/PLGA system,the employed long venting time(over 40 min)and high pressure(over 250 bar)foaming strategy is suitable for the fabrication of scaffolds with favorable highly interconnected macroporous morphology.

Fig.4.SEM images of scaffolds with MBG content of 0,5 wt%,10 wt%,15 wt%and 20 wt%prepared at fixed pressure of 250 bar,venting time of 40 min and various temperatures of 38,42,46,and 50°C,respectively.

Fig.5.(a)Pore size,(b)porosity and(c)interconnectivity of scaffolds with MBG content of 0,5 wt%,10 wt%,15 wt%and 20 wt%prepared at 250 bar,40 min and temperatures of 38,42,46,and 50°C,respectively.

Table 2 Scaffolds with proper and similar morphology for physicochemical and biologic properties analyses
3.3.Structure modulation of scaffolds with varied MBG content
Apart from pressure and venting time,the structure of scaffolds is also highly related to the content of incorporated inorganic particles.Since the incorporated inorganic particles can influence foaming process by working as nucleation agents [45,46].Furthermore,foaming temperature,as one of vital foaming parameters,usually has profound effects on the porous structure by influencing the solubility and diffusivity of CO2,as well as the melt strength of foaming matrix[44,52].Herein,to modulate the structure of scaffolds with varied content of MBGs,samples have been foamed at varied temperature from 38 to 50 °C,and fixed pressure and venting time of 250 bar and 40 min.SEM images and relevant morphological characteristics of scaffolds are presented in Figs.4&5.
Notably,all MBG/PLGA scaffolds exhibit a high interconnectivity(around 95%)and a relatively big pore size(over 100 μm),indicating the reliability of the developed foaming strategy for samples with all MBG content(Fig.5c).Besides that,both of pore size and porosity of composite scaffolds show a decreasing trend with increasing content of MBGs (Fig.5b).Usually,the increasing content of MBGs can enhance the nucleation of pores by providing increasing heterogeneous nucleation sites[45,46].Based on the competition between pore nucleation and pore growth,the growth of pores is suppressed at samples with higher MBG content,resulting in a decreased pore size and porosity.On the contrary,all scaffolds show slightly increasing porosity and pore size,when foaming temperature has increased from 38°C to 50°C(Figs.4&5).On the one hand,at higher foaming temperature,the melt strength of MBG/PLGA matrix is weakened,which provides less resistance to the foam expansion.On the other hand,with the increase of temperature,the diffusion of gas into the generated pores can also be improved to enhance the growth of pores[44,52].Therefore,the reduction of melt strength and the enhancement of gas diffusion combined result in the increasing pore size and porosity in samples prepared at higher temperature.Importantly,the increase of foaming temperature offers an effective method to reduce the negative effects of increasing MBG content on the pore size and porosity.
Apart from providing favorable interconnected macroporous structure,the mechanical and biological properties of scaffolds are also crucial for the outcome of tissue regeneration.Based on the developed foaming strategy,we have elaborately designed MBG/PLGA scaffolds with favorable morphology(about 200 μm in pore size,over 95%in interconnectivity,and about 80%in porosity)for the following physicochemical and biologic properties analyses,which is believed to provide suitable pore feature for tissue engineering[26,53].The structure characteristics of scaffolds are shown in Table 2.
3.4.Compressive properties of scaffolds
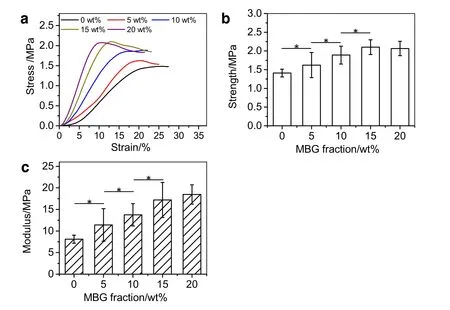
Fig.6.(a)The stress–strain curves,(b)yield strength and(c)Young's modulus of MBG/PLGA scaffolds(*p<0.05).

Fig.7.The concentration changes of released(a)calcium and(b)silicon ions from the MBG/PLGA scaffolds immersed in PBS solutions at 37°C for 5 weeks.

Fig.8.Proliferation of MSCs onto the MBG/PLGA scaffolds on the first 5 days(*p<0.05).
The scaffold as the temporary replacement of aimed tissue is required to provide proper mechanical supporting.To test the mechanical properties of MBG/PLGA scaffolds,a typical compressive testing has been carried out by a universal testing machine,and results are shown in Fig.6.It is clear that with the increase of MBG content from 0 wt%to 15 wt%,both the tensile strength and Young's modulus of the developed scaffolds exhibit a significant increase.Quantitatively,compared with the neat PLGA scaffold,the strength of samples with 15 wt%of MBGs increases by almost 50%(from(1.41±0.10)MPa to(2.10±0.19)MPa),and the modulus dramatically increases by about 120%(from(8.10±0.93)MPa to(17.19±4.05)MPa).The uniform distribution of MBGs into the PLGA matrix provides efficient reinforcement of the polymer matrix,contributing to the enhanced tensile strength and Young's modulus with increasing MBG content from 0 wt%to 15 wt%[54].Meanwhile,PLGA might infiltrate into the mesoporous channels of MBGs,which is also beneficial to the improvement of strength and modulus of the MBG/PLGA composites [19].However,once the content of MBGs is over 15 wt%,the enhancements of both strength and modulus become insignificant.The main reason might be that higher content of MBGs destroys the structural continuity of PLGA matrix,resulting in the strength of scaffolds with 20 wt%is slightly decreased[54].

Fig.9.The adhesion of MSCs onto the MBG/PLGA scaffolds on the 1st day.

3.5.Release properties of scaffolds
The release of bioactive ions has strong relation to the biological properties of scaffolds.Here,the accumulation of calcium and silicon ions in immersing solutions has been detected to test the ion release properties of developed scaffolds(Fig.7).The results demonstrate that samples with higher content of MBGs show a higher concentration of released calcium and silicon ions as expected.Meanwhile,it is also noticed that the concentration of calcium reaches its equilibrium value in the 2nd or 3rd week.One explanation is that the released calcium and phosphorus ions afterwards precipitate as Ca-phosphate[55].
3.6.In vitro cellular response of scaffolds
For optimal tissue regeneration,scaffolds are required to provide favorable microenvironment for adhesion,proliferation and ingrowth of seeded cells[25,26].Here,to evaluate the in vitro biological properties of prepared scaffolds,MSCs have been seeded onto the prepared samples,and their further proliferation,adhesion and distribution onto the scaffolds have also been examined.
First,the proliferation of MSCs onto the samples has been examined by CCK-8 assays,and the results are shown in Fig.8.It can be clearly found that the amount of MSCs onto all the samples shows continuing increase during the 5 days.The good proliferation of MSCs onto the prepared scaffolds demonstrates that the porous materials fabricated via the developed scCO2foaming strategy have no noticeable cytotoxicity.More importantly,compared with neat PLGA scaffold,MBG/PLGA scaffolds exhibit improved proliferation capability,especially for scaffolds with 15 wt%MBGs.The enhanced proliferation is attributed to the release of bioactivity ions,agreeing with reported results that the ionic dissolution products of MBGs can up-regulate gene expression related to proliferation[56].
Then,the adhesion of MSCs onto the samples has been studied by CLSM on the first day of seeding.As shown in Fig.9,MSCs onto neat PLGA scaffolds present less-spread f-actin filaments,while MSCs adhered onto MBG/PLGA scaffolds show extended filopodia-like structure.Especially,cells onto scaffolds with 15 wt%and 20 wt%exhibit a more organized polymerical cytoskeleton.The above results demonstrate that the incorporation of MBGs provides scaffolds with a favorable environment for cell adhesion,and the enhanced cellular adhesion might also be ascribed to the stimulation of released bioactive ions[2].
Further,the 3D distribution of MSCs onto the samples has been also tested by CLSM on the fifth day,shown in Fig.10.It is delightful that MSCs shows observable ingrowth onto all prepared scaffolds,indicating that the well-designed highly interconnected macroporous structure provides essential channels and pore size for the migration of cells into scaffolds.In addition,the ingrowth of cells indicates a sufficient use of scaffold,which is a beneficial aspect for achievement of integral tissue regeneration.
4.Conclusions
In summary,we have successfully fabricated highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via a green and effective scCO2foaming strategy.The high pressure and long venting foaming method is clarified to be suitable for obtaining interconnected macropores,where the increase of pressure is beneficial to generate interconnected structure via enhanced plasticizing effects of CO2,and extended venting time favors the growth of pores to big pore size by improving pore growth.Furthermore,the incorporation of MBGs enhances the pore nucleation,resulting in reduced porosity and pore size of scaffolds.To effectively compensate the negative effects of MBGs,foaming temperature is slightly increased.Meanwhile,the improvement of the strength and stiffness are confirmed with the addition of MBGs.The addition of MBGs shows positive effects on the cellular response,including the proliferation and adhesion due to the release of calcium and silicon ions.In addition,the highly interconnected macroporous structure of prepared scaffolds provides essential channels and pore size for ingrowth of cells.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors appreciate the experimental support and suggestion from Department of Stomatology of Renji Hospital,and the discussion with Dr.Xun P from Flinders Institute for NanoScale Science&Technology at Flinders University.The authors are grateful to the National Natural Science Foundation of China(Grant No.21676083),the Fundamental Research Funds for the Central Universities and 111 Project(Grant No.B20031).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation and properties of a low-cost porous ceramic support from low-grade palygorskite clay and silicon-carbide with vanadium pentoxide additives
- Perspectives and challenges of hydrogen storage in solid-state hydrides
- Wet flue gas desulfurization performance of 330 MW coal-fired power unit based on computational fluid dynamics region identification of flow pattern and transfer process
- EMMS-based modeling of gas–solid generalized fluidization:Towards a unified phase diagram
- Using expansion units to improve CO2 absorption for natural gas purification-A study on the hydrodynamics and mass transfer
- Simulation and experimental study on the surface morphology and energy lost of the target material under non-overlapping impact of angular particles
