Highly Stretchable and Transparent Hydrogel as a Strain Sensor
2021-04-08WANGJilong王霁龙HUXuefeng胡雪峰ZHANGXintian张新添QIUJingjing邱婧婧
WANG Jilong(王霁龙), HU Xuefeng(胡雪峰), ZHANG Xintian(张新添), QIU Jingjing(邱婧婧)
1 Key Laboratory of Textile Science & Technology, Ministry of Education, Donghua University, Shanghai 201620, China
2 College of Textiles, Donghua University, Shanghai 201620, China
3 Department of Mechanical Engineering, Texas Tech University, Lubbock TX 79409, USA
Abstract: The rapid developments of artificial intelligence have attracted attention in designing electronic skin (e-skin) to realize the mechanical and sensory properties of human skin. To better imitate the tactile sensing properties of human skin, a stretchable and transparent hydrogel is produced. Thus, an elastic and capacitive strain sensor was successfully produced through the as-prepared hydrogel. The sensor was elastic with a high conductive stability and could detect the strain changes in different states, which had very short response time that could be applied into the detection of large and small deformations and would shed light on its application in e-skin.
Key words: electronic skin (e-skin); hydrogel sensor; strain; ion conductivity
Introduction
Skin is one of the largest sensory organs of human body, which contains various sensors that can sense the air temperature and the humidity by converting physical or chemical stimuli into nerve impulses[1-2]. The wide ranges of sensory capabilities and environmental stretchability of human skin have received enormous attention in recent two decades[3-6]. Enormous efforts have been made to develop soft electronics or actuators in order to mimic the skin’s unique characteristics for health monitoring and human-machine interfaces[7]. Such devices, also known as“artificial skin”or“electronic skin (e-skin)”, can transduce environmental stimuli such as strain[3, 7-10], pressure[11-15], moisture[16-17], temperature[18-20], and vibration[21]into some measurable electronic signals like current, resistance, or capacitance. In these sensors, rigid electrical materials such as graphene sheets[22], liquid metals[23], and metal nanostructures[24-25]are applied as conductors to transmit signals. These materials improve the conductivity that is necessary for the e-skin, while they compromise the elasticity and the transparency, which is quite important for the future application.
Traditionally, e-skin is usually fabricated into a stretchable sheet with sensitive sensor materials and provides signals through electrical conductors like graphene sheets[22], silver nanowires[26],etc., which transmits signals via electrons. Human skin conducts the signals via ions, which offers a novel method to advance the ionic conductors based on many sensory sheets called as “ionic skin”[27-28]. Sunetal.[27]proposed the concept of “ionic skin” for the first time that counts on ionic transduction through hydrogels. They had successfully combined ionic conductors with stretchable dielectrics to make actuators, which had some deformation response to the external forces. They constructed sheets of dispersed sensors to exhibit the ability of the sensor in detecting the location and the pressure of touch. Furthermore, Leietal.[28]discovered a bioinspired mineral hydrogel and fabricated it into a novel kind of ionic skin sensors that were adaptable to the mechanical force. The sensors they had produced were elastic and self-healable, which could sense the subtle pressure variations, such as a mild finger touch, or even small water droplets.
Common sensors conduct external signals through stretchable electrical conductors that transmit signals via electrons. Researchers fabricated the electrical conductors by adding carbon materials such as graphene sheets[29], carbon grease[30], and carbon nanotubes[31]to improve the conductivity of the hydrogel. These methods not only improved the conductivity, but also compromised the elasticity. Lietal.[32]prepared a conductive hydrogel by combining polyaniline and polyacrylamide (PAAm) viainsitupolymerization. They studied the effects of two different acids (hydrochloric acid and p-toluene sulfonic acid) doped polyaniline on the properties of hydrogels, but the added materials compromised the flexibility of the hydrogel.
Hydrogels, of which the polymeric networks swollen with water, are very stretchable and transparent. They can act very tough as elastomers[33], which can detect large deformations, such as pressure changes caused by a joint motion. In addition, lots of hydrogels are biocompatible that can be applied as biometric sensors[34], and the high transparency of hydrogels can make these sensory sheets be capable of conducting electrical signals without impeding optical signals[27].
In this paper, we explore the e-skin that has the potential in the progress of new kinds of stretchable and transparent sensory sheets. Acrylamide (AAm) has been used as a gel material for a long time due to its excellent polymerization properties, and it can polymerize into transparent gel with good biocompatibility via a simple way. Elastic hydrogels grounded on PAAm polymeric networks with sodium chloride (NaCl) solution are achieved by the free radical reaction. In addition, a strain sensor is produced to detect some small deformations and subtle pressure variations.
1 Experiments
1.1 Materials
AAm is used as a monomer,N,N′-methylenebis (acrylamide) (MBAA) is used as a crosslinker,N,N,N′,N′-tetramethylethylene diamine (TEMED) is used as a crosslinking accelerator, and ammonium persulfate (APS) is used as a radical initiator. NaCl is used as an electrolyte and polyethylene (PE) film is used as a dielectric. They were purchased from Sigma Aldrich (Shanghai, China).
1.2 Preparation of ionic hydrogel
Elastic conductive hydrogels are prepared as shown in Fig. 1. PAAm network hydrogels are achieved through the simple and effective free radical reaction and immersing process[27].
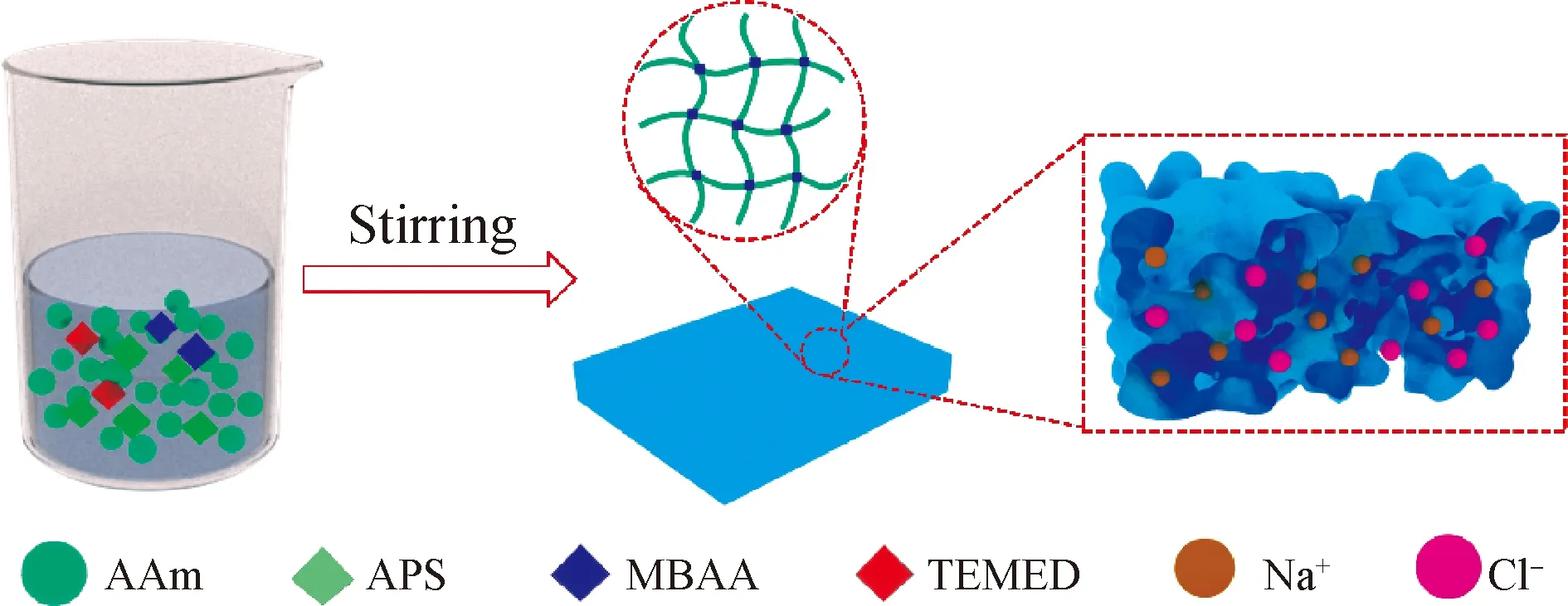
Fig. 1 Schematic diagram of preparation process
A mass of 2.4 g AAm was added into 10 mL deionized (DI) water. MBAA (0.002 5) and APS (0.015) in regard to the mass of AAm monomer were appended as the crosslinker for AAm and the radical initiator, respectively. The hydrogels were synthesized by dissolving AAm monomer powder and NaCl into DI water. The molar concentrations of NaCl were fixed as 2.74 mol/L throughout the entire experiments. After being completely dissolved, 5 μL TEMED was added into the mixture as an accelerator, which was cooled in an ice-bath. After 10 min strong stirring, the solution was poured into a mould to achieve the desired shape. After being placed overnight at room temperature, the achieved gels were kept in a humid box(a box with a bottle of water to keep the environment moist) for further testing. A multi-meter was used to measure the resistance and the capacitance changes of strain sensors.
1.3 Characterization
The conductivity test of ionic hydrogels was measured by a multi-meter under different strains, and the details can be summarized as follows. The multi-meter was set to the ohmmeter and zeroed. The two pointers of the multi-meter were placed on both sides of the hydrogel respectively, and the results were recorded after the number was unchanged. For the hydrogel under a tensile state, the clips were clamped on both sides of the hydrogel and stretched to a certain elongation, and the results were recorded until the number was unchanged.
Morphology of the hydrogels was characterized by a JEOL JSM-5600LV scanning electron microscope (SEM).
2 Results and Discussion
Different from rigid strain sensors, our hydrogel sensors have an excellent application prospect in soft strain responsive materials of wearable devices because of their excellent stretchability and flexibility. As shown in Fig. 2(a), the PAAm hydrogels can withstand a high-level deformation of twisting without any mechanical failure and can be stretched twice of its original length without breaking[shown in Fig. 2(g)]. It can be seen from Figs. 2(d) and (h) that the hydrogel can knot and do not crack after continuous stretching. In Figs. 2(e) and (f), the hydrogel can be compressed without any observable damages, indicating that our hydrogels possess a remarkable stretchability and elastic recoverability. NaCl in water makes the hydrogel be an excellent ion conductor. The main reason for the excellent tensile properties of the hydrogel is that the AAm monomer forms a linear molecular network after free radical reaction. The macromolecular network can deform at the external force and the crosslinking point will not be destroyed. After the external force is removed, it will recover to its original state. The network hydrogel achieves an extremely high stretchability, excellent transparency, conductivity and biocompatibility. The conductivity of the ionic hydrogels can reach up to 267 mS/cm, which is higher than that of the common hydrogels.
In addition, it is indispensable to illustrate that the hydrogels can be applied tightly to the skin surface or glove[shown in Fig. 2(b)] and can extend along the direction of skin movement[shown in Fig. 2(c)], indicating that these hydrogels can be applied as the possible candidates for wearable sensors and devices in the future. The prepared hydrogel has such a good deformability mainly because its network structure can absorb the tensile or compressive energy, and it can still recover after the release of external force.
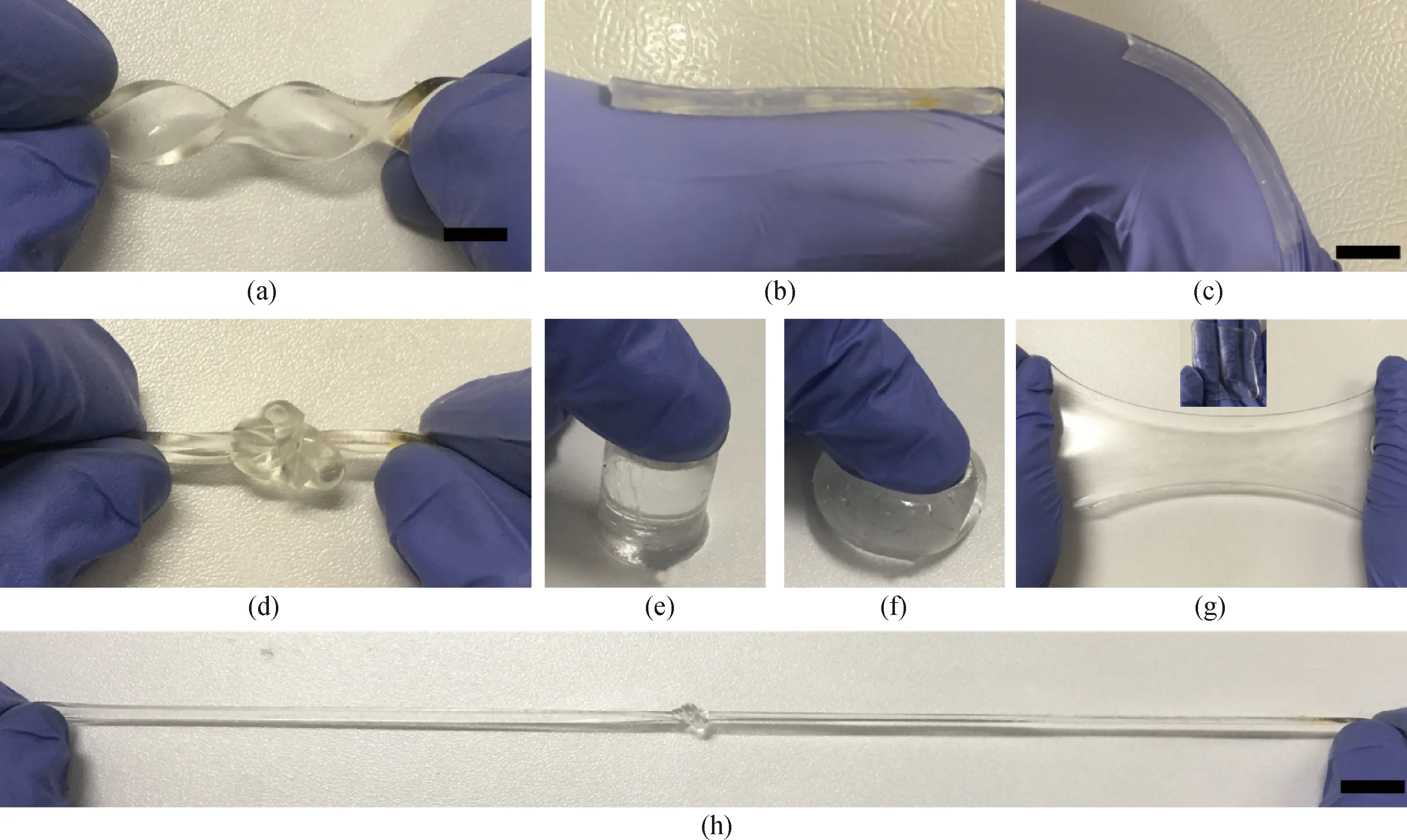
Fig. 2 Remarkable elasticity for PAAm hydrogels: (a) twisting; (b) wrist bending at 0°; (c) wrist bending at 60°; (d) knotting; (e) before compression; (f) in compression; (g) a large expansion without fracture, with original sample (5 cm × 5 cm) inserted; (h) stretching; (scar bar: 2 cm)
As shown in Fig. 3(a), the highly transparent PAAm hydrogel was produced, and it could be clearly observed. Also the transparent hydrogel allows this sensory sheet to convey electrical signals without affecting photosensitive signals[shown in Fig. 3(b)]. The conductive hydrogel could be utilized as ionic wires connecting the circuit by inletting Na+and Cl-into the hydrogel. The bright light was shown when the transparent PAAm hydrogel was connected to wires. Our hydrogels had an excellent electric conductivity owing to a large number of mesopores containing lots of Na+and Cl-. Interestingly, the light became dark when the hydrogel was stretched, and turned bright again when the hydrogel was recovered to its original shape[shown in Fig. 3(c)]. In the initial state, the lamp is quite bright. With the gradual stretching of the hydrogel, the brightness of the lamp gradually decreased until it was finally extinguished. With the recovery of the hydrogel deformation, the brightness of the lamp gradually returned to its original state. That is because the stretchable elongation along the planar direction leads to a higher resistance. In the light of the formula of resistance,
whereR,ρ,l, andSstand for resistance, resistivity, length, and area, respectively. When the hydrogel was strained, the length of the hydrogel increased greatly; while the cross-section area only had a slight decrease. And they led to a growth in resistance. Besides, during the stretching process, the micro networks are stretched and the ion channel is continuously compressed, and thus the ion flow in the hydrogel is hindered, causing the lamp gradually dimer.

Fig. 3 Transparent PAAm hydrogel with NaCl solution: (a) high transparence; (b) conductivity;(c) high stretchablility
Figure 4 shows the SEM photograph of the as-prepared hydrogel in NaCl aqueous solution. It could be clearly observed that there were numerous interconnected mesopores inside of the hydrogel, which could be explained by the continuous stirring in the preparation process so that the holes were arranged along a certain direction. These mesopores formed an ion channel so that the conductivity of hydrogels was greatly improved.
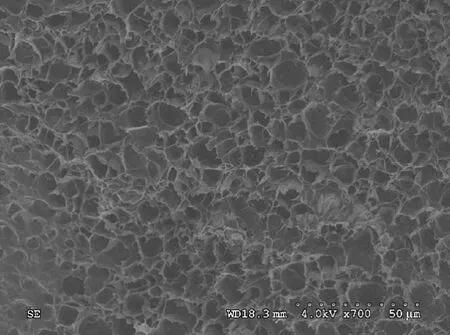
Fig. 4 SEM photograph of as-prepared hydrogels
Based on this phenomenon, a simple strain resistive sensor was fabricated via connecting conductive hydrogel films onto electrical wires. As shown in Fig. 5(a), a transparent ionic hydrogel film was stretched with different strains and did not show any fractures in the entire stretching process, showing an excellent stretchability and elasticity. The relative resistance changeQwas calculated by
Q=(R-R0)/R0×100%,
whereRandR0stand for stretched resistance and original resistance, respectively. The relative resistance changeQwas achieved in a loading-unloading cycle[shown in Fig. 5(b)]. When the hydrogel was stretched to the half of its original length, its resistance increased by 30%. When the strain reached 100%, the resistance increased only to 50%. However, when the strain reached 150%, the resistance almost doubled. During the stretching process, the ion channel is continuously compressed and the ion flow is hindered, which makes the resistance increase. In addition, the hydrogel was cyclically stretched by 100% during the streching-recovery process, and a stable resistive change was achieved[shown in Fig. 5(c)]. It indicated that the PAAm hydrogel was elastic with a high conductive stability, and it could be used for a long time without performance degradation.

Fig. 5 Conductivity test results of hydrogel sensors: (a) elongation change connected in electric circuit; (b) relative resistance change versus time at corresponding strain; (c) repeated response of conductive hydrogels
3 Conclusions
In conclusion, we have successfully prepared capacitive sensors and new kinds of sensory sheets by the PAAm hydrogel. This hydrogel is highly stretchable, transparent and conductive, and the sodium chloride in water makes it an excellent ion conductor. The prepared ionic hydrogel is successfully fabricated into a strain sensor to detect the strain variations in different states. The stable resistive change shows that the hydrogel sensor is stable in the conductivity, which will shed light on its application in e-skin.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Polyacrylonitrile/Graphene-Based Coaxial Fiber-Shaped Supercapacitors
- Effect of Processing Parameters on Morphology and Mechanical Properties of Hollow Gel-Spun Lignin/Graphene Oxide/Poly (Vinyl Alcohol) Fibers
- Characteristic Analysis and Harmonic Feature Identification of Micro-Vibration on Flywheels
- Defect Detection Algorithm of Patterned Fabrics Based on Convolutional Neural Network
- Tufting Carpet Machine Information Model Based on Object Linking and Embedding for Process Control Unified Architecture
- Multiple Needle Electrospinning for Fabricating Composite Nanofibers with Hierarchical Structure
