Preparation of hybridizing zeolitic imidazolate frameworks with carboxymethylcellulose for adsorption separation of n-hexane/3-methylpentane
2021-04-13
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology,Advanced Catalysis and Green Manufacturing Collaborative Innovation Center,Changzhou University,Changzhou 213164,China
Keywords:Zeolitic imidazolate frameworks Carboxymethylcellulose(CMC)n-Hexane Adsorption separation
ABSTRACT A novel ZIF-8-CMC hybrid material was fabricated from the hybridization of ZIF-8 and carboxymethylcellulose(CMC) by impregnation method for n-hexane/3-methylpentane separation.The surface properties of ZIF-8 were tailored by introducing CMC into ZIF-8 nanoparticles.In this work,adsorption separation of n-hexane(nHEX)and 3-methylpentane(3MP)on ZIF-8-CMC were investigated by batch vapor-phase adsorption and liquid-phase breakthrough adsorption.The adsorption selectivity of nHEX/3MP reversed from preferable adsorption of nHEX to preferable adsorption of 3MP upon the increasing of CMC containing in the hybrid materials.As the temperature increases,the adsorption amounts of nHEX and 3MP decrease.With the increasing of CMC contents,the nHEX uptake decreased,the uptake capacity of 3MP increased gradually.For liquid-phase breakthrough adsorption,the dynamic adsorption capacity of nHEX also decreased with the increasing of temperature.
1.Introduction
The naphtha and straight kerosene mainly contains n-alkanes of C6to C20,in which n-C6to n-C14are usually known as the light liquid paraffins and n-C14to n-C20are usually known as heavy liquid wax.n-Alkanes are very important chemical raw materials for the production of alkyl benzene,benzenesulfonate synthetic detergent,lubricant additives,paraffin oil,and a series of chemicals[1–3].Among the light liquid paraffins,C6alkane isomers have similar chemical and physical properties.The kinetic diameters of n-hexane(nHEX)and 3-methylpentane(3MP)are 0.43 and 0.50 nm,respectively[4,5].nHEX is widely used to produce high quality solvent oils and obtain high yields of ethylene during steam cracking,while branched isomers are preferred ingredients in gasoline due to their high research octane number(RON)[6].Therefore,the separation of linear hydrocarbons from their branched isotherms becomes extremely important.
C6alkane isomers could be separated through adsorptive separation techniques using fixed bed absorbents such as zeolites and MOFs(metal–organic frameworks)[7–13].In 1956,the laboratories of Linde Air discovered the synthesis of molecular sieve zeolite 5A that has the ability to completely separate n-paraffins from other hydrocarbons,which is a major success even today in the petrochemical industry[14].The main applications are the total isomerization process from UOP[15,16]and the Ipsorb process from Axens[17].In these processes,low RON normal paraffins [i.e.nHEX (RON 30)]are separated from high octane branches [i.e.2,2-dimethylbutane (22DMB) (RON 94),2,3-dimethylbutane (23DMB) (RON 105)][13],and the maximum working capacity of zeolite 5A for the adsorption of the linear nHEX is close to 13 wt%[18].Meanwhile,MOFs have been extensively studied for gas storage and separation due to their high porosity and pore size,fascinating tunability of shape and surface function [19–23].Among them,zeolite imidazolate frameworks(ZIFs)are of particular interest due to their chemical and thermal stability[24].Specially,ZIF-8 adopts the sodalite (SOD) topology bearing zinc cations and methylimidazolate ligands,which possesses permanent porosity involving large spherical cavities(1.14 nm)connected by a flexible six-member ring of free aperture of 0.34 nm.Structure of ZIF-8 consists of a cubic arrangement with 8 sodalite cages in the corners.The higher specific surface area,larger pore volume as well as the adjustable pore size of ZIF-8 make it a good medium to sieve branched alkanes from linear alkanes[25].
The ability to alter the size and shape of the pores within MOFs has led to these architectures being used as shape-selective materials that preferentially retain compounds with specific dimensions,a particularly useful property during the separation of constitutional isomers of hexane.Previous studies introduced porous crystalline MOFs into carboxymethylcellulose (CMC) solution to produce a hierarchically porous MOF[26].Herein,we attempted to combine ZIF-8 with CMC to obtain ZIF-8-CMC composite to separate nHEX and its isomer 3MP.
In this work,the synthesis and application of ZIF-8-CMC microcrystals were investigated.Systematic study on the adsorption separation of hexane isomers was developed using ZIF-8-CMC series adsorbents.Adsorption equilibrium,kinetics and multicomponent breakthrough adsorption experiments were carried out to measure the adsorbed amount of nHEX and 3MP.The influence of temperature on the single adsorption isotherms and multicomponent breakthrough curves were also analyzed.
2.Materials and Methods
2.1.Materials
2-Methylimidazole,zinc nitrate hexahydrate,acetone,ethanol,N,N-dimethylformamide (DMF),carboxymethylcellulose(CMC),acetonitrile,dichloromethane(CH2Cl2),nHEX and 3MP were all purchased from Aladdin Reagent Corporation.nHEX and 3MP were ≥99%purity.
2.2.Synthesis of ZIF-8 and ZIF-8-CMC
ZIF-8 nanoparticles were initially prepared in an aqueous system at room temperature described in our previous report[27].The obtained ZIF-8 powder (0.6 g) was then dispersed in a 15 cm3solution of mixed DMF/acetone (V/V=2:3) with sonication for 20 min in a 100 cm3round-bottom flask.The suspension was collected after rotary evaporation to remove acetone,and 9 cm3water was added to the mixture followed by another 20 min sonication.After stirring with pre-prepared CMC solutions (0.3 g,0.6 g,0.9 g in 10 cm3water,600 rpm for 10 min)for 1 h,a series of ZIF-8-CMC gel with different CMC loadings was achieved.CH3CN was added to the resulting gel and ZIF-8-CMC hybrid solids were finally obtained.The as-synthesized ZIF-8-CMC samples were soaked in dichloromethane for three days and dried at 353 K to complete activation.The CMC loadings were 33 wt%,50 wt% and 60 wt%,respectively,denoted hereafter as ZIF-8-CMC (33%,50%,60%).
2.3.Characterization
Powder X-ray diffraction(PXRD)patterns were collected at room temperature using a Rigaku D/MAX-2500PC diffractometer (Rigaku Co.,Japan)with Cu Kα1radiation(λ=0.15406 nm)operated at 40 kV and 100 mA.Scanning electron microscopy(SEM)images were taken at an acceleration voltage of 5 kV with a Zeiss Supra-55 microscope.Thermogravimetric analysis(TG)(TG/DTA-6300)of the activated ZIF-8,CMC and ZIF-8-CMC(33%,50%,60%)samples were performed from room temperature to 1023 K with a scan rate of 10 K·min−1under N2atmosphere.N2adsorption isotherms were measured at 77 K using a Micromeritics ASAP2460 sorption analyzer.
2.4.Adsorption experiments
Vapor-phase batch adsorption measurements were performed on an Intelligent Gravimetric Analyzer(Model IGA-100B,Hiden Isochema Instrument) with a high sensitivity of 0.1 μg.The instrument has an ultrahigh vacuum system precisely controlled by a computer allowing the accurate gravimetric changes to be recorded in gradually increased relative pressure values.And the change of the adsorbed amounts upon time was also recorded.Before the measurement,each sample was vacuum-degassed overnight at 353 K to remove water molecules and other guest species adsorbed in the pores.Batch adsorption experiments were performed at 298 K and 393 K,respectively.
2.5.Breakthrough experiments
The dynamic adsorption separation of nHEX and 3MP were investigated using breakthrough experiments of binary mixtures in liquid phases.To testify the reproducibility,parallel dynamic experiments were carried out three times.The breakthrough experimental flow diagram is shown in Fig.S1.Before the adsorption experiments,samples were activated at 353 K for 4 h to remove the physically adsorbed water molecules.The stainless steel column (10 cm length,1.0 cm inner diameter) entirely filled with adsorbent (1–2 g) was operated by continuously introducing hexane isomer mixtures with known composition[the mass fraction nHEX/3MP=1:1,and isooctane was used as the solvent(60%)in the inlet mixture]at a fixed total pressure(0.5 MPa and 1.2 MPa),and was adjusted to keep a total flow rate of 0.5 cm3·min−1.A gas chromatography with FID detector (Shimadzu GC2010 chromatography)was used to determine the composition of exit liquid mixture from the adsorption column.
The equilibrium dynamic adsorption capacity(qc)of the adsorbents is calculated from the breakthrough curves according to the equation as follows:

where Ciand C0are the outlet and inlet mass concentrations(%)of the stream through the fixed bed column,respectively;u(cm3·min−1)is the volume flow rate of hexane isomer mixtures;m(g)is the dosage of the adsorbent;V′(cm3)is the dead space volume of adsorbent bed void and around the pipe;ρ(g·cm−3)is the density of the binary mixture.TheCidt is the total outlet mass of component i by integration of the breakthrough curves measured in terms of outlet concentration as a function of time.
The separation factor S,for a binary mixture of components i and j,is defined as follows:

3.Results and Discussion
3.1.Characterization
The PXRD patterns of synthesized ZIF-8 and ZIF-8-CMC hybrid materials are displayed in Fig.1.The PXRD patterns with unique peaks at 2θ=7.5°,10.4°,12.7°,and 18.4°are in accordance with the simulation patterns of ZIF-8 from the literature[24].After the hybridization of CMC,the characteristic diffraction peaks of ZIF-8-CMC were similar to those of ZIF-8.This indicated the topology of ZIF-8 was well retained in ZIF-8-CMC sample.
The morphologies of ZIF-8-CMC composite materials were measured by SEM and displayed in Fig.2.In comparison of pure phase CMC material (Fig.S2,Supporting information),there are many aggregated polyhedral nano-scale particles on the surface of ZIF-8-CMC samples.As the concentration of CMC increases,higher crystallinity is detected.
Fig.3 shows the thermal stability of the synthesized hybrid samples.It can be seen that ZIF-8-CMC(33%,50%,60%)have similar TG curves.The slight mass loss below 473 K is attributed to the removal of physically adsorbed water and solvent,and the predominant mass loss is observed at~573 K,which is attributed to the decomposition of organic ligands and collapse of frameworks.Mass loss doesn't stop up to 1073 K with the remaining amount of only 20%.The higher CMC contents within ZIF-8-CMC,the more mass loss occurred for the hybrid materials.

Fig.1.PXRD patterns of ZIF-8,ZIF-8-CMC(33%),ZIF-8-CMC(50%),ZIF-8-CMC(60%)and simulated ZIF-8.

Fig.2.SEM images of(a,b)ZIF-8-CMC(33%),(c,d)ZIF-8-CMC(50%),(e,f)ZIF-8-CMC(60%).

Fig.3.TG curves of ZIF-8,ZIF-8-CMC(33%),ZIF-8-CMC(50%),ZIF-8-CMC(60%)and CMC samples.

Fig.4.N2 adsorption–desorption isotherms of ZIF-8 and ZIF-8-CMC samples.
The porous properties of the synthesized materials were analyzed with nitrogen adsorption–desorption isotherms,and the results were displayed in Fig.4 and summarized in Table 1.The Brunauer–Emmett–Teller(BET)method was utilized to calculate the specific surface areas using adsorption data in a relative pressure ranging from 0.05 to 0.30.The pore size distributions and pore volume were derived from the adsorption branches of the isotherms.For ZIF-8,the nitrogen uptake increases rapidly at low relative pressures,and the isotherm becomes smooth at relative pressures higher than 0.1,indicating a typical type I isotherm of microporous materials.However,an enhancement in uptake could be observed at relative pressures higher than 0.8,this indicates multilayer adsorption occurred at high relative pressures.For the hybrid ZIF-8-CMC materials,the isotherms exhibit similar characteristics of ZIF-8,that is,high uptake at low relative pressures and certain uptake at high relative pressures.It should be noted that with the increase of CMC content,the nitrogen uptake decreases gradually.Moreover,the micropore diameters of ZIF-8 and ZIF-8-CMC are all at about 0.50–0.55 nm and 0.77 nm as shown in Fig.5.For the hybrid materials which have high amounts of CMC (60 wt%),a peak at about 1.1 nm could be observed with a new pore generating upon the addition of CMC.As indicated in Table 1,the surface area and total pore volume of micropores for the ZIF-8-CMC materials significantly decrease with the increasing of the CMC loading,and the average pore diameters become slightly wider.

Table 1 Pore structure parameters of ZIF-8 and ZIF-8-CMC
3.2.Adsorption isotherms
The single component vapor-phase isotherms of nHEX and 3MP adsorbed on ZIF-8-CMC at 298 K and 393 K are shown in Fig.6.With the increasing amount of CMC in ZIF-8-CMC,the nHEX uptake decreases,while the 3MP uptake increases.For the hybrid material with 33 wt%CMC,the largest adsorption amounts of nHEX is higher than 80 mg·g−1.It is worth noting that the adsorbed amounts of 3MP(41 mg·g−1)is larger than that of nHEX(17 mg·g−1)for ZIF-8-CMC(60 wt%CMC).Obviously,the adsorption selectivity of nHEX/3MP was reversed from preferable adsorption of nHEX to preferable adsorption of 3MP with the increasing amount of CMC in the hybrid materials,which have significant affinity for isoalkane with larger polarity due to the OH and COOH groups of the CMC material.On the other hand,the adsorbed amounts of nHEX and 3MP measured at 298 K are larger than their corresponding value at 393 K.

Fig.5.Pore size distribution curves of ZIF-8 and ZIF-8-CMC samples.
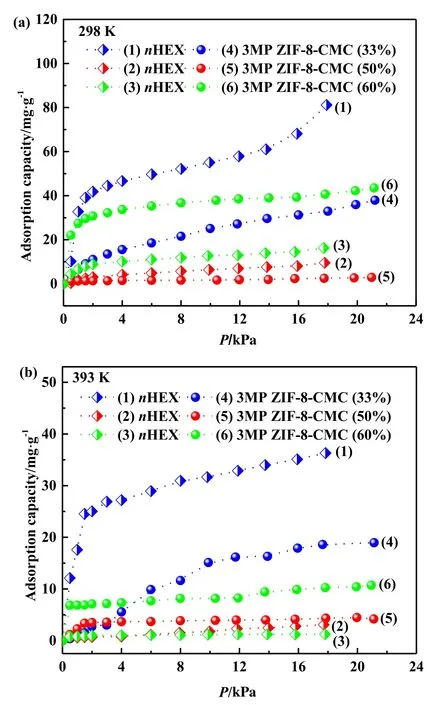
Fig.6.Single compound vapor-phase adsorption isotherms for nHEX and 3MP on ZIF-8-CMC (33%,50%,60%) at (a) 298 K and (b) 393 K.
The batch adsorption data obtained are better fitted with the Freundlich equation.The fitted curves are depicted in Fig.S3 and the Freundlich isotherm constants are presented in Table S1 and S2.Generally,the value of n can be used to denote whether the adsorption is favorable.When n >1,it is favorable adsorption;when n <1,it is difficult to adsorb.It can be seen from Table S1 that the adsorption isotherms of 3MP on ZIF-8-CMC(33%and 60%)at 298 K are obviously suitable for the Freundlich adsorption isotherm equation,the fitting degree R2reaches 0.99 and n >1,indicating that the process is favorable adsorption.As indicated in Table S2,at 393 K,all of the Kfparameters decrease,which is in accordance with the general rule that Kfdecreases with the increasing of temperature.
3.3.Adsorption kinetics

Fig.7.Adsorption rate curves of nHEX and 3MP on ZIF-8-CMC(33%,50%,60%)(a)298 K and(b)393 K.
The adsorption rate plots obtained using an IGA-100B Gravimetric Analyzer are shown in Fig.7.The adsorption amount of nHEX is superior to 3MP on ZIF-8-CMC(33%)at 298 K and 393 K.At 298 K,the equilibrium adsorption capacities of nHEX on ZIF-8-CMC(33%,50%,60%)is ZIF-8-CMC(33%)>ZIF-8-CMC(50%)>ZIF-8-CMC(60%),while that of 3MP is the complete opposite.From Fig.7,we can see that the adsorption of 3MP reached equilibrium on ZIF-8-CMC(60%) in the shortest time.With the increasing of the CMC fraction,the adsorption time for 3MP to reach saturation becomes shorter,while that of nHEX becomes longer and the adsorption capacity decreases from >80 mg·g−1to 15 mg·g−1.ZIF-8-CMC(33%) has the largest adsorption capacity for nHEX,and ZIF-8-CMC (60%) has the highest adsorption capacity for 3MP.The equilibrium adsorption capacities of nHEX and 3MP at 298 K on ZIF-8-CMC (33%) are 81.1 and 37.9 mg·g−1,respectively.On the other hand,the adsorption capacities decrease significantly with the increasing of the temperature.At 393 K,the equilibrium adsorption capacities of nHEX and 3MP on ZIF-8-CMC(33%)fall to 36.3 and 18.9 mg·g−1,respectively.It is clear that the adsorption separation selectivity of nHEX and 3MP decreases with the increasing of the temperature.The equilibrium adsorption capacities of nHEX and 3MP at 298 K on ZIF-8-CMC(60%)are 16.2 and 43.5 mg·g−1,respectively.At 393 K,the equilibrium adsorption capacities of nHEX and 3MP are 1.2 and 10.7 mg·g−1,respectively.In addition,the specific surface area of ZIF-8-CMC(60%)is the smallest,but the adsorption amount for 3MP at 293 K is the largest,probably due to its highest content of CMC,which makes the pore size of hybrids more favorable for 3MP adsorption.
To analyze the adsorption rate of nHEX and 3MP on ZIF-8-CMC under different temperatures,both the pseudo-first-order kinetic model and the pseudo-second-order kinetic model were used to describe the kinetic data.For these two models,all steps including internal diffusion,external diffusion,and adsorption were considered,assuming that the difference between the average concentration in solid phase and the equilibrium concentration was the driving force of the adsorption.The total adsorption rate was proportional to either the driving force as in the pseudo-first-order equation or the square of the driving force as in the pseudo-second-order equation.
The relevant kinetic parameters are presented in Tables S3 and S4,and the fitted curves are depicted in Figs.S4–S7.Qmis the maximum adsorption capacity measured by Gravimetric Analyzer.It can be seen that the adsorption amount of nHEX on ZIF-8-CMC(33%)is the largest,and the adsorption kinetic data at 298 K fitted the pseudo-first-order kinetic model better,while those at 393 K were more suitable for the pseudosecond-order kinetic model.The better fitness of pseudo-first-order model indicates that the adsorption of nHEX on ZIF-8-CMC (33%) at 298 K is only a surface adsorption process.And with the increasing of adsorption temperature,all the processes extended from surface adsorption to external or internal diffusion through the pores.
The adsorption kinetics of nHEX on ZIF-8-CMC(50%)at 298 K and 393 K is more in accordance with the pseudo-first-order kinetic equation,while the adsorption kinetics of 3MP adsorption is more in accordance with the pseudo-second-order kinetic equation.Obviously,ZIF-8-CMC(50%)is not suitable for adsorption separation of nHEX and 3MP because its adsorption capacities and selectivity for nHEX and 3MP are too small.
The adsorption of 3MP on ZIF-8-CMC(60%)was larger than that of nHEX at 298 K.The adsorption process of 3MP on ZIF-8-CMC(60%)at 298 K and 318 K can be described well by the pseudo-second-order kinetic model,the R2values of which are both larger than 0.99.From Tables S3 and S4,it can be seen that the deviation of the fitted value Qeby the pseudo-second-order kinetic equation from experimental data is small.This further indicates that the pseudo-second-order kinetic model can better describe the adsorption process.
3.4.Dynamic breakthrough curve measurements
On the basis of the industrial separation conditions[13,28],a set of breakthrough experiments of binary nHEX/3MP mixtures with equivalent mass were performed at 298 K/0.5 MPa and 393 K/1.2 MPa to evaluate the adsorption dynamics behavior and separation potential of ZIF-8-CMC(33%,50%,60%)materials.Breakthrough curves are shown in Figs.8 and 9.

Fig.8.Breakthrough curves for the separation of equivalent mass binary mixtures of nHEX/3MP on(a)ZIF-8-CMC(33%),(b)ZIF-8-CMC(50%),(c)ZIF-8-CMC(60%)at 298 K/0.5 MPa.

Fig.9.Breakthrough curves for the separation of equivalent mass binary nHEX/3MP mixtures on(a)ZIF-8-CMC(33%),(b)ZIF-8-CMC(50%),(c)ZIF-8-CMC(60%)at 393 K/1.2 MPa.
Fig.8 illustrates the breakthrough curves of nHEX/3MP mixtures on ZIF-8-CMC materials at 298 K/0.5 MPa.ZIF-8-CMC(33%)exhibits the largest dynamic adsorption capacity towards nHEX.However,the dynamic adsorption amount of nHEX and 3MP on ZIF-8-CMC (50%)seems little,and the separation effect is negligible.Furthermore,the dynamic saturated adsorption capacity of 3MP is better than nHEX on ZIF-8-CMC(60%).The dynamic separation selectivity of nHEX/3MP is as follows:ZIF-8-CMC (33%) >ZIF-8-CMC (60%) >ZIF-8-CMC (50%),which is consistent with the pure component isotherm.Owing to the strong temperature effect for pure component adsorption,the dynamic adsorption experiments were also conducted at 393 K for the separation of nHEX/3MP binary mixture(Fig.9).
Dynamic adsorption capacities and separation factors at 298 K and 393 K are listed in Table 2.The selectivity as well as the dynamic adsorption capacities of nHEX/3MP over ZIF-8-CMC materials decrease with the increasing of the temperature.The less CMC-loaded materials preferentially adsorb nHEX,exhibiting the normal adsorption hierarchy of hexane isomers over reported MOFs materials,which typically follow the order of the normal boiling points of these hexane isomers(linear >monobranched >dibranched)with the exception of ZIF-76[29,31–33].In comparison with ZIFs and zeolite 5A adsorbents(Table 2),the separation factors of these hybrid materials for nHEX over 3MP are either comparable with or slightly inferior to that of ZIF-8[29].The result indicated that the composites of ZIF-8 and CMC could retain the separation effect for nHEX and 3MP of the pristine ZIF-8 to some degree.
4.Conclusions
Different amounts of CMC were incorporated into the zeolitic imidazolate framework material ZIF-8,producing a new kind of hybridmaterials[ZIF-8-CMC(33%),ZIF-8-CMC(50%),and ZIF-8-CMC(60%)]using impregnation method.The selectivity of nHEX/3MP adsorbed on ZIF-8-CMC was reversed from preferable adsorption of nHEX to preferable adsorption of 3MP with the increasing loading amounts of CMC.Meanwhile,with the increasing of the CMC content,the adsorption capacity of nHEX decreases and 3MP increases.The batch adsorption capacity of nHEX on ZIF-8-CMC (33%) is the highest at 298 K,about 81.1 mg·g−1,while the adsorption capacity of 3MP on ZIF-8-CMC(60%)at 298 K is the largest,more than 40 mg·g−1,which indicated that the added CMC has strong affinity for branched 3MP with larger polarity due to the robust OH and COOH groups within CMC.The content of CMC has an effect on the pore size of hybrid materials.ZIF-8-CMC(33%)has the best separation effect of nHEX and 3MP with the largest separation selectivity,probably because of its largest specific surface area and well shape-matching with straight chain nHEX.The total pressure of 0.5 MPa and the temperature of 298 K is the better experimental condition for dynamic adsorption separation.

Table 2 Overview of dynamic adsorption capacity and separation selectivity of binary nHEX/3MP adsorbed on ZIF-8-CMC materials(iso-octane as solvent,c0 (nHEX):c0 (3MP)=1:1,total flow rate of 0.5 cm3·min−1)and zeolite 5A obtained from breakthrough curves
Nomenclature
CiOutlet mass concentrations of the stream through the fixed bed column,%
C0Inlet mass concentrations of the stream through the fixed bed column,%
KfFreundlich adsorption constants,mg·g−1·kPa−1
m Dosage of the adsorbent,g
QeEquilibrium adsorption capacity,mg·g−1
QmMaximum adsorption capacity,mg·g−1
QtAdsorption capacity at time t,mg·g−1
qcEquilibrium dynamic adsorption capacity,mg·g−1
S Separation factor
u Volume flow rate of hexane isomer mixtures,cm3·min−1
V′ The dead space volume of adsorbent bed void and around the pipe,cm3
ρ Density of the binary mixture,g·cm−3
Acknowledgments
This research was supported by the National Natural Science Foundation of China(Nos.11775037 and 21676030),the Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology(BM212110),and The Postgraduate Innovation Project of Changzhou University(KYCX19_1782).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2020.05.023.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Perspectives and challenges of hydrogen storage in solid-state hydrides
- Wet flue gas desulfurization performance of 330 MW coal-fired power unit based on computational fluid dynamics region identification of flow pattern and transfer process
- EMMS-based modeling of gas–solid generalized fluidization:Towards a unified phase diagram
- Using expansion units to improve CO2 absorption for natural gas purification-A study on the hydrodynamics and mass transfer
- Simulation and experimental study on the surface morphology and energy lost of the target material under non-overlapping impact of angular particles
