Kinetic study for the oxidation of cyclohexanol and cyclohexanone with nitric acid to adipic acid
2021-04-13DetaoPanGuangxiaoLiYuanhaiSuHuilongWeiZhenghongLuo
Detao Pan,Guangxiao Li,Yuanhai Su,Huilong Wei,Zhenghong Luo
Department of Chemical Engineering,School of Chemistry and Chemical Engineering,State Key Laboratory of Metal Matrix Composites,Shanghai Jiao Tong University,Shanghai 200240,China
Keywords:Adipic acid Nitric acid Tubular reactor Kinetic model
ABSTRACT The adipic acid is an important intermediate in the production of nylon,polyurethane and polyester resins.The industrial approach for preparing adipic acid is through the liquid catalytic oxidation of KA oil with nitric acid.In this work,a comprehensive model is developed for this reaction based on the kinetic study conducted in a continuous flow tubular reactor.The kinetic model fits well with the experimental results across the experimental conditions,and the average relative error between the calculated and experimental values is 5.7%.Results show that there was an induction period at the early stage of reaction.Moreover,it is found that at temperature range of 328–358 K,the formation rate of adipic acid strongly dependents on the temperature and nitric acid concentration.The developed model is used to predict the yield of adipic acid at 359–368 K.The work in this study could provide much knowledge for industrial tubular reactor design.
1.Introduction
Adipic acid(AA)is a key intermediate in the production of nylon polyurethane and polyester resins,which is also an important link in the chemistry of intermediates for its price and market availability[1–3].Interest in the development of more efficient process has been continuing to inspire researchers to explore new synthesis strategy and resources[4–9].In industry,the manufacture of AA relies on the nitric acid oxidation of the mixture of cyclohexanol and cyclohexanone(KA oil),which can be derived from liquid catalyzed oxidation of cyclohexane with air[10–14],hydrogenation of cyclohexene[15–18]or hydrogenation of phenol[19,20].The second step results in a yield of above 88%AA in the presence of copper(II)and vanadium(V)catalysts[21].
Although the oxidation of KA oil with nitric acid to AA has been industrialized for many years,the understanding of the reaction mechanism is still limited.It has been found that several intermediates reacting in series or competing steps are crucial to the product distribution.An overall scheme that describes a consensus view of the reaction mechanism has been proposed by Godt et al.[22]and van Asselt et al.[23]as shown in Fig.1.In Fig.1,there are two reaction pathways for the formation of AA.One pathway is through the hydrolysis route.KA oil is firstly oxidized into cyclohexanone and then cyclohexanone reacts with nitric acid to form 6-hydroxyimino-6-nitrohexanoicacid (NA)which is subsequently hydrolyzed to AA in acid aqueous.The other pathway is through the oxidation route.KA oil is firstly oxidized into cyclohexanone,and then cyclohexanone reacts with nitric acid to form dione which is catalytically oxidized to AA as well as a small portion of glutaric acid and succinic acid.With increasing temperature,various nitrates and nitrites are formed in proceeding of the reactions which may via the complex radical mechanism [24].Castellan et al.[21,25,26]discussed for the possible reactions that could happen under various temperature and nitric acid concentration.It is found that at low temperature and low nitric acid concentration,the generation of dione is increased with time prolonged.While at high temperature,the formation of NA is predominant.
In order to have a deeper understanding of the reaction mechanism,as well as gain better insight into the effects of various conditions on the progress of the reaction,the investigation on the kinetic characteristic is required.Besides,the study of the kinetic models is essential to the design of the reactor and the optimization of the process[27].Several investigations concerning the reaction kinetic characteristics have been reported in the literature.Van Asselt et al.[28–30]presented powerlaw models for the simplified reaction network as shown in Fig.1.The authors conducted most of their experiments in a batch reactor under the temperature range below 303 K to make sure that NA and dione were stable and the reaction rates were measurable.Qu et al.[31]studied the kinetics in a CSTR reactor at temperature range of 323–363 K and developed empirical power law rate equations for AA,and the byproducts.The authors also discussed the catalytic effect of Cu (II)and V(V)on the selectivity of the gas products including N2,NO2and N2O.Their results showed that copper and vanadate have a synergetic effect in suppressing the production of the by-products and decreasing the generation of gas products N2O,which is unable to be recovered by absorption with water and recycled into the process.
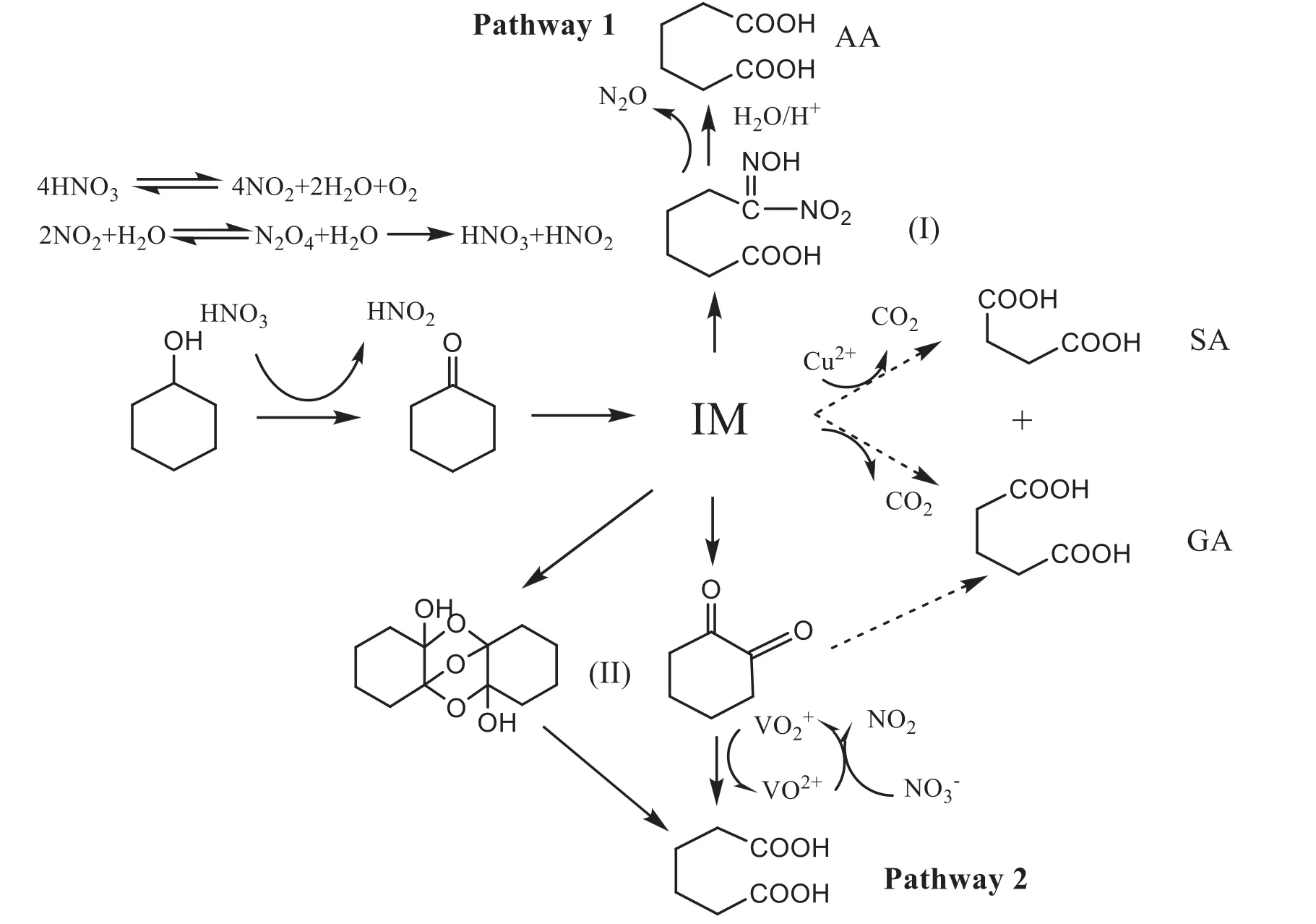
Fig.1.Adipic acid reaction pathways
The oxidation of KA oil with nitric acid is a strongly exothermic reaction.Besides,the gas generation becomes more serious at higher temperature.It is difficult to acquire accurate conversion within a short residence time in the stirred reactor at high temperature range.Moreover,the heat and mass transfer is often limited in the large reactor,which results in the high-energy consumption while low yield of adipic acid.Thus,more attentions should be taken into account to the development of energy saving and easily controllable reactor.The tubular reactor has a lot of advantages in performing the strongly exothermic and volatized reactions.For example,the pressure,temperature as well as residence time can be precisely controlled within the tubular reactor,due to the easy achievement for the fast heat and mass transfer.Our interest in the nitric acid oxidation of KA oil to AA has led us to investigate the reaction in a continuous flow tubular reactor,which inner diameter is 1 mm.However,to the best of our knowledge,the existed kinetic models have disadvantages when applied in the tubular reactor.The semi-empirical kinetic model by van Asselt et al.[23,28,29]is derived from low temperature experiments in the stirred reactor.The empirical kinetic model by Qu et al.[31]is not suitable for the tubular reactor.Therefore,there is an urge to acquire the accurate kinetic model,which can be applied for the industrial tubular reactor.
In this work,an overall kinetic model is developed for the oxidation of KA oil with nitric acid,which is conducted in the tubular reactor.The effects of various reaction conditions on the yield of AA are investigated.Meanwhile,the model is used to predict the yield of AA under various conditions.The results of this work would be favored for the achievement of high yield of adipic acid and for the design of the industrial reactor.
2.Experimental
2.1.Materials
Commercial glutaric acid(RG),succinic acid(RG),adipic acid(RG),cooper(99.9 wt%),and vanadium pentoxide(99.9 wt%)were provided by Sinopharm Chemical Reagent Co.,Ltd.Nitric acid (AR) was purchased from Macklin Biochemical Co.Ltd.Cyclohexanol and cyclohexanone(99.0%,AR)were provided by Yonghua Chemical Technology Co.,Ltd.The regents were used as received without any further purification as the chromatography investigations do not show any significant impurities.
2.2.Kinetic measurements
This work has established a complete continuous flow tubular reactor and test system for the purpose of precise control of the various reaction conditions,and accurate quantification of the product and main byproducts.The reaction system is composed of three parts:the reactor unit,the liquid analysis unit and the gas analysis unit.The concentration of the nitric acid solution and the KA oil(cyclohexanone:cyclohexanol=20:80 wt%)were accurately prepared by using an electronic balance.The initial concentration of KA oil is 0.45 mol·L−1.Catalyst Cu(electrolytic copper powder)and V(vanadium pentoxide,V2O5)which ratio was 0.4:0.04 wt%,were added to the nitric acid to dissolve.The two streams of KA oil and nitric acid were quantitatively fed into the tubular reactor by the precision syringe pumps(2PB-1040II,Beijing Xingda Science and Technology Development Co.,Ltd.).The volume flow of nitric acid was 10 ml·min−1,and that of KA oil is 0.5 ml·min−1.The two streams were joint in a T-mixer before entering the reactor.The reactor was made by the stainless steel(di=1 mm).The reaction temperature was controlled by water bath thermostat(BWS-0510,Shanghai Bluepard Instruments Co.,Ltd.).Samples at the reactor outlet were collected in bottles and they were immediately cooled down by adding cold water.
The concentrations of liquid components in the samples were measured by a high-performance liquid chromatography(LC-16,Shimadzu Chromatography).The column was WondaSil C18-WR,4.6 mm× 150 mm,5 um,which was optimized for analyzing.The flow rate for the carrier solution(mixture of ultrapure water and acetonitrile)was 0.8 ml·min−1.The detection wavelength of the detector was 210 nm.The gas product was analyzed by gas chromatography(GC-6600,Shanghai Fanwei Instrument Equipment Co.,Ltd),and TCD detector was applied.The total volume flow of gas phase at the reactor outlet was measured by a precise volume flow meter.The yield of AA is calculated by the following equations:

According to the quasi-homogeneous and quasi-steady assumption,the residence time is calculated as follows:

where V is the volume of the reactor,which is changed by varying the length of tubular reactor.Qland Qvrepresent liquid and gas volume flow rate respectively.
3.Results and Discussion
3.1.General course of the reaction
Fig.2 shows the typical curves of the dynamic development during the course of KA oil oxidation with nitric acid,which is carried out in a continuous flow tubular reactor at 338 K.As can be seen from Fig.2,AA is the main product in the liquid phase at whole reaction time,and glutaric acid and succinic acid are the byproducts.The reaction rate increases with time prolonged and the products build up within the system.The formation of glutaric acid and succinic acid is due to the conversion of intermediates[23,32–34].The addition of copper and vanadate not only inhibited the formation of glutaric acid but also improved conversion of intermediates to AA.In this work the total yield of glutaric acid and succinic acid is less than 5%in all conditions,thus their formation pathway is not discussed in detail and their content is specified.
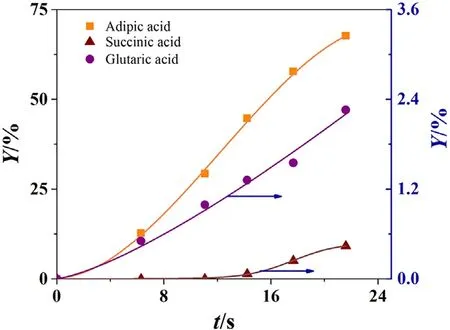
Fig.2.Products profiles at 338 K,CH0 =10.35 mol·L−1.
It also can be seen from Fig.2 that the profiles are S-type curves,in which the lag of reaction is observed during the early stage.The kinetic experiments show that the delay becomes shorter as the reaction temperature increases.Hence it is reasonable to conclude that the lag is not caused by the resistant of heat transfer.We assume the lag is attributed to an induction period,which is related to the decomposition of nitric acid to nitrous acid,which acts as the initiator of the oxidation of cyclohexanone at whole temperature range[23,32].The induction period is affected by temperature,nitric acid concentration,as well as catalysts according to this study.The catalyst shows positive effect on the reduction of the induction period.The KA oil may be oxidized by the HNO3under the catalysis of the vanadium to form carbine radicals[21].High temperature and high nitric acid concentration favors for shortening the induction period.In addition,the way to adding catalysts in the reactor also affects the early stage.It should be noted that although the presence of induction period has only minimal impact on the final yield of adipic acid,the reaction mechanism at the early stage should be paid attention to.In our further work,we will discuss these aspects in more detail.
3.2.Reaction kinetics
The kinetic study of KA oil oxidation with nitric acid within the tubular reactor was conducted at temperature range of 328–358 K,with the nitric acid concentration varying from 8.01 to 10.35 mol·L−1.The concentration profiles at various conditions are shown in Fig.3.It is seen in Fig.3 that after an induction period,there was a transition to a much higher activity regime occurs in the course of the reaction.For example,as shown in Fig.3(a),at 328 K there is less than 1%of the formation of AA before 25 s,after which the formation rate is suddenly increased.Apparently,low temperature is not suitable for industrial application.Hence in the following part,328 K is out of consideration when the kinetic model is discussed.Fig.3(b)–(d)shows that temperature and nitric acid concentration play important roles on reaction rate.The increase of temperature and nitric acid concentration as well as residence time favors for improving the reaction rate and furthermore,the yield of AA.The yield is increased from 67%to 95%when temperature is increased from 338 to 358 K,under which the length of reactor is 22 m,and CH0is 10.35 mol·L−1.Nitric acid concentration also has a positive effect on the yield.However at temperature as high as 358 K,increasing the nitric acid concentration has no significant improvement on the yield,indicating an upper limit for nitric acid concentration.It should be mentioned that the effect of mass transfer on the yield of AA was eliminated by optimizing the total liquid volume flow rate before the conduction of kinetic experiments.It is observed that as the liquid flow rate is more than 10.5 ml·min−1,the yield of adipic acid was not increased with increasing flow rate.Thus,under the condition of liquid flow rate of 10.5 ml·min−1,the mass transfer between the water–oil phase can be neglected.
The kinetic model parameters are obtained by polynomial fitting the data at various conditions.Before modeling,there are the following four basic settings:(1)It is assumed that there is no temperature gradient in the whole reactor.(2)The axial and radial diffusion can be negligible.(3) The reaction system is quasi-steady state.(4) The whole system can be treated as quasi-homogeneous.The overall kinetic model is obtained as follows:firstly establish mass balance equation according to the reaction mechanism;secondly,simplify the mass balance equations based on some reasonable assumptions;thirdly,determine the apparent reaction orders of the reactants and the corresponding apparent parameters by multiple iterations.The differential equations in this study are calculated by using MATLAB with the ode 23 function.
3.2.1.Establishing mass balance equation
According to Fig.1,the reaction network contains parallel reactions in which AA could be produced through the conversion of dione and nitrolic acid.However,the quantification of dione and nitrolic acid by HPLC was failed in this study since the pure sample was hard to be obtained.In addition,the HPLC profiles show that there are other intermediates in the reaction mixer.Therefore in this study,we do not discuss the difference between the two reaction pathways of AA.Instead,the total concentration of intermediates CKwas applied in mass balance equations.

Fig.3.Experimental adipic acid concentrations as(a)T=328 K;(b)T=338 K;(c)T=348 K;(d)T=358 K,CH0 =8.01–10.35 mol·L−1.
The decomposition of nitric acid occurs during the reaction.Along with nitrous acid,oxygen is produced by the reverse decomposition of nitric acid.Here,the influence of oxygen is not considered since the pressure of the system was low and the amount of oxygen was small.NOxis produced during the course of the reaction,however they are considered as recoverable under the experimental conditions.N2O,N2and CO2are the gas products that should be taken into account.
Accordingly,the mass balance equations are written as:
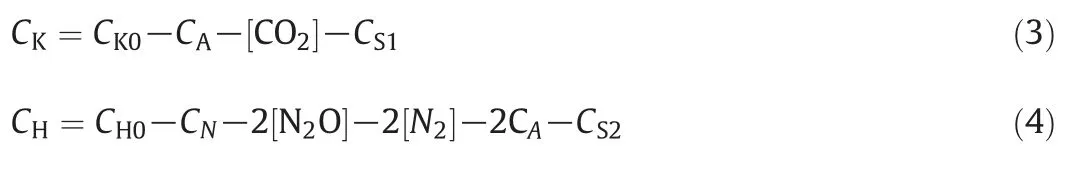
where S1 and S2 refer to intermediates and nitric acid consumed in the formation of side reactions.
Initial concentration of nitric acid,KA oil,the yield of gas products as well as the yield of AA are known,whereas the concentration of intermediates,nitric acid during the course of reaction is unknown.
3.2.2.Simplifying mass balance equation
According to the gas profiles,one can conclude that the amount of CO2and N2produced by the reaction was 10–20 times less than that of N2O,thus except N2O,the concentration of gas products is assumed to be zero in Eqs.(3)and(4).Although the stoichiometric ratio of N2O to AA was unknown,we observed from the GC profiles that the concentration of N2O and AA was almost in a 1:1 ratio under the experimental conditions.In addition,the nitrous acid was first produced by the decomposition of nitric acid,after which it was consumed in the following reactions.Therefore the concentration of nitrous acid is treated as zero.Assuming the byproducts were generated by the reaction of KA oil with HNO3,and the stoichiometric ratio of KA oil to HNO3was 1:2 during the reaction.Since the selectivity of reaction was above 95% across the experimental situations,we assume 5%reactant which did not participate in the formation of AA was consumed in the formation of other intermediates and by products.The mass balance is now simplified as follows:

3.2.3.Determining the kinetic parameter
The rate equation is written as the equation:

Due to the initial concentration of HNO3is obvious larger than that of CK,CHis assumed constant and the rate equation is simplified as follows:

Logarithm of both sides of Eq.(8),it is converted into:

According to Eq.(9),ln(dCA/dt)is linear with lnCKwhen the reaction time varies from t1 to t2,and the slope of the ln(dCA/dt)-lnCKcurve is the reaction order of CK.Here,the experimental values after the induction period are used to evaluate the formation rate of AA.By comparing different fitting method,the polynomial fit method is the most accurate way to fit the experimental data.The equation is shown as Eq.(10).The corresponding regression parameters are listed in Table 1.


Table 1 The regression parameters of cubic polynomial at different initial nitric acid concentrations at temperature of 348 K
The fitting results are shown in Fig.4.As can be seen from Fig.4,cubic polynomial method fitted the experimental value well with R2more than 99.5%.
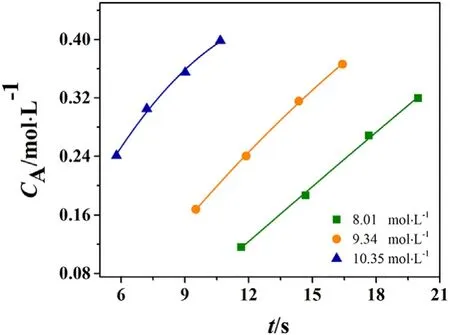
Fig.4.The experimental and calculated adipic acid concentrations as(c)T=348 K,CH0 =8.01–10.35 mol·L−1.
The calculated reaction order α is 1.06,1.1and 0.23 as the initial nitric acid concentration is 8.01 to 10.35 mol·L−1.However,1.06 is selected as the reaction order of CKsince the lower nitric acid concentration is closer to the actual condition in reactor.
When the reaction time ranges from t1 to t2,Eq.(7)is transformed to:


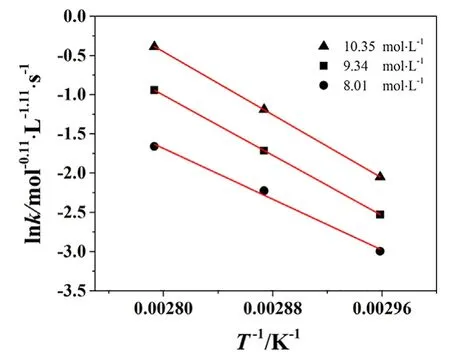
Fig.5.Activation energy at various initial nitric acid concentrations.
The rate constants as a function of temperature with initial nitric concentration varying from 8.01 to 10.35 mol·L−1are shown in Fig.5.As can be seen from Fig.5,the linear of the straight lines is satisfactory,and the reaction energy can be read from the slopes of lines,which is 81,677 J·mol−1.The pre-exponential factor is calculated from the intercept of the lines which is 1.1×1012mol−0.11·L−1.11·s−1.The kinetic model is written as:

3.3.Model validation
The calculated and experimental AA concentrations are shown in Fig.6.The initial point of simulation is changed from t=0 to the point at the beginning of the fast reaction stage.The choice of the new initial point depends on the trend of the S-type curves of the formation rate of AA.It should be noted that the new initial point has no influence on the calculated finial yield of AA.The corresponding values of ω at various conditions are shown in Table 2.
According to Table 2,the range of ω is from 0.85% to 22.05% at temperature range of 338 to 358 K with initial nitric acid varying from 8.01 to 10.35 mol·L−1.The average relative error between the calculated and experimental values is 5.7%.The largest error appears at 338 K,at which when the initial concentration of nitric acid increases from 8.01 to 10.35 mol·L−1,ω decreases from 22.5% to 2.45%.With increasing temperature,the values of ω decrease,indicating a better performance of the developed model under these conditions.At 338 K,the calculated values are larger than the experimental values.The possible reason may be that at lower temperature,the simplification of the mass balance equations may not be accurate.Besides,the length of the induction period has a significantly influence on the relative error between the simulated result and the experimental results at low temperature range.However,in practical industrial applications,the reaction is often carried out under higher temperature range.The developed model could be used to predict the reactor performance at industrial conditions.
3.4.Application of the developed model
The formation rate of AA depends on the nitric acid concentration and reaction temperature.The developed model is used to calculate the concentration profiles of AA at temperature range of 359–368 K with nitric acid concentration varying from 8.01 to 10.35 mol·L−1.The results are shown in Fig.7.It should be noted that the effect of the induction period is out of consideration in the prediction.However,when the developed model is applied in the industrial reactor design,the lag of reaction should be considered,since it has a certain effect on the calculation of the reactor volume.

Fig.6.Calculated and experimental adipic acid yields as(a)CH0 =8.01 mol·L−1,(b)CH0 =9.34 mol·L−1,(c)CH0 =10.35 mol·L−1 at 338–358 K.
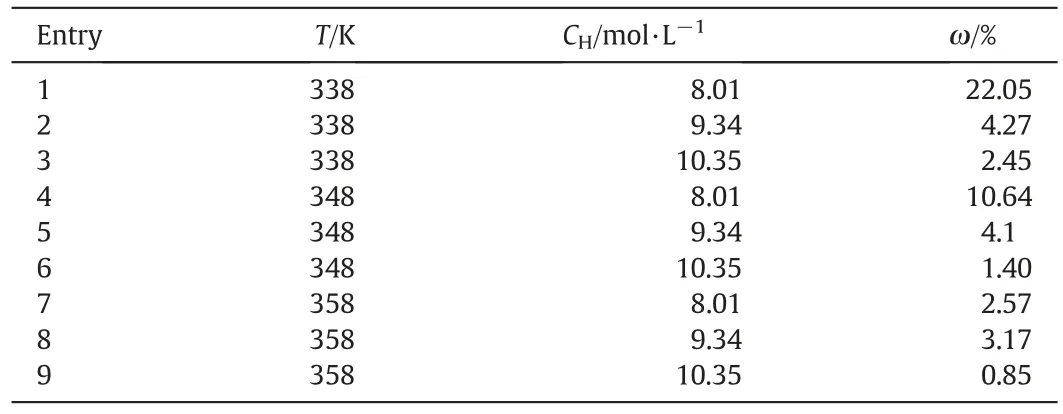
Table 2 The relative errors between the experimental and calculated values at different reaction conditions
As can be seen from Fig.7,at temperature range of 359–368 K,the formation rate and yield of AA is significantly influenced by temperature.When the initial nitric acid concentration is 8.01 mol·L−1,the reaction time decreases from 11.3 to 5.6 s with temperature increasing from 359 to 368 K.However,the increase in nitric acid concentration has no significant effect on the yield.The reason may be that when temperature is above 358 K,the water content at high nitric acid concentration decreases in liquid phase,resulting in the inhibition of hydrolysis of the intermediates.It should be noted that some side reactions might become serious with increasing temperature.The selectivity in the high temperature range may be lower than 95%.It should be very important to control the temperature in the industrial process.
4.Conclusions
In summary,the oxidation of KA oil with nitric acid is enhanced when it is performed in a tubular reactor.The induction period at the early stage of reaction is observed,which has only minimal impact on the yield of adipic acid.At 328 K,the induction period is longer than 25 s at low nitric acid concentration.The kinetic study shows that in the temperature range of 328–358 K,the reaction rate is strongly dependent on temperature,residence time and nitric acid concentration when the nitric acid concentration varies from 8.01 to 10.35 mol·L−1.An overall kinetic model that can be applied to calculate the formation rates and yields has been developed.The apparent reaction orders of the intermediates and nitric acid are 1.06 and 0.05 respectively.The apparent activation energy is 81.677 kJ·mol−1.The kinetic model fits well with the experimental results across the experimental conditions,and the average relative error between the calculated and experimental values is 5.7%.The developed model has been used to predict the formation rate of adipic acid at temperature range of 359–368 K.It is shown that at temperature range of 359–368 K,the increase in nitric acid concentration has no significant effect on the yield.Moreover,an excess of nitric acid might have a negative effect due to that the hydrolysis of intermediates was suppressed with low water content in the liquid phase.
Nomenclature
CAConcentration of adipic acid,mol·L−1
CciCalculated concentration of adipic acid,mol·L−1
CHConcentration of nitric acid,mol·L−1
CKConcentration of all the intermediate,mol·L−1
CKA0,CH0Initial concentration of KA oil and nitric acid,mol·L−1
k Rate constant,mol-0.11·L-1.11·s−1
n Number of data point
Ql,QvTotal volume flow of liquid phase and gas phase,ml·min−1
T Temperature,K
t Residence time,s
V Volume of reactor,ml
Y Yield of the product
α,β Parameters of kinetic equations
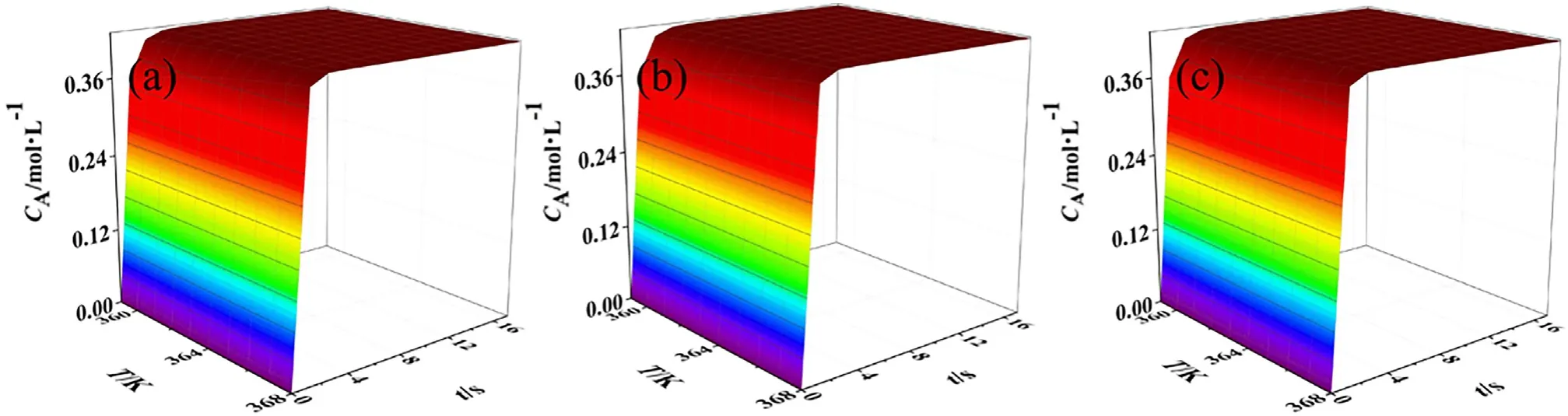
Fig.7.The predicted adipic acid concentration profiles at 359–368 K:(a)CH0 =8.01 mol·L−1,(b)CH0 =9.34 mol·L−1,(C)CH0 =10.35 mol·L−1.
ω Average relative error
Acknowledgements
The authors thank the Ningbo Science and Technology Plan Project(2018B10013),the Program of Shanghai Subject Chief Scientist(18XD1402000) and the computational support from the Center for High Performance Computing,Shanghai Jiao Tong University for supporting this work.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Perspectives and challenges of hydrogen storage in solid-state hydrides
- Wet flue gas desulfurization performance of 330 MW coal-fired power unit based on computational fluid dynamics region identification of flow pattern and transfer process
- EMMS-based modeling of gas–solid generalized fluidization:Towards a unified phase diagram
- Using expansion units to improve CO2 absorption for natural gas purification-A study on the hydrodynamics and mass transfer
- Simulation and experimental study on the surface morphology and energy lost of the target material under non-overlapping impact of angular particles
