单头饲养和群体饲养的西花蓟马实验种群生命表比较
2021-03-26李欣华王登杰雷仲仁王海鸿
李欣华,王登杰,雷仲仁,王海鸿
单头饲养和群体饲养的西花蓟马实验种群生命表比较
李欣华1,王登杰2,雷仲仁1,王海鸿1
1中国农业科学院植物保护研究所植物病虫害生物学国家重点实验室,北京 100193;2绵阳农业科学研究院,四川绵阳 621000
【】对西花蓟马()实验种群生命表的研究大多采用单头饲养(individual-rearing,IR)的方式,研究结果可用于田间自然情况下种群发生的预测。然而西花蓟马在自然情况下常常是群聚发生,而非单头发生。论文旨在比较单头饲养和群体饲养(group-rearing,GR)两种方式建立起的西花蓟马实验种群的生命表参数,探讨何种方式建立起的生命表用于预测自然条件下西花蓟马的发生情况可能更为准确。分别用单头饲养和群体饲养的方式构建西花蓟马实验种群在菜豆豆荚上的年龄-阶段两性生命表,比较两种饲养条件下西花蓟马的生活史和种群参数;采用bootstrap方法计算种群生长参数的平均数和标准误;应用检验(Mann-Whitney test)(Sigmaplot 12.5)估计单头饲养和群体饲养条件下西花蓟马种群参数、发育历期和繁殖力间的差异。单头饲养和群体饲养对若虫期、蛹期、雄虫寿命、总产卵前期、单雌产卵量、蛹重、成虫体长有显著影响, 对卵期、成虫期、成虫产卵前期、雌虫寿命、蛹长、蛹宽、成虫体宽的影响不显著。单头饲养西花蓟马的若虫期(4.49 d)、蛹期(4.03 d)、雄虫寿命(22.82 d)、总产卵前期(11.37 d)显著长于群体饲养(3.05、3.32、18.64、10.00 d);单头饲养西花蓟马的蛹重(0.03 mg)、雌成虫体长(203.72 μm)、雄成虫体长(149.74 μm)、单雌产卵量(48粒)显著低于群体饲养(0.07 mg、288.81 μm、203.39 μm、133.39粒)。内禀增长率()、周限增长率()、净增殖率(0)、总生殖率()和平均世代时间()在单头饲养的情况下分别为0.161 d-1、1.175 d-1、20.730、35.699、18.70 d,在群体饲养情况下分别为0.242 d-1、1.274 d-1、60.499、102.342、16.88 d。群体饲养的西花蓟马种群增长比单头饲养的快。相对于单头饲养的西花蓟马,群体饲养的西花蓟马种群增长速度更快、单位时间内产生后代数更多,若使用单头饲养方式建立生命表对西花蓟马种群发生动态进行预测预报可能会延误最佳防治时机,以群体饲养的方式建立的生命表对田间种群的动态预测应该更准确。
西花蓟马;年龄-阶段两性生命表;单头饲养;群体饲养
0 引言
【研究意义】西花蓟马()是一种世界性发生的,危害蔬菜、花卉及经济作物的重要害虫[1-3],起源于美国西部洛基山脉,2000年在中国云南省首次发现[4],并迅速传播,已造成了巨大的经济损失[4-6]。昆虫生命表是研究昆虫种群在一定生态条件下各阶段个体的存活数、死亡数和死亡原因的重要手段[7]。到目前为止,对西花蓟马生命表的研究均采用单头饲养(individual-rearing,IR)方式,包括寄主[8-12]、温度[13-15]、杀虫剂[16]、病原物[17]、生殖方式[18]、种群[19]以及复合因素[20-21]的影响。但事实上,在自然条件下西花蓟马常群聚发生[22]。单头饲养和群体饲养(group-rearing,GR)的昆虫生活史之间存在显著差异。因此,根据上述两种饲养方式分别建立生命表,对比生命表数据,可为西花蓟马生命表研究探索更可靠的方法,也为西花蓟马预测预报及防治提供更详实的理论依据。【前人研究进展】单头饲养的雄性太平洋甲虫蟑螂()要比群体饲养(8—10头)的发育历期更长、成虫体型更大[23]。群体饲养(4—35头每组)与单头饲养的西南龟瓢虫()在生长速度、产卵前期、成虫体型等方面有差异[24]。4龄松尺蠖()幼虫群体饲养时的生长速度快于单头饲养[25]。群体饲养的斑点木蝶()比单头饲养的个体成虫体型小[26]、雌虫产卵量多[27]。因上述饲养方式的不同而产生生活史差异的现象在家蟋蟀()[28]、西方玉米根虫()[29]、黏虫()[30]、亚洲玉米螟()[31]、二点委夜蛾()[32]、桃小食心虫()[33]、玉米蛀茎夜蛾()[34]、梨豆夜蛾()[35]、黄粉虫()[36]、冈比亚按蚊()[37]、玻里尼西亚斑蚊()[38]、稻纵卷叶螟()[39]、小地老虎()[40]中也有发现。【本研究切入点】单头饲养西花蓟马的生命表得到了广泛的研究,但目前为止,还没有关于群体饲养西花蓟马的生命表研究。【拟解决的关键问题】通过设置相同饲养环境,比较单头饲养和群体饲养两种方式下的生物学特性和生命表参数,以期为西花蓟马种群动态监测及其综合治理决策提供依据。
1 材料与方法
试验于2019—2020年在中国农业科学院植物保护研究所植物病虫害生物学国家重点实验室完成。
1.1 供试虫源
西花蓟马2018年采自北京昌平区辣椒()田中。在中国农业科学院植物保护研究所植物病虫害生物学国家重点实验室建立实验种群。饲养于三洋人工气候箱内(MLR-351H,SANYO Electric Co.,Ltd),环境温度(24±1)℃,光周期14 h﹕10 h(L﹕D),相对湿度(70±10)%。以菜豆()为寄主植物和产卵基质。供试全部虫源为10对西花蓟马在菜豆上产卵12 h后取得。
1.2 单头饲养和群体饲养西花蓟马各虫态发育历期测定
试验在三洋人工气候箱中进行(气候条件设定同1.1)。将上述含卵寄主转移至双开口圆柱形玻璃罐(底部直径×高=10 cm×30 cm,本试验所用玻璃罐均为同一规格)中,玻璃罐两端用尼龙纱网(200目)封住保持透气,内部放置一张滤纸吸收罐内多余水分。每日在体视显微镜(SZX16,OLYMPUS)下观察卵的孵化情况,将新孵出的1龄若虫转移至新玻璃罐中并记录,作为试验虫源分别进行单头饲养和群体饲养。剩余同批孵化西花蓟马分别以上述两种饲养方式留作备用虫源。每日定时更换新鲜菜豆,观察记录单头饲养和群体饲养西花蓟马羽化前的发育与存活情况。试验虫源配对后,每日定时取出含卵寄主(菜豆)至新玻璃罐中,卵孵化后,将1龄若虫的数量记为产卵量[17]。
1.2.1 单头饲养 将新孵出的1龄若虫分装至玻璃罐中,每罐1头,共40罐。待羽化后,在体视显微镜下鉴定成虫性别并记录,然后雌雄配对,若新羽化的试验虫源雌雄数量不对等,则使用备用虫源配对补齐。配对后,继续记录试验虫源的寿命、产卵量,直至其死亡。若试验虫源配偶先死亡,则随机从备用虫源中为其另选配偶进行配对。蛹期取40头备用虫源,测量其蛹重、蛹长、蛹宽,羽化后各取40头雌雄备用虫源分别测量体长、体宽。
1.2.2 群体饲养 所用方法参考文献[41-42]并加以改进,将新挑出的1龄若虫共40头[17]装入玻璃罐中,逐日观察,羽化后鉴定成虫性别,记录其产卵量和寿命。在饲养过程中,若所有雌虫全部死亡,则随机从备用虫源中选取与存活雄虫数量相等的雌性与其进行配对,记录剩余雄性试验虫源的寿命;若所有雄虫全部死亡,则随机从备用虫源中选取与存活雌虫数量相当的雄性进行配对,记录剩余雌性试验虫源的产卵量和寿命。试验虫源全部死亡后试验停止。其蛹重、蛹长、蛹宽,雌雄虫体长、体宽的测量方法同1.2.1。
1.3 数据分析
计算得出上述值后,再根据下列公式分别计算,内禀增长率(,intrinsic rate of increase,,初始年龄为0)、周限增长率(,finite rate of increase,λ=e)、总生殖率(,gross reproductive rate,=∑m)、净增殖率(0,net reproductive rate,)、平均世代时间(,mean generation time,=(lnR)/)等参数。其中代表年龄,代表阶段,代表龄期数。s′代表一个年龄阶段的个体存活到年龄阶段的概率,假设s=1,计算方法依据CHI等[43]。
根据上述计算公式,原始数据可使用TWOSEX- MSChart[47](http://140.120.197.173/Ecology,Visual BASIC环境,国立中兴大学)完成相关运算。种群参数的平均值和标准误使用bootstrap[48]法进行运算。采用检验(Mann-Whitney test)(Sigmaplot12.5,Systat Software Inc.,Chicago,IL)估计单头饲养和群体饲养的西花蓟马种群参数、发育历期和繁殖值间的差异。
体长、体宽、体重等数据使用SPSS 23.0计算平均值及标准误,采用配对-test进行显著性分析。
2 结果
2.1 饲养方式对西花蓟马生长发育和繁殖的影响
单头饲养和群体饲养中每阶段各指标如表1所示。其中若虫期、单雌平均产卵量(<0.001),总产卵前期、蛹重、雌成虫体长、雄成虫体长(<0.01),蛹期、雄虫寿命(<0.05)差异显著。单头饲养和群体饲养的卵期、雌成虫期、雄成虫期、雌成虫产卵前期、雌虫寿命、蛹长、蛹宽、雌成虫体宽、雄成虫体宽均无显著差异。单头饲养西花蓟马的若虫期(4.49 d)、蛹期(4.03 d)、雄虫寿命(22.82 d)、总产卵前期(11.37 d)显著长于群体饲养(3.05、3.32、18.64、10.00 d);单头饲养西花蓟马的蛹重(0.03 mg)、雌成虫体长(203.72 μm)、雄成虫体长(149.74 μm)、单雌产卵量(48粒)显著低于群体饲养(0.07 mg、288.81 μm、203.39 μm、133.39粒)。

表1 单头饲养和群体饲养的西花蓟马生活史
:该时期存活的西花蓟马个数the number of surviving individuals ofof the specific period。雌成虫产卵前期代表雌虫从羽化到产卵第1天;
总产卵前期代表雌虫从卵期一直到产卵第1天Adult preoviposition period (APOP) is defined as the duration from eclosion to the first day of oviposition.
Total preoviposition period (TPOP) is defined as the duration from eggs to the first day of oviposition
采用SPSS中的成对-test进行差异显著性分析Significant difference was analyzed by paired-sample-test of SPSS,***:<0.001;**:<0.01;*:<0.05;ns:无显著差异No significant difference。表2同The same as Table 2
2.2 饲养方式对西花蓟马存活率和繁殖率的影响
存活率曲线s描述的是个体存活到年龄阶段的可能性,可观察到重叠现象[49]。如图1所示,单头饲养若虫总历期(74 d)、蛹总历期(84 d)长于群体饲养。第8天时,单头饲养若虫已有72.5%化蛹,12.5%的个体仍为若虫,且未出现羽化个体,而群体饲养若虫已全部化蛹,且有20%的已羽化为成虫。第9天时,单头饲养若虫已全部化蛹,但无羽化个体,群体饲养37.5%个体已完成羽化。第10天时,单头饲养有7.5%的个体羽化,此时群体饲养个体已全部羽化。饲养至第14天时,单头饲养个体全部羽化。
雌虫特定年龄繁殖力(f)、特定年龄存活率(l)、特定年龄繁殖力(m)、特定年龄繁殖值(lm)如图2所示。f是年龄阶段的单日平均产卵量[49],雌成虫位于生活史的第4阶段,故为f。单头饲养于f出现最大值4.26,群体饲养于f出现最大值为15.56,但f出现次高峰值,与峰值接近,其值为15.44。
l是年龄的存活率;m是年龄时所有个体平均生产子代数;lm是l与m的乘积,是年龄时所有存活个体的繁殖值[49]。从图2可以看出单头饲养的l值一直匀速下降,第35天,大部分蓟马的寿命达到最大值,曲线开始急剧下降;群体饲养的l曲线在第16天开始急剧下降,此时试验虫源雄虫个体的数量开始锐减,在饲养至第23天时,试验虫源雄虫全部死亡。
特定年龄生命期望(e)(图3)是指在年龄阶段个体的剩余存活时间,生命期望值会随着年龄增长逐渐降低[49],单头饲养的生命期望(25.721.8 d)高于群体饲养。单头饲养雄虫的生命期望从第16天起出现反常并在第24天超过雌虫,原因为其中两头雄虫的寿命超过了平均寿命(22.82 d),分别为24、30 d,与之配对的雌虫均未产卵。
特定年龄-阶段繁殖值(v)(图4)是指在年龄阶段的个体对以后的种群贡献,初孵若虫的繁殖值(v)等于周限增长率()[49]。单头饲养的v值为1.175,群体饲养的v值为1.274。相对于单头饲养(v= 18.36),群体饲养(v=27.70)的繁殖值高峰较高,出现得更早。饲养至23 d时,群体饲养的试验虫源雄虫已全部死亡,使用试验虫源雌虫以雌雄比1﹕1进行配对后,第25天又出现一次峰值(v=20.52)。

图1 单头饲养和群体饲养西花蓟马的特定年龄-阶段特定存活率

图3 单头饲养和群体饲养西花蓟马的特定年龄-阶段生命期望
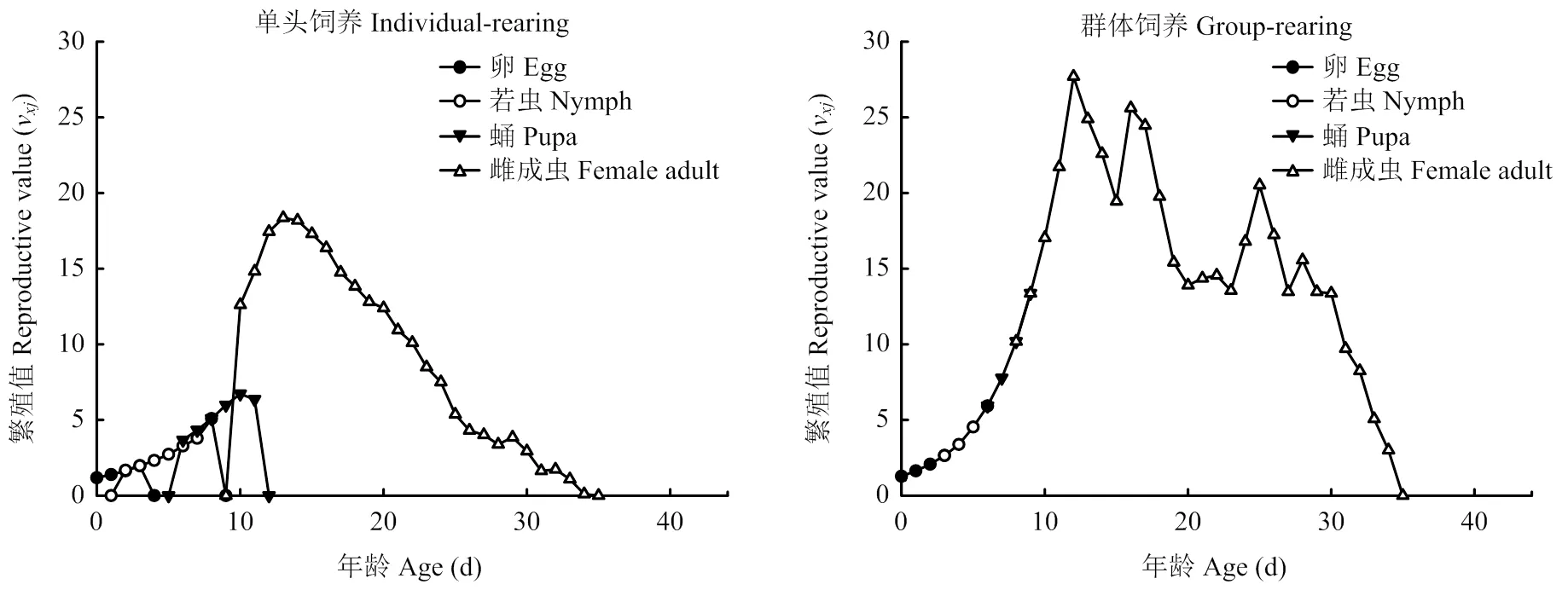
图4 单头饲养和群体饲养西花蓟马的特定年龄-阶段繁殖值
2.3 饲养方式对西花蓟马后代种群参数的影响
单头饲养的内禀增长率(0.161 d-1)、周限增长率(1.175 d-1)、净增殖率(20.730)和总生殖率(35.699)均极显著低于群体饲养(0.242 d-1、1.274 d-1、60.499、102.342),而平均世代时间(18.70 d)极显著高于群体饲养(16.88 d)(表2)。
3 讨论
单头饲养和群体饲养的西花蓟马在发育历期、成虫体长、蛹重、单雌平均产卵量、成虫寿命等方面存在显著差异。
群体饲养的西花蓟马发育历期显著短于单头饲养个体,蛹重和成虫体长显著大于单头饲养个体。此种由于饲养密度不同而引起的发育历期差异的现象在秀丽线虫()上也有发现[50]。群体饲养较单头饲养昆虫发育时间短的现象在太平洋甲虫蟑螂[23]、珠腹珀蟋()[51]、西南龟瓢虫[24]、亚洲玉米螟[31]中也有发现。

表2 单头饲养和群体饲养西花蓟马的种群参数
与单头饲养相比,群体饲养的昆虫发育历期短、蛹重较重可能与其幼虫期取食成功率较高有关[52-54]。例如,觅食中的黑腹果蝇()幼虫会通过视觉信号和化学信号(气味等)追踪到正在进食的同种其他个体,减少觅食行为所花费的精力、时间[52]。同样,昆虫的胰岛素信号通路会对其营养摄取量进行反馈,营养摄取量多时该通路相关基因表达量升高,发育时间缩短,体尺增大。反之,发育时间增长,体尺减小[53]。在七叶树蝴蝶()中,其类固醇激素和蜕皮激素同样可起到调节发育速度和体尺的作用[53]。对于西花蓟马,其发育历期、蛹重、体尺与取食成功率、营养摄取量的关系,以及内在的分子机制,仍有待进一步研究。
群体饲养西花蓟马的单雌平均产卵量显著高于单头饲养。类似的现象在螺旋粉虱()上也有发现[55]。成虫体型和体重影响昆虫繁殖力[56-59]。埃及伊蚊()在产卵最多时体型大的个体单雌平均产卵量、产卵次数显著高于体型小的个体[56]。斑点木蝶中体型较大的雌性个体体内拥有较多的成熟卵[57]。地中海实蝇()的雌体体型大小与最大日产卵量显著正相关[58,60]。日本黑蝇()的体型(翅长、头宽)与繁殖力成正比[59]。黑腹果蝇成虫体重低的个体产卵量少[61]。与单头饲养相比,群体饲养的西花蓟马产卵量较高,可能与其体型较大,繁殖力较强有关。
单头饲养西花蓟马的平均寿命长于群体饲养西花蓟马,类似的现象在秀丽线虫上也有发现[50]。大多数生物(包括昆虫在内)生存能量的分配会在繁殖和寿命间进行权衡,生殖能力强的个体寿命短[62]。当限制黑腹果蝇交配机会(切除雄性的外生殖器)时,雌虫寿命显著增长[63];限制其产卵(移除产卵基质)时,黑腹果蝇的寿命会延长[62,64]。与单头饲养相比,群体饲养的西花蓟马单雌平均产卵量较高、寿命较短,可能与此有关。
单头饲养方式会造成产卵延迟。单头隔离饲养的西花蓟马首次产卵时间比群体饲养平均推迟0.81 d。同样,在秀丽线虫中,单头饲养的群体首次产卵时间显著晚于群体饲养[50]。有报道指出,西花蓟马产卵时间的延迟会造成产卵量的下降[65],可能是因为卵母细胞发育不良或被吸收。单头饲养西花蓟马产卵量低于群体饲养可能与其产卵时间存在延迟有关。
单头饲养西花蓟马的内禀增长率、周限增长率、净增殖率和总生殖率显著小于群体饲养,平均世代时间显著长于群体饲养。由此可见,群体饲养西花蓟马种群扩增要显著优于单头饲养种群。若用单头饲养的方式建立生命表,对西花蓟马种群动态进行预测预报,很可能错过最佳防治时机。
4 结论
单头饲养和群体饲养的西花蓟马生命表参数存在显著差异。群体饲养西花蓟马生命表参数可能更符合田间种群发生动态,建议使用群体饲养的方式建立西花蓟马生命表。
[1] REITZ S R, GAO Y, KIRK W D J, HODDLE M S, LEISS K A, FUNDERBURK J E. Invasion biology, ecology, and management of western flower thrips. Annual review of entomology, 2020, 65: 17-37.
[2] REITZ S R. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): The making of a pest. Florida Entomologist, 2009, 92(1): 7-13.
[3] CHILDERS C C, LEWIS T. Feeding and oviposition injuries to plants//Thrips As crop pests.Wallingford, UK:CAB International, 1997:505-537.
[4] WU S, TANG L, ZHANG X, XING Z, LEI Z, GAO Y. A decade of a thrips invasion in China: lessons learned. Ecotoxicology, 2018, 27(7): 1032-1038.
[5] GAO Y, LEI Z, REITZ S R. Western flower thrips resistance to insecticides: detection, mechanisms and management strategies. Pest Management Science, 2012, 68(8): 1111-1121.
[6] ZHANG B, QIAN W, QIAO X, XI Y, WAN F. Invasion biology, ecology, and management ofin China. Archives of insect biochemistry and physiology, 2019, 102(3): e21613.
[7] MORRIS R F, MILLER C A. The development of life tables for the spruce budwormCanadian Journal of Zoology, 1954, 32(4): 283-301
[8] ZHANG Z J, WU Q J, LI X F, ZHANG Y J, XU B Y, ZHU G R. Life history of western flower thrips,(Thysan., Thripae), on five different vegetable leaves. Journal of Applied Entomology, 2007, 131(5): 347-354.
[9] SORIA C, MOLLEMA C. Life-history parameters of western flower thrips on susceptible and resistant cucumber genotypes. Entomologia Experimentalis et Applicata, 1995, 74(2): 177-184.
[10] 郅军锐, 李景柱, 盖海涛. 西花蓟马取食不同豆科蔬菜的实验种群生命表. 昆虫知识, 2010, 47(2): 313-317.
ZHI J R, LI J Z, GAI H T. Life table for experimental population offeeding on leguminous vegetables. Chinese Bulletin of Entomology, 2010, 47(2): 313-317. (in Chinese)
[11] HULSHOF J, KETOJA E, VäNNINEN I. Life history characteristics ofon cucumber leaves with and without supplemental food. Entomologia Experimentalis et Applicata, 2003, 108(1): 19-32.
[12] GAUM W G, GILIOMEE J H, PRINGLE K L. Life history and life tables of western flower thrips,(Thysanoptera: Thripidae), on English cucumbers. Bulletin of Entomological Research, 1994, 84(2): 219-224.
[13] ULLAH M S, LIM U T. Life history characteristics ofand(Thysanoptera: Thripidae) in constant and fluctuating temperatures. Journal of Economic Entomology, 2015, 108(3): 1000-1009.
[14] JIANG S, ZHANG N, WANG S, WANG J, LI J, ZHANG B, ZHENG C. Effects of heat shock on life parameters of(Thysanoptera: Thripidae) F1offspring. Florida Entomologist, 2014, 97(3): 1157-1166.
[15] 王海鸿, 薛瑶, 雷仲仁. 恒温和波动温度下西花蓟马的实验种群生命表. 中国农业科学, 2014, 47(1): 61-68.
WANG H H, XUE Y, LEI Z R. Life tables for experimental populations of(Thysanoptera: Thripidae) under constant and fluctuating temperature. Scientia Agricultura Sinica, 2014, 47(1): 61-68.(in Chinese)
[16] 杨广明, 郅军锐, 李顺欣, 刘利. 乙基多杀菌素和印楝素对西花蓟马生长发育及繁殖的亚致死效应. 应用生态学报, 2016, 27(11): 3698-3704.
YANG G M, ZHI J R, LI S X, LIU L. Sublethal effects of spinetoram and azadirachtin on development and reproduction of(Pergande). Chinese journal of applied ecology, 2016, 27(11): 3698-3704. (in Chinese)
[17] ZHANG T, REITZ S R, WANG H, LEI Z. Sublethal effects of(Ascomycota: Hypocreales) on life table parameters of(Thysanoptera: Thripidae). Journal of Economic Entomology, 2015, 108(3): 975-985.
[18] DING T, CHI H, GOKCE A, GAO Y, ZHANG B. Demographic analysis of arrhenotokous parthenogenesis and bisexual reproduction of(Pergande) (Thysanoptera: Thripidae). Scientific reports, 2018, 8(1): 3346.
[19] NIELSEN M C, TEULON D A J, CHAPMAN R B, BUTLER R C, Drayton G M, PHILIPSEN H. Comparison of life history parameters of two(Thysanoptera: Thripidae) strains in New Zealand. Environmental Entomology, 2010, 39(2): 303-311.
[20] LOWRY V K, SMITH J W, MITCHELL F L. Life-fertility tables for(Hinds) and(Pergande) (Thysanoptera: Thripidae) on peanut. Annals of the Entomological Society of America, 1992, 85: 744-754.
[21] HOLLINGSWORTH R G. Life history observations on(Thysanoptera: Thripidae) infesting gardenia in Hawaii, and a comparison of the humidity requirements forandproceedings of the hawaiian entomological society, 2003, 36: 79-87.
[22] TERRY L I.(Thysanoptera: Thripidae) oviposition in apple buds: Role of bloom state, blossom phenology, and population density.Environmental Entomology, 1991, 20(6):1568-1576.
[23] WOODHEAD A P, PAULSON C R. Larval development ofreared alone and in groups. Journal of Insect Physiology, 1983, 29(9): 665-668.
[24] OMKAR S, PATHAK. Crowding affects the life attributes of an aphidophagous ladybird beetle,. Bulletin of Insectology, 2009, 62(1): 35-40.
[25] ŠMITS A. Performance of pine looperlarvae under population build-up conditions. Entomologia Experimentalis et Applicata, 2002, 104(1): 117-124.
[26] GIBBS M, LACE L A, JONES M J, MOORE A J. Intraspecific competition in the speckled wood butterfly: Effect of rearing density and gender on larval life history. Journal of Insect Science, 2004, 4: 16.
[27] GIBBS M, BREUKER C J. Effect of larval-rearing density on adult life history traits and developmental stability of the dorsal eyespot pattern in the speckled wood butterfly,. Entomologia Experimentalis et Applicata, 2005, 118(1): 41-47.
[28] MCFARLANE J E, ALLI I, STEEVES E. Studies on the group effect in(L.) using artificial diets. Journal of Insect Physiology, 1984, 30(2): 103-107.
[29] YU E Y, GASSMANN A J, SAPPINGTON T W. Effects of larval density on dispersal and fecundity of western corn rootworm,LeConte (Coleoptera: Chrysomelidae). PLoS One, 2019,14(3): e0212696.
[30] 蒋善军, 罗礼智, 胡毅, 张蕾. Cry1Ac毒蛋白对粘虫生长发育、繁殖及飞行能力的影响. 昆虫学报, 2010, 53(12): 1360-1366.
JIANG S J, LUO L Z, HUy, ZHANG L. Effects of Cry1Ac protein on growth and development, reproduction and flight potential of the oriental armyworm,(Lepidoptera: Noctuidae). Acta Entomologica Sinica,2010, 53(12): 1360-1366. (in Chinese)
[31] 乔利, 潘兹亮, 卢兆成, 张丽霞, 仵均祥. 单头饲养与群体饲养对亚洲玉米螟生长发育与繁殖的影响. 西北农业学报, 2011, 20(10): 204-206.
QIAO L, PAN Z L, LU Z C, ZHANG L X, WU J X. Growth development and reproduction of the(Guenee) under single raising and group raising. Acta Agriculturae Boreali-occidentalis Sinica, 2011, 20(10): 204-206. (in Chinese)
[32] 李艳, 江幸福, 张蕾, 程云霞, 刘彦群, 罗礼智. 幼虫密度对二点委夜蛾生长发育及繁殖的影响. 应用昆虫学报, 2014, 51(3): 623-629.
LI Y, JIANG X F, ZHANG L, CHENG Y X, LIU Y Q, LUO L Z.Effects of larval density on the development and reproduction of. Chinese Journal of Applied Entomology, 2014, 51(3): 623-629. (in Chinese)
[33] LI X, FENG D, XUE Q, MENG T, MA R, DENG A, CHI H, WU Z, ATLIHAN R, MEN L, ZHANG Z. Density-dependent demography and mass-rearing of(Lepidoptera: Carposinidae) incorporating life table variability. Journal of Economic Entomology, 2019,112(1): 255-265.
[34] FANTINOU A A, PERDIKIS D C, STAMOGIANNIS N. Effect of larval crowding on the life history traits of(Lepidoptera: Noctuidae).European Journal of Entomology, 2008, 105(4): 625-630.
[35] FESCEMYER H W, HAMMOND A M. Effect of larval density and plant age on size and biochemical composition of adult migrant moths,Hübner (Lepidoptera: Noctuidae). Environmental Entomology, 1988, 17(2): 213-219.
[36] WEAVER D K, MCFARLANE J E. The effect of larval density on growth and development of. Journal of Insect Physiology, 1990, 36(7): 531-536.
[37] LYIMO E O, TAKKEN W, KOELLA J C. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of. Entomologia Experimentalis et Applicata, 1992, 63(3): 265-271.
[38] HAPAIRAI L K, MARIE J, SINKINS S P, BOSSIN H C. Effect of temperature and larval density on(Diptera: Culicidae) laboratory rearing productivity and male characteristics. Acta tropica, 2014,132(Suppl.): S108-S115.
[39] YANG F, HU G, SHI J J, ZHAI B P. Effects of larval density and food stress on life-history traits of(Lepidoptera: Pyralidae). Journal of Applied Entomology, 2015, 139(5): 370-380.
[40] SAPPINGTON T W, SHOWERS W B. Lack of translation of density-induced morphological polyphenism to long-duration flight behavior of black cutworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America, 1992, 85(2): 188-194.
[41] CHI H, YOU M S, ATLıHAN R, SMITH C L, KAVOUSI A, ÖZGöKçE M S, GüNCAN A, TUAN S J, FU J W, XU Y Y,. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomologia Generalis,2020, 40(2): 103-124.
[42] NING S, ZHANG W, SUN Y, FENG J. Development of insect life tables: comparison of two demographic methods of(Diptera: Anthomyiidae) on different hosts. Scientific reports, 2017, 7(1): 4821.
[43] CHI H, LIU H. Two new methods for the study of insect population ecology.Bulletin of the Institute of Zoology, Academia Sinica, 1985, 24(2): 225-240.
[44] CHANG C, HUANG CY, DAI SM, ATLIHAN R, CHI H. Genetically engineered ricin suppresses(Diptera: Tephritidae) based on demographic analysis of group-reared life table. Journal of Economic Entomology, 2016, 109(3): 987-992.
[45] CHI H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environmental Entomology, 1988,17(1): 26-34.
[46] CHI H, SU H Y. Age-stage, two-sex life tables of(Ashmead) (Hymenoptera: Braconidae) and its host(Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environmental Entomology, 2006, 35(1): 10-21.
[47] CHI H. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. 2009, http://140.120.197.173/Ecology/.
[48] EFRON B, TIBSHIRANI R J. An introduction to the bootstrapMonographs on Statistics and Applied Probability,1993, 57: 436.
[49] CHI H, YANG T C. Two-sex life table and predation rate ofThunberg (Coleoptera: Coccinellidae) fed on(Sulzer) (Homoptera: Aphididae). Environmental Entomology, 2003, 32(2): 327-333.
[50] LUDEWIG A H, GIMOND C, JUDKINS J C, THORNTON S, PULIDO D C, MICIKAS R J, DORING F, ANTEBI A, BRAENDLE C, SCHROEDER F C. Larval crowding acceleratesdevelopment and reduces lifespan. PLoS genetics, 2017,13(4): e1006717.
[51] DAKSHAYANI K, MATHAD S B. A comparative study of growth, development and survival of the cricketwalker reared singly and in groups. Experientia, 1973, 29(8): 978-979.
[52] DURISKO Z, KEMP R, MUBASHER R, DUKAS R. Dynamics of social behavior in fruit fly larvae. PloS one, 2014, 9(4): e95495.
[53] NIJHOUT H F. The control of body size in insects. Developmental Biology, 2003, 261(1): 1-9.
[54] KOYAMA T, MIRTH C K. Unravelling the diversity of mechanisms through which nutrition regulates body size in insects. Current Opinion of Insect Science, 2018, 25: 1-8.
[55] TARAVATI S, MANNION C. Effect of aggregation and cage setting on some life-history parameters of(Hemiptera: Aleyrodidae). Journal of Economic Entomology, 2016,109(1): 249-254.
[56] TSUNODA T, FUKUCHI A, NANBARA S, TAKAGI M. Effect of body size and sugar meals on oviposition of the yellow fever mosquito,(Diptera: Culicidae). Journal of Vector Ecology, 2010, 35(1): 56-60.
[57] BERGER D, WALTERS R, GOTTHARD K. What limits insect fecundity? Body size- and temperature-dependent egg maturation and oviposition in a butterfly. Functional Ecology, 2008, 22(3): 523-529.
[58] BLAY S, YUVAL B. Oviposition and fertility in the Mediterranean fruit fly (Diptera: Tephritidae): Effects of male and female body size and the availability of sperm. Annals of the Entomological Society of America,1999, 92(2):278-284.
[59] BABA M. Oviposition habits of(Diptera: Simuliidae), with reference to seasonal changes in body size and fecundity. Journal of Medical Entomology,1992, 29(4):603-610.
[60] EdwardsA W. The genetical theory of natural selection. Genetics, 2000, 154(4): 1419-1426.
[61] MIN K, FLATT T, KULAOTS I, TATAR M. Counting calories indiet restriction. Experimental Gerontology, 2007,42(3): 247-251.
[62] FLATT T. Survival costs of reproduction in.Experimental Gerontology, 2011, 46(5):369-375.
[63] FOWLER K, PARTRIDGE L. A cost of mating in female fruitflies. Nature, 1989, 338: 760-761.
[64] PARTRIDGE L, GREEN A, FOWLER K. Effects of egg-production and of exposure to males on female survival in.Journal of Insect Physiology, 1987, 33(10): 745-749.
[65] 杨广明, 郅军锐, 李顺欣, 张宇羽. 延迟交配对西花蓟马成虫寿命及繁殖力的影响. 山地农业生物学报, 2015, 34(4): 31-34.
YANG GM, ZHI JR, LI SX, ZHANG YY. Effect of delayed mating on adult longevity and reproduction of. Journal of Mountain Agriculture and Biology, 2015, 34(4): 31-34. (in Chinese)
Comparison of Life Tables for Experimental Populations of Individual-rearing and Group-rearing
Li Xinhua1, Wang Dengjie2, Lei Zhongren1, Wang Haihong1
1State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193;2Mianyang Academy of Agricultural Sciences, Mianyang 621000, Sichuan
】The method of individual-rearing (IR) was often used to study the life table of western flower thrip (), and the results can be used to predict the population occurrence under natural conditions in the field.However,often occur in groups rather than single head under natural conditions. The objective of this study to compare the life table parameters of individual-rearing and group-rearing (GR), and to explore the accuracy of population occurrence dynamics under natural conditions based on the data from individual-rearing and group-rearing.【】The age-stage, two-sex life table of the experimental population ofreared on bean pod was constructed by individual-rearing and group-rearing, respectively, and the life history and population parameters ofwere compared under the two conditions. The means and standard errors of population growth parameters were calculated using the bootstrap method. The Mann-Whitney test (test) was used to evaluate the differences in the population parameter, development period, and fecundity of individual-rearing and group-rearing.【】individual-rearing and group-rearing had significant effects on nymph stage, pupal stage, male longevity, total preoviposition period, per female oviposition, pupal weigh and adult body length, but not on egg stage, adult stage, adult preoviposition period, female longevity, pupal length, pupal width, adult width. The nymph stage (4.49 d), pupal stage (4.03 d), male longevity (22.82 d), total preoviposition period (11.37 d) of individual-rearingwere significantly longer than group-rearing ones (3.05, 3.32, 18.64 and 10.00 d, respectively). The pupal weigh (0.03 mg) of individual-rearingwas significantly lower than that of group-rearing(0.07 mg). The adult body lengths of individual-rearing(female: 203.72 μm, male: 149.74 μm) were significantly lower than those of group-rearing(female: 288.81 μm, male: 203.39 μm). The per female oviposition of individual-rearing(48) was significantly lower than that of group-rearing(133.39). The intrinsic rate of increase (), finite rate of increase (), net reproductive rate (0), gross reproductive rate () and mean generation time () of individual-rearingwere 0.161 d-1, 1.175 d-1, 20.730, 35.699, 18.70 d, respectively, while those of group-rearingwere 0.242 d-1, 1.274 d-1, 60.499, 102.342, 16.88 d, respectively. The population growth of individual-rearingwas slower than that of group-rearing.【】Compared with the individual-rearing, the population of group-rearinggrew faster and produced more offspring per unit time. Using the individual-rearing feeding method to establish a life table to predict the population dynamics ofmay delay the best control time. The life table established by the group-rearing method should predict the population dynamics more accurately.
; age-stage two-sex life table; individual-rearing; group-rearing

10.3864/j.issn.0578-1752.2021.05.008
2020-05-19;
2020-06-28
国家重点研发计划(2017YFD0201205)、国家现代农业产业技术体系专项资金(CARS-25-B-07)
李欣华,E-mail:15733275161@163.com。通信作者王海鸿,Tel:010-62815930;E-mail:wanghaihong2020@sina.com
(责任编辑 岳梅)
