Interaction of tetradecyltrimethylammonium bromide with bovine serum albumin in different compositions:Effect of temperatures and electrolytes/urea
2021-04-13JoynalAbedinShamimMahbubMohammadMajiburRahmanAnamulHoqueDileepKumarJavedMasoodKhanAhmedElSherbeeny
Joynal Abedin,Shamim Mahbub,Mohammad Majibur Rahman,Anamul Hoque,Dileep Kumar,Javed Masood Khan,Ahmed M.El-Sherbeeny
1 Department of Chemistry,Jahangirnagar University,Savar,Dhaka 1342,Bangladesh
2 Department of Chemistry&Physics,Gono Bishwabidyalay,Savar,Dhaka 1344,Bangladesh
3 Department of Environmental Sciences,Jahangirnagar University,Savar,Dhaka 1342,Bangladesh
4 Division of Computational Physics,Institute for Computational Science,Ton Duc Thang University,Ho Chi Minh City,Viet Nam
5 Faculty of Applied Sciences,Ton Duc Thang University,Ho Chi Minh City,Viet Nam
6 Department of Food Science and Nutrition,Faculty of Food and Agricultural Sciences,King Saud University,2460,Riyadh 11451,Saudi Arabia
7 Industrial Engineering Department,College of Engineering,King Saud University,P.O.Box 800,Riyadh 11421,Saudi Arabia
Keywords:TTAB BSA Conductivity Electrolyte Exothermic interaction
ABSTRACT Herein,the interaction of a protein,bovine serum albumin(BSA),with tetradecyltrimethylammonium bromide(TTAB,a cationic surfactant),has been investigated using the conductivity measurement technique in pure water and some sodium salts(NaCl,Na2 SO4, Na2 CO3 ,and Na3 PO4 )solutions at temperature range of 295.15–320.15 K.Results reveal that,in the plot of specific conductivity versus the concentration of TTAB,only a single critical micelle concentration(cmc)was found for the TTAB+BSA mixed system in all solvents media studied.The addition of BSA in aqueous TTAB solution,the value of cmc undergoes a change from its pure form,which indicates the presence of strong interaction operating between the BSA and TTAB molecules.In aqueous system,the cmc values of the TTAB+BSA mixtures are obtained higher compared to the values found for single TTAB surfactant.However,the addition of salt decreases the cmc value of mixed TTAB+BSA system.The values of cmc of the BSA+TTAB mixed system at 310.15 K and 1.00 mmol·kg−1 ionic strength of salt followed the order:cmcNa2CO3 >cmcNa3PO4 >cmcNaCl >cmcNa2SO4 .The cmc values of TTAB+BSA mixture were found to be lowered in urea solution within the concentrations studied.The values of degree of dissociation(α)and fraction of counter ion binding(β)were found to be dependent on additives and temperature.The free energy of micellization(ΔGm o)is negative for all the systems,which manifests that the micellization phenomenon is energetically spontaneous.The enhancement of the negative value of ΔGm o in aqueous salt solutions reveals an increase of spontaneity of the TTAB+BSA micellization process.The values of ΔGm o also reveal that the spontaneity of micelle formation is enhanced at higher temperatures in all media studied.The values of free energy of transfer(ΔGm o,t )were also determined for numerous solvent media used in the present study and described with appropriate reasoning.
1.Introduction
The monitoring on the interaction of surfactants with solutes,such as drugs,polymers,proteins,amino acids,etc.,has received a greater attention by the chemists,biologists,pharmacists,and technologists with wider ranges of applications[1–4].The surfactants are amphiphilic molecules which are extensively used in numerous formulations in applied and technological fields.In these latter cases,the applications of such molecules fall in the categories of solubilizing enhancer,emulsifying and stabilizing agents,drug carriers and many more.Due to a spontaneous self-aggregation nature of these molecules in solution,their solution properties undergo a drastic change after aggregate/micelle formation.The initiation of the aggregate formation takes place within a very narrow concentration of amphiphile species,which is identified as the critical micelle concentration(cmc)[5–8].Usually,the surfactants act as supportive recipients in the drug transportation phenomenon.The solubilities,along with the bioavailability characteristics of the hydrophobic drug,have been developed most likely due to the nature of solubilization process of the drug in the core of micelles,which,thereby,diminishes the degradative and toxic properties of drug molecules.The cationic amphiphiles are extensively employed as anti-microbial agents in the formulation of different disinfectants,sanitizers,and fragrances,suchasbeautifyingemulsions[9,10].The tetradecyltrimethylammonium bromide(TTAB,Scheme 1),one of the common cationic surfactants,has long been used in capillary electrophoresis to separate acid derived from anionic species [11].TTAB is also used as model surfactant in the study of surfactant-solute(drug/protein/polymer)interactions in different chemical industries and research laboratories.TTAB has the capability to prohibit the growth of cancer cell[12].TTAB also plays significant role to stabilize the nanoparticles.The insertion of additives to surfactant micelles would alter the physicochemical properties of the assembly,such as the formation of critical micelle concentration,the degree of counter ion binding and thermal reaction,and the clouding behavior of surface-active substances[10,13,14].
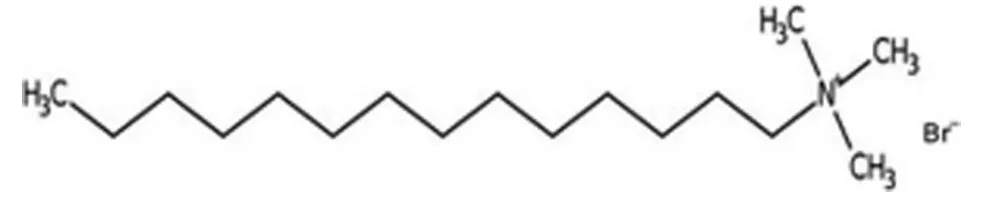
Scheme 1.Structure of TTAB.
The bovine serum albumin(BSA),a protein mainly derived from bovine plasma,is enriched with amino acids,along with certain sulfur compounds containing the–S-S–linkage and–SH functional groups.It is also familiar for its biopolymeric activity.The BSA possesses attractive applications in medicine,biology,pharmaceutical industry,as well as in paper mills and cellulosic films[15,16].The BSA contains both positively(such as,lysine and histidine)and negatively(such as,glutamic and aspartic acids)charged amino acid residues in its skeletal structure,which help it to stay attached with the charged surface[17].For its extraordinary adsorptive power on both hydrophobic and hydrophilic surfaces,the BSA is generally utilized as the blocking agent in numerous bioanalytical assays[18–20].The adsorptive surface of BSA allows it to hold hydrophilic species quite strongly and the subsequent desorption from the adsorbed surface is not facile even with a wash by phosphate-buffered saline(PBS),one molar NaCl,and sodium dodecyl sulfate(SDS)solutions[21].The BSA can also bind other substrates,like fatty acids,metals,amino acids,and drugs.Thus,the BSA molecules possess the ability to perform a substantial amount of tasks to transport,along with the expulsion of wider ranges of different endogenous and exogenous compounds,in the body fluid[22].Proteins are mostly used in the stabilization and enhancing the amphiphilic properties of common detergents [23].Also,the idea of protein-surfactant interaction is applied in various sectors of industrial and pharmaceutical studies[24].Surfactants are entered in the human body as these are widely used in the drug formulations.So there is a possibility of interaction between albumin and surfactants in the body fluids.It is reported that about 76%sequence homology exists between human serum albumin(HSA)and BSA[25].It was described that anionic species could bind by the electrostatic interaction in the subdomain-IIA hole of BSA whereas that in HSA takes place by both electrostatic and nonelectrostatic forces[26].There is also probability of binding interaction of different cationic species with BSA/HSA through similar or dissimilar binding modes.So,a thorough study involving BSA and cationic species in various media similar to biological fluids is indispensable to have knowledge on the physico-chemical properties of the resultant mixed systems.Such model study might also help in generating idea for the effect of BSA+other additives on the micellar properties of surfactants.
Although a substantial amount of work is available in the literature on the protein-surfactant interactions,it is still important to further explore the area involving the BSA+TTAB mixtures and a diversified range of additives.There are numerous methods for calculating the critical micelle concentration(cmc),like the conductivity measurement,tensiometric measurement,fluorescence spectroscopy,SANS and NMR techniques[27–29].But the conductivity method is very simple,most common,and reliable technique compared to other approaches for studying the micellization behavior of ionic surfactant molecules.In the previous studies,the aggregation of BSA,with CPC/CDMEAB in salt medium,has been carried out and the thermodynamic parameters of micellar systems have been reported[4].Many electrolytic species are available in the body fluid,and their concentrations can vary to a large extent,but the presence and content of these electrolytes could affect the mode of interaction of amphiphile mixtures.It is,therefore,crucial to know the consequence of electrolytes on the association behavior of solute+surfactant systems in the laboratory mimicking conditions.The mixtures of polymer and surfactant are used explosively in different industries where the surfactant-polymer interaction is affected as a function of temperature and polymer concentration.Moreover,accurate estimation of cmc of surfactant/surfactant+polymer mixture is essential from pharmaceutical formulation viewpoint,as the solubilization of drugs in the blood stream critically depends on cmc of the surfactants[3].In persuasion of the present study,the mode of interaction of BSA protein with an important surfactant TTAB(cationic),in water,and in the presence of electrolytes (NaCl,Na2SO4,Na2CO3,Na3PO4:12H2O)and urea,was undertaken via the conductivity measurement method.In the present study,cmc,counter-ion binding(β),standard free energy changeand free energy of transferof the TTAB+BSA aggregation in pure H2O,electrolyte and urea solutions have been evaluated and discussed appropriately.
2.Experimental
2.1.Chemicals
The purchased materials were of analytical grades and have been utilized with no pre-treatment.Table 1 summarizes the relevant information of the materials used in the present study.The empirical formula of TTAB is C17H38BrN and the molar mass is 336.4 g·mol−1,while the molecular weight of BSA(lyophilized form)is 66500.Distilled-deionized water having the specific conductivity(κ)of 1.0 to 1.5 μS·cm−1(temperature range 298.15–318.15 K) was used to all practical purposes.
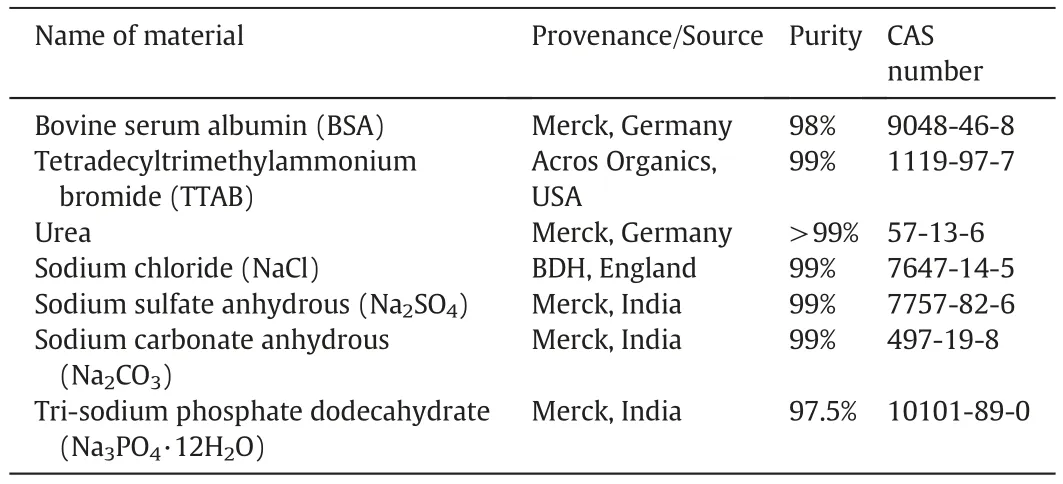
Table 1.The type,provenance,purity,and CAS Number of different materials.
2.2.Conductivity measurement
The conductometric measurement of the samples was accomplished via a Jenway(UK)4510 conductivity meter(keeping a default cell constant of 1.0 cm−1).The conductivity meter was calibrated using KCl solution of definite concentration.The values of κ of the TTAB+BSA system in salts and urea solutions have been detected in the present study.The accuracy of measurement falls within ±0.5%.At first,we have prepared a concentrated solution of TTAB in different solvents(BSA/BSA+salts/BSA+urea solutions having particular concentration of BSA/Salt/urea)as stock solutions,and then the prepared solution of surfactant was progressively added to the fixed volume (20 ml) of solvent(BSA/BSA+salts/BSA+urea solutions having particular concentration of BSA/Salt/urea)keeping the temperature constant.Subsequently,on each addition,a complete mixing was ensured letting enough time to achieve temperature equilibrium before the accomplishment of conductivity measurement.The desired temperature was sustained via the RM6 Lauda thermostat water bath having a correctness of ±0.1 K.The working solutions were prepared to make sure that the concentrations of BSA/salts/urea remained constant in the solvent/TTAB mixture in all of the cases studied.The cmc value of studied system was found from the sharp break point obtained in the κ versus[TTAB]Plot[1,2].
3.Results and Discussion
3.1.Effect of BSA on the cmc of TTAB in aqueous medium
After administering the medicine(containing surfactant for formulation purposes)in the human body,various types of binding interactions are obvious between albumin molecule and surfactant which might affect the solution property of the surfactant.So elucidation of the mechanism of interaction between albumin and surfactant is important and such a study was carried out in the current work.The interactions of BSA protein with cationic surfactant TTAB can be elucidated from the behavior of micelle formation of TTAB in attendance of BSA.Thus,it is important to understand the value of cmc in the surfactant micellar systems.In the present study,the cmc value was obtained by using the conventional conductivity method described in Section 2.2.The cmc value of the surfactant solution can be measured from the discontinuity of the curve that shows the variation of analyte property against the concentration of surfactant.
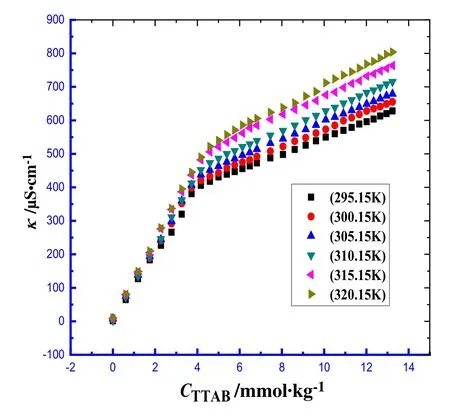
Fig.1.The specific conductivity(κ)against[TTAB]for the TTAB+BSA mixed systems having 0.005%(w/w)of BSA in water at various temperatures.
Fig.1 displays the plot of κ versus TTAB concentration in attendance of BSA(TTAB+BSA mixture)in water at various studied temperatures.Usually,at natural pH or close to pH=7,BSA is found in anionic form (present study condition).For all experimental cases of TTAB surfactant,one breakpoint was obtained and the concentration of surfactant at the breakpoint has been denoted as the cmc.Fig.1 clearly displays that below the cmc,the κ values undergo a sharp increase with the increase of TTAB concentration,whereas overhead the cmc,the corresponding upsurge of κ is rather slow.At lower[TTAB](below cmc),the primary rise in κ is attributed to the contribution made by the presence of free TTA+and Br¯ions,which are in rapid movement in the solution system.But,overhead the cmc,the gradual enhancement of κ occurs.The slow increment in κ after cmc,as compared to the value observed before cmc,becomes apparent for two reasons:(i)owing to the TTAB micelles formation,and(ii)the formation of Helmholtz layer through the compression of bromide ions via the TTAB micelles.The latter factor helps in stabilizing the self-associated surfactant system via the neutralization of surface charge of formed micelles,consequently,dropping in intermolecular repulsive potency[30,31].Thus,the assembled monomers possess a reduced suppleness than the free monomers of TTAB.
The available cmc values in the literature for individual TTAB in aqueous system at various working temperatures range from 3.74 to 4.39 mmol·kg−1[32–36].In the current study,the concentration range of 0.001%–0.30% (w/w)for BSA has been selected randomly and within this concentrations BSA is completely soluble in water.The addition of the BSA to TTAB increases the cmc of pure TTAB in H2O,and the cmc values experience a further rise with the enhancement of BSA concentration.At relatively higher concentration of BSA,the cmc experiences a sharp increase,giving a pattern analogous to an inverse sigmoid S-shaped curve.The results are depicted in Fig.2 and Table S1(Supplementary Materials).Therefore,the observation indicates that the micellization of pure surfactant in aqueous system is disfavored in BSA solution,and this phenomenon happens due to the existence of strong interactions between the BSA and TTAB.The possible interactions between BSA and TTAB are both hydrophobic interaction (between nonpolar portion of BSA and hydrophobic tail of TTAB)and electrostatic interaction(between polar heads of TTAB and ionic groups(-and–COO−) of amino acid residues of BSA).The ion-ion and ion-dipole forces might also be present between BSA and TTAB during micellization.Ionic groupsand–COO−) of amino acid residues of BSA may penetrate in the stern layer of TTAB micelles[37].The degrees of ionization (α) of micelle were obtained by the ratio of slope's above and below the cmc.Conversely,the degree of counter ion binding (β) at the cmc was computed through the deduction of α value from unity(β=(1 −α))[38].The values of β for TTAB+BSA mixed system having BSA concentrations of 0.001%,0.005%,0.015%,0.05%,0.1% and 0.3% (w/w) in water at 310.15 K are found to be 0.76,0.75,0.75,0.73,0.72 and 0.66,respectively.
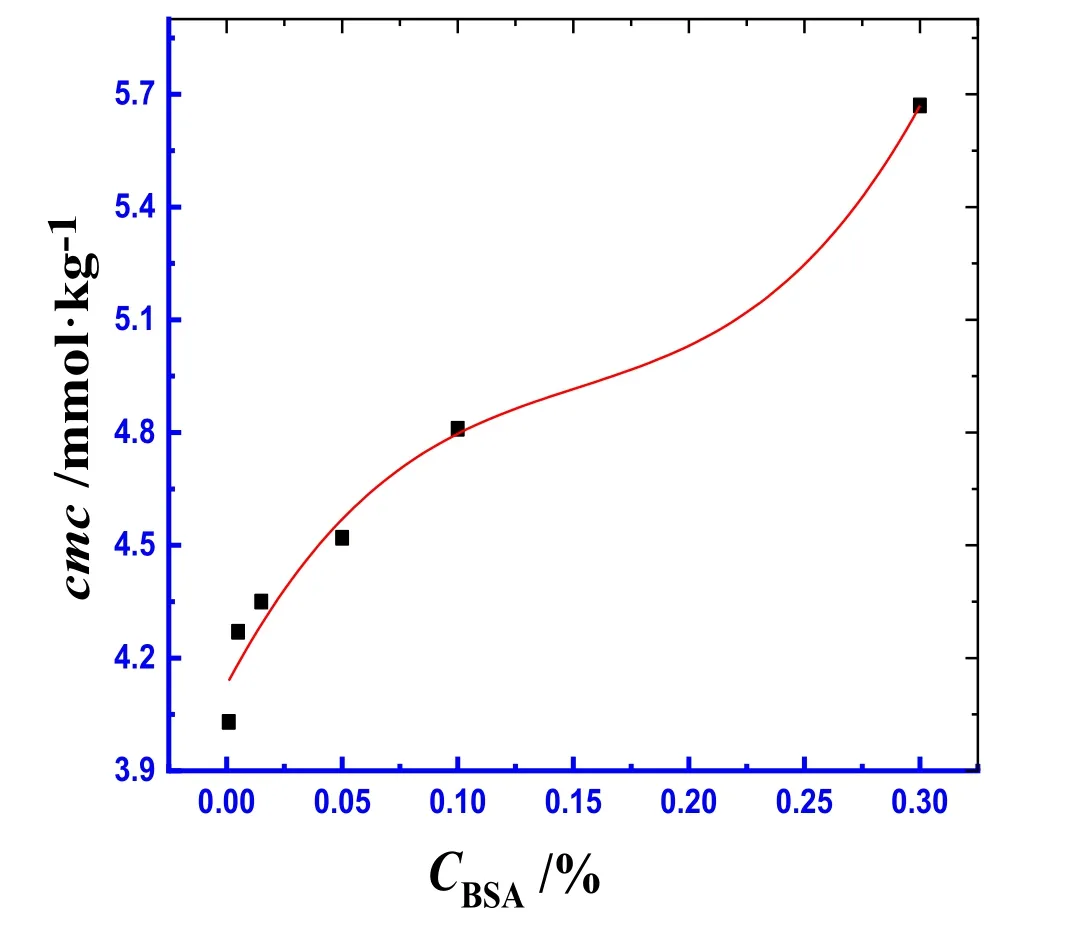
Fig.2.The cmc values versus the BSA mass concentration for the TTAB+BSA mixed system in aqueous system at 310.15 K.
3.2.Effect of salts on the cmc and degree of counter ion binding(β)of TTAB+BSA
The variation of cmc values of TTAB+BSA mixture at a temperature of 310.15 K in different salt media having different ionic strengths of salts have been sketched in Fig.3 and also in Table S2(Supplementary Materials).In the current study,the ionic strength(I)of all electrolytes was selected in the range from 0.05 to 7.0 mmol·kg−1to study micellization of TTAB+BSA mixture at 310.15 K,while the ionic strength(I)of 1.00 mmol·kg−1of all electrolytes was considered at varying temperatures.The cmc values of TTAB+BSA mixed system at a temperature of 310.15 K in salt solution(NaCl,Na2SO4,Na2CO3,and Na3PO4)were observed rather low compared to the corresponding values found in aqueous medium.However,the values of cmc were also observed to undergo a decline with an enhancement of salt concentration with an exception at 0.05 mmol·kg−1of Na2CO3solution(Table S2(Supplementary Materials)).The results disclose that the association of TTAB+BSA mixed system is favored in the presence of inorganic salts.Noteworthy,the decrease of cmc of ionic surfactants with the addition of electrolytes,e.g.,NaCl/Na2CO3/Na3PO4etc.,fairly agrees with the values reported for other surfactant aggregations[39–42].The drop down of cmc values in these observations is mainly attributed to the thinning of ionic layer that surrounds the ion head groups of surfactant.In addition,in the presence of more electrolytic species,the overall decrease of electric repulsion operating among the added electrolytes may contribute a smaller cmc[39,40].Due to the salting out effect of electrolytes,the solubility of surfactants is decreased in aqueous system and thus the hydrophobic interaction among the surfactants becomes enhanced.So TTAB+BSA micellization in the presence of electrolytes is accelerated and the magnitude of cmc gets lowered.

Fig.3.The cmc values versus the ionic strength (I) of salts for the BSA+TTAB mixed system having a BSA concentration of 0.005%(w/w)in NaCl(▲),Na2 SO4 (▼),Na2 CO3 (■),and Na3 PO4 (●)solutions at 310.15 K.
At 310.15 K and salt ionic strength(I)of 1.00 mmol·kg−1,the cmc values of TTAB+BSA mixed system follow the order:(Table S2(Supplementary Materials)).The observed change in the cmc values might have been caused due to the presence of ions with different ionic characteristics.Since the cation is essentially the same (Na+) in all salt solutions,the variation of observed cmc values of different electrolytes could be attributed to the differences in nature of anionic species.A similar trend,the addition of salt to pure and mixed amphiphile solutions causes a decrease in the value of cmc,has also been detected in earlier observations [43,44].The chloride ion(Cl−),a moderate chaotropic agent having a huge singly charged ionic density and little charge crowd,breaks down the structure of water and weakens the stability of hydrophobic interactions.The consequence of these effects causes an unstability in the micellar aggregation.On the other hand,the sulfate ionbeing a strong kosmotropic anion having a high density of multi-charge ionic nature and a big charge crowd,interacts powerfully with water molecules,thereby,making the spatial orientation of water even more stabilized.These synergistic effects create a strong hydrophobic aggregate of TTAB molecules in the presence of sulfate ion.Thus,the Na2SO4molecule induces a salting out effect on the hydrophobic interaction of surfactants,which significantly lowers down the cmc values of micellar system in comparison to the value found in case of NaCl salt.The values of β for TTAB+BSA mixed system having 0.005%(w/w)BSA at 310.15 K are found to be lower in salt solution compared to aqueous solution and the values tend to decrease with increase of concentration of salts,with a few exceptions.
3.3.Effect of urea on the cmc and β of TTAB+BSA mixture

Fig.4.The cmc values versus the concentration of urea(mmol·kg−1)for the TTAB+BSA mixed system having a BSA concentration of 0.005%(w/w)at 310.15 K.
The plausible mechanistic mode by which urea significantly contributes to the formation of micellar aggregates with biomolecules and surfactants has yet to be disclosed fully.It is,therefore,important to have a solid understanding on how urea poses an effect on the micellization of surfactant,especially the behavior of urea on the association of surfactant-protein mixed systems.In the current study,the role of urea on the aggregation of TTAB+BSA mixture was also observed.The value of micellar parameters(e.g.,cmc and β)for the TTAB+BSA mixture at a temperature of 310.15 K in urea solution having different compositions are presented in Fig.4 and Table S3(Supplementary Materials).The cmc of TTAB+BSA mixed matrix in the presence of urea are relatively smaller than those values found in case of aqueous solution.With the insertion of urea into water,the dielectric constant of water+urea mixed system undergoes an increase [45].Urea molecules spread throughout the medium homogeneously and the water+urea mixed solvent exist as a single phase[46].It was reported that the addition of urea to water increases the dielectric constant of water[47].The Coulomb's law says,the attraction/repulsion among the charged entities is inversely proportional to the dielectric constant of solvent.As the dielectric constant of water+urea mixed solvent is higher than that of pure water,the Br−–Br−and TTA+–TTA+repulsion as well as TTA+–Br−attraction are lower in water+urea mixed solvent compared to water.Due to decline of head groups repulsion among TTA+ions,micelle formation takes place at lower surfactant concentration i.e.,the cmc value is reduced[47,48].Singh et al.reported that in case of the micellization of surfactant in polar solvents the cmc of the same surfactant decreases with the increase of dielectric constant of media[49].Singh et al.[49]also discussed that in urea solution an increased H-bonds (between urea and water)and a decreased solvation of nonpolar chains of ionic surfactant would enhance the hydrophobic effect which might decrease the cmc of surfactant at low urea concentration.The micellar aggregation of amphiphilic species,like the one observed in case of urea,was supposed to have been caused due to an intricate compensating equilibrium between the“hydrophobic”and the“hydrophilic”interactions[47,48].In addition,the micellar aggregates make the hydrophobic moieties more soluble.This is believed to happen due to a collateral alteration in the surrounding environment(e.g.,polarity and viscosity)of surfactant molecules.The structure and physicochemical nature of micelles,like the critical micellar concentration(cmc),the number of aggregation(Nagg),thermodynamic properties on micelle formation,and the counter ion binding factor(β)all depend on the extent of compensation of interactions between the“hydrophobic”and the“hydrophilic”ends[47,48,50–52].However,the equilibrium between the“hydrophobic”and“hydrophilic”effects are greatly reduced in the presence of organic compounds,in the present case urea.
The urea is the most frequently used organic compound whose contribution has long been studied on the micellization process of various substances.The reason behind the choice of urea originates on the fact that urea is commonly employed to observe the denaturation of proteins at high urea concentration.But in low urea concentrations,it might act as an agent to renature the structure of proteins[47].A recent study reveals that the addition of urea in the micellar systems causes an augmentation in the hydrophilic(or,the dielectric)characteristics of water.The induced hydrophilic property of water renders the polar head groups of amphiphilic compounds even more solvated [46].If so happens in practice,then,it is expected that,in aqueous medium,the cmc value of amphiphilic molecules undergoes a gradual decrease.The ongoing result warrants a strong relationship between the interaction of urea with that of amphiphiles which has the propensity to make a bilayer.
The observed variation of cmc of TTAB+BSA mixed system in urea solution compared to aqueous medium is attributed to the fact that,unlike water,the urea molecule induces a direct and/or indirect effect on the molecular aggregation of surfactant and proteins[53–55].The role of urea on the micellar system has produced a good number of corollaries.At first,urea possesses the ability to form strong H-bond with water and other aqueous media and polar solvents.The synergistic effect of urea pushes the water molecules away from the solvated shell at the hydrophobic end of the surfactant,thereby,ensuring an increased solubility.Secondly,the alternate or indirect mechanism predicts that urea potentially could annihilate the three-dimensional arrangement of water,which,in turn,reduces the energy demand to solubilize the hydrophobic moiety in the bulk of solution mixture[56].The β values of TTAB+BSA mixed system having 0.005%(w/w)BSA at 310.15 K in urea solution are found to be almost similar to those in aqueous solution and the values also remain almost unchanged with an increase of urea concentrations(at lower urea concentrations).
3.4.Effect of temperature on the cmc and β of mixed system in H2O and salts/urea solutions
The impacts of the change of temperatures of solvents on the micellar properties of TTAB+BSA mixture were investigated by selecting the range of temperature from 295.15 to 320.15 K.From the area of utilization,there is a possibility to experience fixed/variable temperatures for TTAB+BSA mixtures in the utilized areas.So in this study the temperature range(295.15–320.15 K)was selected so that the range covers the general temperatures of applied fields,human body(37.4°C or 310.55 K)and also room temperature(around 298.15 K).The specific conductivity(κ)for the TTAB+BSA mixture having a concentration of 0.005%(w/w) BSA was found to be dependent on temperatures(295.15–320.15 K).Fig.1 shows the effect of temperature on κ within the concentration range of TTAB used at different temperatures.The cmc,along with β of TTAB+BSA in H2O and salt (NaCl,Na2SO4,Na2CO3,and Na3PO4)/urea solutions,have been included in Table 2.The measured cmc values for the TTAB+BSA mixture in water,NaCl,and Na3PO3were found to experience a gradual increase with a rise of temperature.However,in Na2SO4and Na2CO3solutions,the cmc values initially have undergone a decrease up to a definite temperature,then dropped to a minimum point and then have experienced a further rise with increasing temperature.The plot of cmc vs.T for the TTAB+BSA mixed system in aqueous and salts/urea solutions have been presented in Figs.S1–S3(Supplementary Materials).In the presence of salts studied thoroughly,the values of cmc were observed relatively lower compared to those values detected in aqueous medium over the entire ranges of temperatures under study.Aguiar et al.observed an increase of cmc with the rise of temperature for the micellization of TTAB within the temperature range from 293.15 to 313.15 K in aqueous and formamide solution[57].The change in cmc,with respect to temperature,could be illustrated by the alteration in nature of hydration spheres surrounding the surfactant molecules.This also causes a greater degree of BSA induced TTAB micellar formation.In the monomeric surfactant,there is an equal likelihood of the presence of two types of hydrations:hydrophobic(water structure surrounding the alkyl chain of surfactant)and hydrophilic (arrangement of water surrounding head groups ofsurfactants).But in the micellized TTAB only hydrophilic hydration is expected which controls the micellization process.However,both types of hydrations are likely to experience a drop as the temperature goes up.A drop in the extent of H2O structure surrounding the TTAB head groups augments the micelle formation(cmc values are decreased).In contrast,a decrease of H2O arrangement around the hydrophobic chain,with the increase of temperature,disfavors micellization(cmc values are increased)[50].Therefore,the extent of these two factors dictates the feasibility if the values of cmc should undergo an increase or experience a slash beyond a specific range of temperature.The results show that in case of H2O,NaCl,and Na3PO4salts,with increasing temperature,the hydrophobic hydration predominates over the hydrophilic hydration,thereby,increases the value of cmc.In addition,it is evident from the data pool in Table 2,the effect of hydrophilic hydration becomes dominant over the hydrophobic hydration up to a certain range of temperature,and then the opposite phenomenon transpires for Na2SO4and Na2CO3salts.
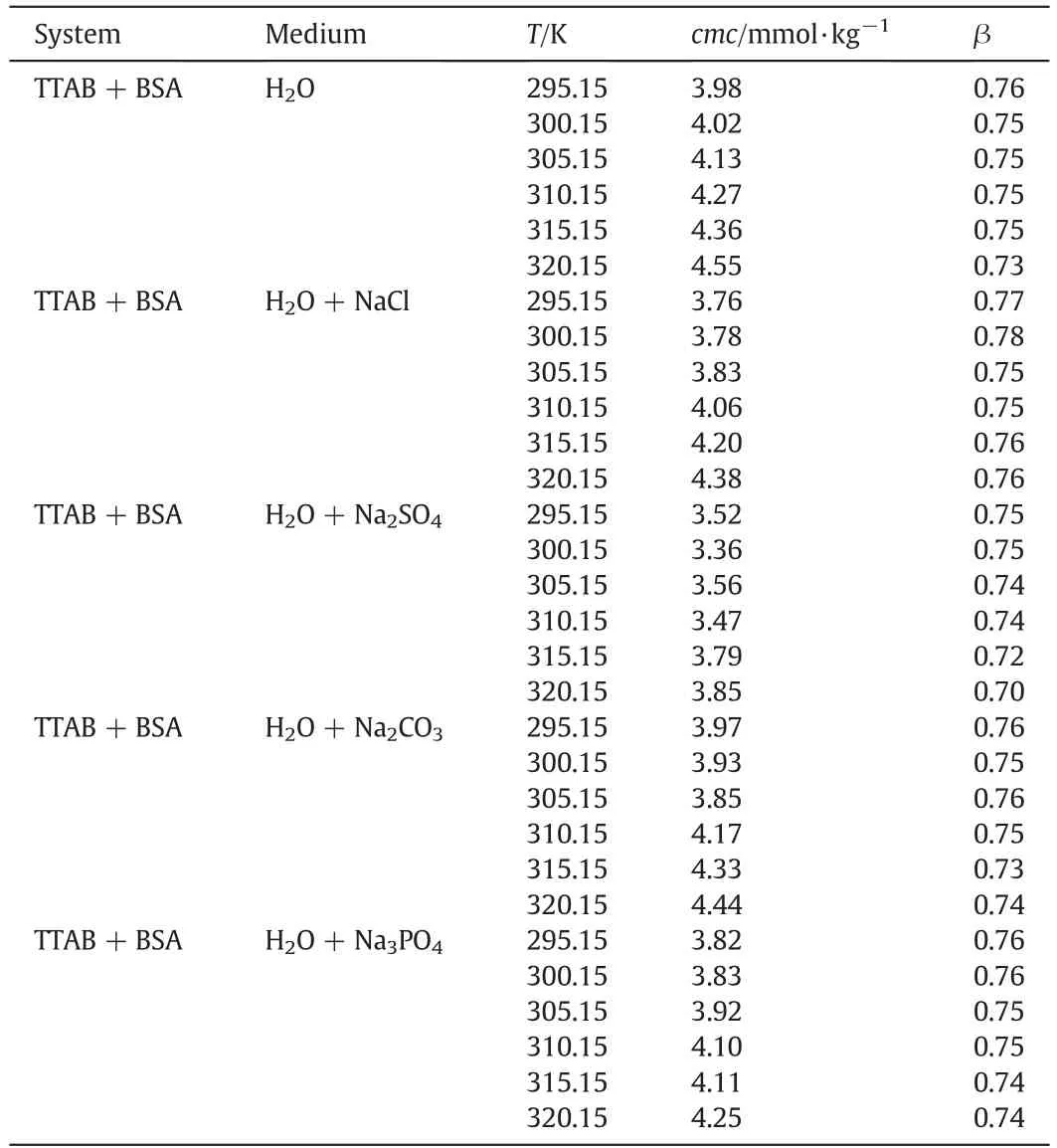
Table 2.The cmc and β value for the TTAB+0.005%(w/w)BSA mixture in aqueous and in different sodium salt solutions with the same ionic strength (I) of 1.00 mmol·kg−1 at various temperatures.①
At elevated temperature,however,the higher solubility of hydrocarbons helps the surfactant monomers to give more stability.Hence,the relatively stable surfactant exhibits a reduced propensity to form micellar aggregates,thereby,causing the cmc value to experience an increase.Table S4(Supplementary Materials)and Fig.S3(Supplementary Materials)show the variation of cmc values for the surfactant+BSA mixture in aqueous as well as urea solution measured at different temperatures.Though the cmc versus T plot is not linear,but the values undergo an increase with increasing temperature.Thus,in urea solution,the effect of temperature on the cmc exhibits the similar trend observed in cases of water and NaCl/Na3PO4solutions.In urea solution,the annihilation of the three-dimensional structure of water around the non-polar end of the surfactant-protein assembly predominates.At elevated temperature,again,the break-down of hydrogen bonds (>O–H——H–N<)between the water molecule and proteins(hydrophobic interaction)creates a hostile environment for micellar formation and causes the cmc values to undergo an increase.
3.5.Thermodynamic parameters of studied system
The evaluation of the standard thermodynamic parameters of micellar system is important,because these values forecast to fully characterize the nature of interactions among different components present in the current study.The spontaneity of any physical process or chemical reaction can be interpreted in terms of the changes in standard free energy(ΔGo)values.

The values of free energy of transferfor the TTAB+BSA micellar system in shifting from aqueous medium to other electrolytes/urea solutions have been computed using the following equations[38,64]:

This indicates that the spontaneity of micellzation of TTAB+BSA mixture dwindles with an increase of BSA concentrations.This observation is also supported by the increase of cmc of TTAB+BSA mixture that lessens down with an increase of BSA concentrations.For TTAB+BSA mixtures having 0.005%(w/w)BSA,the negative values ofincrease with an elevation of temperature.At 310.15 K,for TTAB +BSA mixtures in electrolytes media within the range of 0.005–7.0 mmol·kg−1,the negativevalues increase with increasing the concentration of NaCl and Na2SO4,but the values remain almost unchanged with the increase of Na2CO3and Na3PO4concentrations.For TTAB+BSA mixtures having fixed electrolytes concentration (1.0 mmol·kg−1),the negativevalues increase with an escalation of temperatures.In urea solution having varying urea contents,the negativevalues augment with the rise of urea contents at 310.15 K.Also,at fixed urea concentration,the magnitudes of negativevalues tend to increase with the rise of temperatures,except the one observed at 320.15 K.These observations demonstrate that the micellization process in H2O and electrolytes/urea media is facilitated at elevated temperatures.The augmentation of negativevalues for TTAB+BSA mixture in different electrolytes/urea media is also supported by the reduction of cmc values in different electrolytes/urea(low concentrations)media.The repulsion among the ionic head group of ionic surfactants is the postponing energy in the case of micellization,while hydrophobic interaction facilitates in micellization.The repulsions among the head groups are depressed and hydrophobic environment around the head groups also altered in the existence of salts by surface charge minimization and weakening the probable steric hindrance of the amphiphile head groups[65,66].
4.Conclusions
Surfactants are widely utilized in drug formulations and hence it is important to gain more knowledge on the micellar properties of surfactants in various media,which are similar to body fluids.The current study describes the micellization behavior and solution properties of TTAB+BSA mixtures in H2O and electrolytes/urea media,carried out by means of conductivity measurement.The micellar properties of TTAB solution are observed to be influenced by the addition of additives(BSA/BSA+electrolytes/BSA+urea)and a variation of temperatures.In all cases studied,only one cmc was obtained for the TTAB+BSA mixed system,and the obtained cmc values were found highly dependent on the compositions of salts/urea,as well as the change of temperature.The cmc values of TTAB+BSA mixture experience a reduction in both salt and urea solutions,which ensure an earlier micellization.Thus,a less quantity of TTAB is required to perform micellar actions in different formulations.Urea solution having low concentrations favors the micellization of TTAB+BSA mixture.The negativevalues in all cases reveal that the micellization of current system is spontaneous andthe spontaneity is enhanced with the rise of temperatures in H2O and salt/urea solutions.Almost negative values of free energy of transfer support the increased spontaneity of the micellization in salt/urea solutions.This study will be helpful for the formulation of drug in pharmaceutical industries taking into account the influence of different additive species that exist in the body fluid.Here,the knowledge of micelle formation behavior of surfactant will offer an added benefit,emphasizing the idea to be applicable to other similar types of industrial formulations.
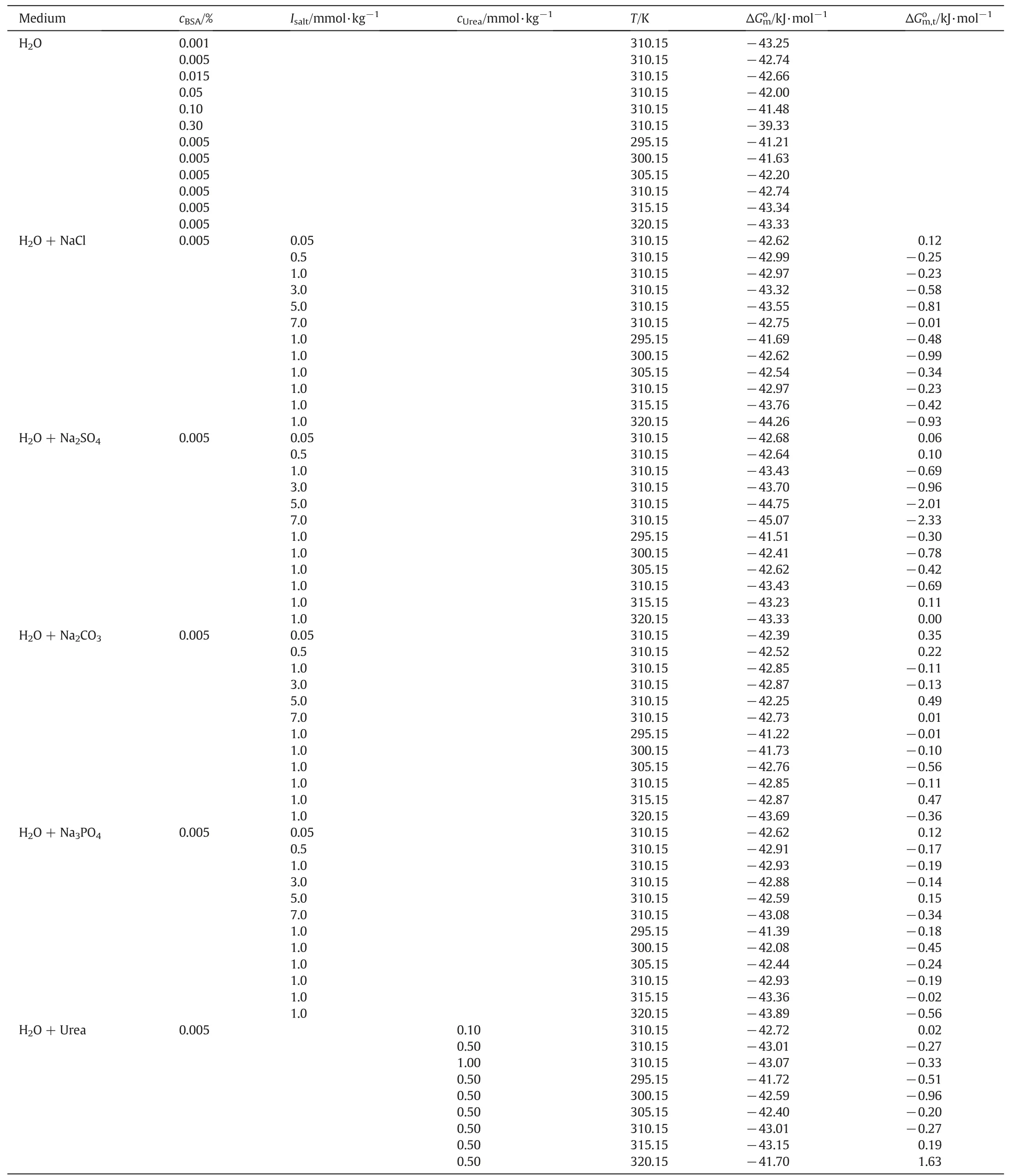
Table 3.The thermodynamic parameters for the TTAB+BSA mixed system containing 0.005%(w/w)BSA in water,electrolytes and urea solutions.①
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to King Saud University for funding this work through Researchers Supporting Project number(RSP-2020/133),King Saud University,Riyadh,Saudi Arabia.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2020.07.062.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Perspectives and challenges of hydrogen storage in solid-state hydrides
- Wet flue gas desulfurization performance of 330 MW coal-fired power unit based on computational fluid dynamics region identification of flow pattern and transfer process
- EMMS-based modeling of gas–solid generalized fluidization:Towards a unified phase diagram
- Using expansion units to improve CO2 absorption for natural gas purification-A study on the hydrodynamics and mass transfer
- Simulation and experimental study on the surface morphology and energy lost of the target material under non-overlapping impact of angular particles
