High nitrogen carbon material with rich defects as a highly efficient metal-free catalyst for excellent catalytic performance of acetylene hydrochlorination
2021-04-13FangjieLuDongXuYushengLuBinDaiMingyuanZhu
Fangjie Lu,Dong Xu,Yusheng Lu,Bin Dai,2,Mingyuan Zhu,2,
1 School of Chemistry and Chemical Engineering,Shihezi University,Shihezi 832000,China
2 Key Laboratory for Green Processing of Chemical Engineering,Xinjiang Production and Construction Corps,Shihezi 832000,China
Keywords:Carbon defects material High nitrogen content Acetylene hydrochlorination Catalyst Chemical reaction Nanomaterials
ABSTRACT In this work,we developed a simple strategy to synthesize a carbon material with high nitrogen and rich carbon defects.Our approach polymerized diaminopyridine(DAP)and ammonium persulfate(APS).Following a range of different temperature pyrolysis approaches,the resulting rough surface was shown to exhibit edge defects due to N-doping on graphite carbon.A series of catalysts were evaluated using a variety of characterization techniques and tested for catalytic performance.The catalytic performance of the N-doped carbon material enhanced alongside an increment in carbon defects.The NC-800 catalyst exhibited outstanding catalytic activity and stability in acetylene hydrochlorination(C2 H2 GHSV=30 h−1,at 220°C,the acetylene conversion rate was 98%),with its stability reaching up to 450 h.Due to NC-800 having a nitrogen content of up to 13.46%,it had the largest specific surface area and a high defect amount,as well as strong C2 H2 and HCl adsorption.NC-800 has excellent catalytic activity and stability to reflect its unlimited potential as a carbon material.
1.Introduction
Vinyl chloride is a monomer of polyvinyl chloride(PVC).Because of China's energy structure,acetylene hydrochlorination is a major method for producing vinyl chloride[1].However,HgCl2is currently still used as the main catalyst for the acetylene hydrochlorination[2].It is greatly harmful to the environment.The country has led to the proscription of mercury use in polyvinylchloride(PVC)production as of 2020[3,4].Therefore,it is exigent that environmentally friendly nonmetal catalysts be developed for acetylene hydrochlorination.In this regard,it is a feasible method to replace HgCl2with other metals.Based on multitude systematic research,Hutchings and colleagues come up with the conclusion that Au catalyst has high conversion and excellent stability;it is capable to replace HgCl2catalyst on acetylene hydrochlorination [5,6].Subsequently,many catalysts,including Cu[7],Pd[8,9],Pt[10,11],Ru[12,13]and so on,have been investigated.However,metal catalysts are generally restricted due to their cost and based on metal reserves.
Carbon-based materials have received a significant degree of attention owing to their unique capabilities.In our previous work,we found that C3N4catalyst can achieve 75% of the catalytic activity of HgCl2catalyst.According to theory calculations,the adsorption sites of acetylene and hydrogen chloride are carbon and nitrogen atoms,respectively[14].We considered the effect of nitrogen in carbon catalysts for the acetylene hydrochloride.The obtained PANI-AC series catalyst revealed exceptional acetylene conversion (76.27%) when nitrogen content was increased to 2.39%.Contrastingly,the nitrogen content of AC is 0.67%and a low acetylene conversion rate(49.85%)[15].Subsequently,nitrogen-doped carbon catalysts have been synthesized by many groups,displaying outstanding catalytic performance.Li's group found that Derivatives of ZIF-8 can generate more N species.Moreover,their findings indicate that as loading of ZIF-8 increased,N content increased to 1.10%,and the activity of the catalyst increased from 60%to 80%[16].Thus,the alter in nitrogen content before and after the reaction can be determined as evidence of an effective increase in the catalytic activity of acetylene hydrochlorination.In addition to strongly investigated nitrogen dopants,sulfur [17,18],phosphorus [19]and boron[20]doped carbon have also emerged as potent candidates.Although carbon-based catalysts of heteroatom-doped for the catalytic activity are currently inferior to the most conversion gold-based catalysts[21,22],they still exhibit significant potential to serve as promising catalysts in the sustainable production of VCM.
When devising and synthesizing,carbon-based catalysts have high activity and stability,and establishing the relationship about the physical characteristic of the catalyst and catalytic properties is critical.We investigated the mono-vacancy graphene,di-vacancy graphene and defect graphene on adsorption ability of C2H2and HCl by DFT calculations.We incidentally found that the carbon defects were able to improve the adsorption capacity of C2H2and HCl [23].As a result,we were exceedingly curious as to whether rich carbon defects in nitrogen-doped carbon catalysts might also influence the catalytic properties of acetylene hydrochlorination.In redox reactions,nitrogencontaining materials with carbon defects have an effect on catalytic activity[24–26].Wang et al.[27]prepared a carbon material containing defects by removing a large number of N species at high temperatures;it was found that the defective structure in carbon materials has a positive effect on improving catalytic activity,but its catalytic activity was poor.Additionally,Qiao et al.[28]synthesized a fragmentary g-C3N4framework with a porous structure and rich nitrogen defects,which significantly enhanced catalytic activity.However,its stability was extremely poor.Therefore,we can conclude that when we increase the defect structure by removing N species at high temperatures,the amount of C2H2adsorption increases and the reactivity increases.At the same time,if we introduce nitrogen to improve the adsorption of HCl,the number of defects will be reduced accordingly and catalytic activity and stability were affected.Therefore,questions remain relating to the low catalytic activity and the low stability of these synthesized high nitrogen-carbon materials with rich defects.
In this study,we designed defect-rich high nitrogen carbon materials to have excellent catalytic activity and stability in acetylene hydrochlorination.Previous studies have shown that diaminopyridine(DAP)polymerizes to form a stable carbon-nitrogen network polymer with a self-supporting spherical backbone structure.Under hightemperature carbonization,the carbon defects of the catalyst increase significantly while reducing the loss of nitrogen,the carbon-nitrogen network will be rearranged.After the nitrogen atom is removed,the original position may form a single-atom vacancy.Due to the unstable structure of the single atom vacancies,two adjacent vacancies will merge to form a new vacancy.It is usually considered a G585 carbon defect(topological defect composed of two pentagons and one octagon).If the loss of nitrogen atoms becomes larger,more carbon defects will be generated[29–32].This approach relies on DAP and ammonium persulfate(APS)being polymerized to form stable and uniformly spherical,and also represents an extremely simple and rapid approach for directly synthesizing porous carbon materials(NC)containing high-level nitrogen and significant nitrogen defects at various temperatures,as shown in Fig.1.Following pyrolysis at 800°C,the resulting coarse surface possessed edge defects on carbon,due to N-doping.We evaluated the catalytic performance of carbon-based catalysts with different defect contents and different nitrogen contents calcined at different temperatures.The N species were analyzed according to X-ray photoelectron spectroscopy(XPS).Carbon defects were characterized using Raman spectroscopy.Characterization of reactant adsorption on the surface of the catalyst by temperature programmed decomposition(TPD).
2.Experimental
2.1.Catalyst preparation
Synthesis of precursor(PDAP)and carbon materials(NC):PDAP was synthesized according to steps provided in existing literature,with minor modifications [32].In general,50 mmol (5.46 g) of 2,6-diaminopyridine and 75 mmol(3 g)of sodium hydroxide were mixed and stirred uniformly in 400 ml of distilled water.Once the solution had been clarified,75 mmol (17.11 g) of APS was prepared and dissolved in 80 ml of distilled water,and added to the above-mentioned clear solution.Finally,the mixture was stirred for 24 h at room temperature.Following on,a brown–brown solid was collected,filtered and washed with deionized water several times to reach a neutral and was then dried at 80 °C to obtain an intermediate PDAP product.Finally,the PDAP was pyrolysed at 600°C,800°C,1000°C and 1200°C for 3 h in a N2atmosphere,at a rate of 5°C·min−1,to obtain a series of catalyst NC-T(T=600,800,1000,1200).
2.2.Catalyst characterization
Using the Bruker D8 VENTURE/QUEST,CuKα(λ=0.15406 nm),two theta scanning ranges of 10° to 90° were adopted for XRD analysis.Raman spectra were collected using the Renishaw inVia instrument.Thermogravimetric(TGA)of catalysts was executed using TGDSC synchronous thermal analyzer instruments(NETZSCH STA 449 F3 Jupiter©,Germany)heated to 1000°C in an air atmosphere.Scanning electron microscopy(SEM,JEM2010,200 kV)was used to observe the sample morphology.Additionally,TPD analysis was conducted using the Chem BET Pulsar TPD/TPR (Automated Chemisorption Analyzer)under heating rate of 10°C·min−1in an atmosphere with helium gas flow rate of 100 ml·min−1.The Micromeritics ASAP 2020 instrument was used to analysis the surface area(BET)of sample.
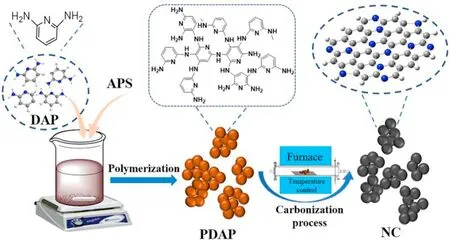
Fig.1.Schematic illustration for the synthesis of NC.

Fig.2.The SEM images of a—NC-600,b—NC-800,c—NC-1000,d—NC-1200,and the TEM images for e-NC-800,and C(f)N(g)and O(h)element mapping for NC-800.
2.3.Description of catalytic tests and analytical criteria
Catalytic performance was probed using a fix-bed reactor(i.d.10 mm).The microreactor with the catalyst (1.0 g,2 ml) was purged with nitrogen to remove water and air before the reaction.The sample was heated for 30 min from room temperature to 220°C,at a heating rate of 5 °C·min−1.Will acetylene and hydrogen chloride gas to 30 h−1space velocity(VHCl/VC2H2=1.15)introduced into heated reactor.The reactor effluent gas through an absorption bottle with a sodium hydroxide solution for removes the unreacted hydrogen chloride.Using a GC-1690F gas chromatograph(GC)equipped with FID detector was carried out online for the gas mixture.
The following formula was used to define the conversion of acetylene(XA)as criteria for catalytic performance.

In the equation,ΦA0is the volume fraction of acetylene in the raw material,ΦAis the volume fraction of acetylene in the product.
The following formula was used to calculate the conversion frequency(TOF)of acetylene as criteria for catalytic performance.

3.Results and Discussion
The diaminopyridine(DAP)was polymerized to take shape stable and uniformly spherical polymer.After high temperature pyrolysis,the precursor(PDSA)was transferred into the NC catalyst,featuring a coarse surface and a diameter of~500 nm spherical structure(Fig.2(a)–(d)).The resulting coarse surface possessed edge defects on carbon due to N-doping,which may increase its catalytic activity.The images of several catalysts show that the morphology of defective carbon catalysts can be changed under different pyrolysis temperature treatments.We speculate that the difference in morphology and structure of nitrogendoped defective carbon catalysts may affect the catalytic activity of acetylene hydrochlorination.Through the elemental map of transmission electron microscopy (TEM),we performed the tracking of the distribution of catalyst elements(Fig.2(f)–(h))and showed even allocation of both C,O and N in the NC-800 catalysts,which indicate large number of N active sites everywhere in the material.

Table 1 texture properties and elemental composition of samples

Fig.3.(a):N2 adsorption–desorption isotherm and(b):XPS spectra of catalysts.
Fig.3(a)shows nitrogen adsorption/desorption isotherms for several catalysts.The materials possessed type I isotherms and,as such,were recognized as belonging to a mesoporous material with a pore size close to that of a micropore.This conclusion was confirmed by the pore size distribution of the materials(see Fig.3(a)),where the average pore diameter was roughly 15 nm.NC-800 has a maximum specific surface area of 449.85 m2·g−1,NC-600 has a minimum specific surface area of 7.68 m2·g−1.The physical properties of other catalysts are listed in Table 1.The chemical ingredient of the materials was then studied.Fig.3(b)shows the full spectrum of XPS for several catalysts,there are no characteristic peaks of any other elements on the full spectrum.Table 1 lists the elemental composition of catalysts.According to Table 1,the nitrogen content in the catalyst decreased from 23.81 to 2.68 when the carbonization temperature was increased from 600°C to 1200 °C,while the carbon and oxygen contents did not change significantly.This clearly shows that during the carbonization of nitrogen-doped carbon catalysts,nitrogen may gradually lose with increasing temperature,a result consistent with the findings in the existing literature[27].If the loss of nitrogen atoms can form atom vacancies during carbonization,more carbon defects will be produced[13,15–17].

Fig.4.(a)XRD patterns and(b)Peak area spectrum fitting for Raman spectra of catalysts.
X-ray diffraction of materials is usually performed to analyze the composition of synthesized carbon materials.Fig.4(a)shows the XRD which illustrate that the positions of the(002)and(101)plane diffraction peaks for these catalysts matched with graphitic carbon;no characteristic peaks were detected for other impurities.When the pyrolysis temperature increases,the half-value width of the two diffraction peaks gradually narrows,and we can infer that the grain size in the catalyst gradually increases.The peak intensities of the two diffraction peaks are gradually increasing,which clearly indicates that the synthetic material has changed from amorphous carbon to graphitized carbon.The NC-600 had no obvious(101)peaks,this indicates that the structure of the carbon framework had not been completely formed.This suggests that the calcination temperature had a significant impact on the carbon framework structure.
To explore the defects caused by N doping,we measured by Raman spectroscopy,and the results are shown in Fig.4(b).All samples showed two peaks near 1365 and 1590 cm−1,(D and G corresponding peaks),respectively[16].The ratio of the D peak and G peak intensity area integration(ID/IG)can reflect the degree of defect of the carbon material.The two vibration peaks of intensity ratio (ID/IG) increased in the order of NC-800(2.81)>NC-600(2.32)>NC-1000(2.23)>NC-1200(2.06),showing that the defect structure in the catalyst gradually increases,while the NC-800 catalyst has the largest number of defects.Moreover,when the calcination temperature exceeded 800°C,alongside the increase in carbonization temperature,the degree of graphitisation of the carbon materials improved,which can be verified by XRD.NC-800 produces a great amount of carbon defects in the carbon catalysts.NC-600 had the lower number of defects than NC-800,the reason for this was that the optimum calcination temperature had not been achieved,leading to a significant amount of nitrogen being retained.
To explore the catalytic performance of the NC catalysts,the temperature optimization test of NC-800 was performed at different reaction temperatures of 180–240°C.Fig.5(a)shows that the highest acetylene conversion increased alongside an increase in temperature.Taking into account the energy consumption during the reaction,the optimal temperature over NC-800 was indicated as 220 °C for acetylene hydrochlorination.At an optimum temperature of 220°C and airspeed of 30 h−1,NC catalysts with different calcination temperatures were evaluated (Fig.5(b)).Among a series of catalysts,NC-800 has the highest acetylene conversion of 97.39%,followed by NC-600(96.31%),NC-1000(85.69%)and NC-1200(70.96%).There was no significant reduction in the activity of NC-800 after 10 h of reaction.It should be noted that nitrogen content can affect the activity of the catalyst.The more nitrogen content in the catalyst,the better the catalytic performance will be(Table 1).Although NC-600 samples had the highest N content (23.81%),their conversion of acetylene and stability was lower than for NC-800 samples.We found that the main reasons for the catalytic activity of NC-600 being lower than NC-800 can be attributed to the lower specific surface area(Table 1)and the carbon defects(Fig.4(b))of NC-600.In addition,by effecting XRD and Raman spectroscopy to characterize the degree of defects for the NC catalyst,we found the catalyst activity sequence to be consistent with the defect content,that is,the higher the defect content,the higher the catalytic activity.These results indicate that the main factors affecting the catalytic activity of the defective carbon material catalyst are the defect structure and nitrogen content in the catalyst.The presence of defects on the carbon material catalyst also changed the electron cloud arrangement on the surface,thereby improving the catalytic activity.Therefore,the excellent catalytic activity of NC-800 was attributed to a high-level N content,a high defect content and a high specific surface area.

Fig.5.(a)Acetylene conversion with NC-800 catalysts(at 180–220°C,GHSV(C2 H2 )=30 h−1,and =1.15).(b)Acetylene conversion with catalysts(at 220°C,GHSV(C2 H2 )=30 h−1,and =1.15).

Fig.6.Relationship between nitrogen content,carbon defects and acetylene conversion.

Table 2 The TOFs of NC-600,NC-800,NC-1000 and NC-1200 catalysts based on single nitrogen atoms
To explore the relationship between nitrogen content,carbon defects and acetylene conversion,a comprehensive comparison was conducted.Fig.6 shows that the nitrogen content decreased as the carbonization temperature increased,and carbon defects initially increased before decreasing as the temperature increased,the acetylene conversion rate initially increased before decreasing as the temperature increased.When the temperature was lower than 800°C,the higher conversion rate of NC-600 was attributed to the maximum nitrogen content;however,due to the lowest number of carbon defects present in this instance,the excessive adsorption of hydrogen chloride will cause the overall catalyst to be too acidic,which rendered it less stable.It can be seen that nitrogen content was the primary reason for increasing catalytic activity at this time.When the calcination temperature was above 800°C,as the temperature increased,the nitrogen content decreased,the carbon defects decreased and the conversion of acetylene decreased.NC-1000 and NC-1200 had poor catalytic activity due to their minimum nitrogen content and the number of defects.It can be seen that carbon deficiency and nitrogen content were the main reasons for an increase in catalytic activity at this time.NC-800 had excellent catalytic activity and long stability,due to it having the largest number of defects and optimal nitrogen content.In summary,the carbon–nitrogen material had excellent catalytic activity and stability when the carbon deficiency and nitrogen content reached optimum levels.
Generally,the catalytic activity performance of materials is tightly related to the amount of active centers.But the types of N-species that participate as active centers have not been demonstrated.In order to define the role of the three forms of nitrogen during the reaction,as shown in Fig.7(a),we performed a N1s XPS analysis of the fresh catalyst.The N1s XPS spectra of four catalysts decomposed into three component peaks,which indicated that graphitic N(401.4 eV),pyrrolic N(400.4 eV)and pyridinic N(398.3 eV)existed in the material[10,17].Fig.7(b) shows the relationship between nitrogen content of three forms and acetylene conversion.It is notable that the content of pyrrolic N and pyridinic N decreased,while graphitic N showed a gradual increase.Surprisingly,the content of graphitic N decreased alongside increasing catalytic activity,showing a completely opposing trend to conversion of acetylene,it shows that the graphitic nitrogen structure has a negative effect on the reaction process.However,the change trend of the content of pyrrolic N in the catalyst is consistent with the trend of acetylene conversion,indicating that the content of pyrrolic N is positively correlated with the conversion of acetylene.Therefore,we believe that pyrrolic N can promote the acetylene hydrochlorination reaction and improve the catalytic activity.[14].In summary,as the number of defects increased,the specific surface area increased,the amount of pyrrolic N increased,and the conversion of acetylene also increased.
Meanwhile,we calculated the conversion frequency(TOF)of several nitrogen-doped carbon defects catalysts based on the mass fraction of nitrogen content and the acetylene conversion of about 10 h.As shown in Table 2,the TOFs of NC-600,NC-800,NC-1000,and NC1200 are 2.17×10−3min−1,4.06×10−3min−1,9.07×10−3min−1,and 1.09×10−2min−1.As the calcination temperature increases,the TOF gradually increases,showing that the amount of acetylene conversion gradually increases in unit time,indicating that the nitrogen content in the carbon-nitrogen material has good dispersibility as the active site.The NC-800 catalyst with the best catalytic performance showed a higher nitrogen content,among which the content of pyrrolic N reaches the maximum(42.36%).For both NC-1000 and NC1200 catalysts,although the TOFs are higher than those of NC-800,this can be attributed to the fact that its nitrogen content is significantly lower than that of NC-800.Therefore,the catalytic activity of NC-1000 and NC1200 catalysts is not as good as that of NC-800.

Fig.7.(a):High-resolution XPS spectra of N1s peak.(b):Relationship between relative atomic percentage of nitrogen bonding states and acetylene conversion.

Fig.8.TPD profiles of catalysts:(a)C2 H2 ;(b)HCl.
To explain why carbon defects in N-doping carbon has excellent catalytic performance,and explore the adsorption of acetylene hydrochlorination by defective carbon materials prepared from different carbonization temperatures.Characterization of reactant adsorption capacity on the catalyst by temperature programmed decomposition(TPD).Fig.8 shows that both HCl and C2H2had obvious adsorption peaks at 250°C.Among them,in C2H2-TPD,there is a smaller desorption peak at 400–600°C(Fig.8(a)).With the increase of the calcination temperature,the desorption peak shows a trend of increasing first and then decreasing,which is consistent with the change pattern of the main peak.We speculate that this peak should come from the decomposition of the carbon deposition polymer formed by C2H2during the adsorption process.In the process,a small amount of polymer is decomposed into gaseous products.[33]We can conclude that as the carbon defects increases,the desorption peak areas of HCl and C2H2increase.NC-800 has the largest desorption peak area for acetylene and hydrogen chloride,which indicates that it has the maximum adsorption capacity for reactive gases.Therefore,we speculate that NC-800 has the largest amount of active sites.In recent years,researchers have generally studied the mechanism of carbon–nitrogen materials used in the acetylene hydrochlorination reaction,especially the key role of nitrogen atoms.In the reaction process,the raw material is first adsorbed.HCl close to N atom and adsorbed on the catalyst[14,34].Due to the interaction between C2H2and C,C2H2adsorbs on the C atom of the catalyst[15].The reaction is then performed at the reaction site,and finally the reaction product is desorbed.Then the reaction is performed at the active site,and finally the desorption process of the reaction product VCM is performed.With the increase of defects,the adsorption amount of C2H2increased,and with the increase of nitrogen content,the adsorption amount of HCl increased,so the catalytic activity was improved[35,36].Comprehensive analysis shows that the adsorption capacity of the reactive gas increases with the increase of carbon defects and N content,thereby improving the catalytic activity.
We performed a catalyst stability test on the optimal performing NC-800(Fig.9(a)).NC-800 remained stable for 200 h.After 450 h,the catalytic activity of NC-800 decreased by 20%,from 98.03%to 78.26%.The five descending curves after 400 h in Fig.9(a)are the activity test dataafter the catalyst is regenerated.In the HCl atmosphere,the catalyst was raised from room temperature to a reaction temperature of 220 °C,thereby activating the catalyst and removing the surface carbon deposits of the catalyst.The stability of the catalyst after several regenerations showed different trends.After the first regeneration,the initial activity of the catalyst reached 97% and dropped to 80% after 10 h.This may be because some carbon deposits on the surface of the catalyst were decomposed after 220°C and HCl treatment,exposing the original pore channels and active sites,thereby restoring acetylene conversion.After the second regeneration,the initial activity of the catalyst reached 94%and dropped to 73%after 20 h.As the number of regenerations increases,the catalytic activity and stability of the catalyst gradually decrease.When the catalyst was regenerated for the fifth time,its initial activity was only 80%.This may be because with the increase of the number of regenerations,the active sites are continuously consumed,and the carbon deposits are continuously accumulated,which cannot be removed by regeneration,and eventually the catalyst cannot achieve its initial activity and quickly inactivated.

Table 3 Pore structure parameters and relative nitrogen content before and after NC-800 reaction

Fig.9.(a):Stability test (at 220°C,and C2 H2 GHSV=30 h−1);(b):TGA curve of the catalyst before and after the reaction.
Fig.9(b)is a TGA test of NC-800 before and after the reaction in an air atmosphere,and the coke content is about 3.22%.Combined with BET and XPS data(Table 3),after the reaction,the specific surface area of the NC-800 catalyst decreased sharply,indicating that some micropores were blocked,and the nitrogen content decreased.In addition,the number of active pyrrolic N sites decreased after the reaction.We speculate that the carbon deposition caused the specific surface area to decrease,covering the active sites,which led to the deactivation of the catalyst and the decrease of the reaction activity.One of the ways to the activity further increase of the catalyst is inhibit the formation of coke.Nevertheless,the high catalytic activity and stability of NC-800 indicate that it has significant potential for non-metallic catalysts.
4.Conclusions
In summary,we investigated the effect of defects in N-doped carbon materials on acetylene hydrochlorination.NC-800 catalyst showed exceptional catalytic activity and stability for acetylene hydrochlorination(at 220 °C,and C2H2GHSV=30 h−1,acetylene conversion rate was 98%),with its stability reaching up to 450 h.The catalysts were characterized by various techniques,and we found that,as the amount of defects increased,the specific surface area increased,the content of active pyridine N and pyrrole N sites increased and catalytic activity increased.Therefore,the excellent catalytic activity of NC-800 is attributed to a high level of N content,a high defect content and a significant specific surface area.The design of high-nitrogen-based catalysts with rich carbon defects has stupendous contributed to the catalytic performance of acetylene hydrochlorination.
Acknowledgements
This work was supported by the National Natural Science Foundation of China(21666033),the State Key Research and Development Project of China(2016YFB0301603),and International Corporation of S&T Project in Xinjiang Production and Construction Corps(2018BC003).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Perspectives and challenges of hydrogen storage in solid-state hydrides
- Wet flue gas desulfurization performance of 330 MW coal-fired power unit based on computational fluid dynamics region identification of flow pattern and transfer process
- EMMS-based modeling of gas–solid generalized fluidization:Towards a unified phase diagram
- Using expansion units to improve CO2 absorption for natural gas purification-A study on the hydrodynamics and mass transfer
- Simulation and experimental study on the surface morphology and energy lost of the target material under non-overlapping impact of angular particles
