眼部药物智能控释系统研究进展
2020-11-23吴彩清余敏斌杨扬帆
吴彩清?余敏斌?杨扬帆

【摘要】药物智能控释系统又称刺激-响应型药物控释系统,是近年药物剂型研究的热点,通过外加磁场、超声波、光等刺激,以及温度、酸碱度等控制,结合相应的高分子材料,达到药物的靶向、实时可控、定量定向给药。眼部的药物智能控释系统研究起步较晚,目前主要仍为基于原位凝胶给药体系的控释系统,其他如磁场、超声、光等外加刺激控释系统仍在起步阶段。精准的靶向治疗是医学发展的必然要求,因此药物精准控释也是未来较为理想的给药方式。本文综述眼部的药物智能控释研究,为眼部给药研究提供参考。
【关键词】眼部;刺激-响应型;药物控释
Research progress on intelligent controlled drug release system for ophthalmic drug Wu Caiqing, Yu Minbin, Yang Yangfan. State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510623, China
Corresponding author, Yang Yangfan, E-mail: yangyangfan@ gzzoc. com
【Abstract】The intelligent controlled drug release system, also known as the stimulus-responsive drug release system, has become a hotspot in the research of pharmaceutical dosage form in recent years. Through combining external magnetic field, ultrasound, light and other stimulants, as well as temperature and pH with corresponding polymer materials, targeted, real-time, quantitative and controlled drug release can be achieved. The research on the intelligent drug release system for ocular drugs has been conducted for a short period of time. The controlled release system based on the in-situ gel drug delivery system remains the major system. Other externally stimulated controlled release systems, such as magnetic field, ultrasound, and light, are still in their infancy. Precise targeted therapy is an inevitable requirement for medical development. Consequently, precise controlled release of drugs will become an ideal way of drug administration in the future. In this article, research progress on the intelligent controlled release for ophthalmic drugs was reviewed, aiming to provide reference for the research of ocular drug delivery.
【Key words】Ocular;Stimuli-responsive;Controlled drug release system
智能藥物控释系统也称为刺激-响应型药物控释系统。智能控释系统的药物载体也称为刺激-响应型载体,能够对外界刺激(如温度、磁场、pH等)产生快速和精确的应答,发生物理结构或者化学性质变化。刺激-响应型载体应用于载药,可实现控制药物的呈递和释放。随着材料化学与药物载体设计的进步,智能药物控释系统的应用使定点、定量和实时控制药物的释放有望成为现实。根据刺激的来源,可将这些智能控释系统的载体分为外源刺激响应型、内源刺激响应型及复合刺激响应型。外源性刺激是独立于病变外的环境,具有可操作及重复性,但需要考虑生物相容性及外界人员操控问题。内源性刺激是通过人体内局部反应的改变激活相应药物载体而释放药物,不需要他人额外的操作,无需考虑生物相容性问题,但为了激活相应载药系统,载体需要与刺激相互作用,不同患者不同疾病内源性刺激类别及强度不同,内源性刺激的应用需要局部病理改变为基础[1]。近年来,智能药物控释系统的眼部应用研究取得一定进展,本文就目前眼部研究相关的智能药物缓释系统进行归纳和综述。
一、外源刺激响应型
1. 温度响应型
温度响应型载体含有使其在温度改变时发生结构变化的组分,引起相变,从而释放所包载的药物。眼部目前研究多为温度响应型原位凝胶控释系统,其在储存温度下是液态,滴入眼内后,由于眼部温度相对高而使其发生相转变,形成凝胶。温度响应型原位凝胶体系中药物载体可由泊洛沙姆407(Poloxamer 407, 即Pluronic F-127)、壳聚糖(Chitosan, CS)、PNIPAAm等温度响应型聚合物构成。该体系使用的载体及所载药物多样,见表1。温度响应型原位凝胶载药系统不仅可用上述凝胶直接载药,也可用凝胶与其他载药体(如脂质体) 结合进行复合载药,见表2。此外,其他温度响应型载体如温控的弹性蛋白样多肽的眼部应用也可以延长药物的眼部作用时间,提高其生物利用度[2]。温度响应型载体眼部应用范围广,可载多种眼药,延长药物眼部作用时间,提高其生物利用度,也可与多种缓释体系结合成复合体系,但其应用会对患者视觉产生影响,只可在不需要用眼如睡眠时使用。
2. 磁响应型
磁响应型智能载药系统是利用磁场作为载药体的刺激,在磁响应的载药系中,可以通过恒定磁场对药物进行远程定向介导,也能通过交变磁场使药物局部升温,或者两者结合协同实现载药体的磁響应。眼部的磁响应载药体系还是处于相对初级阶段,应用磁场控制眼部药物的释放及眼内手术操作的微型机器人是近年研究热点,磁场介导的载药体系主要应用于眼后段。
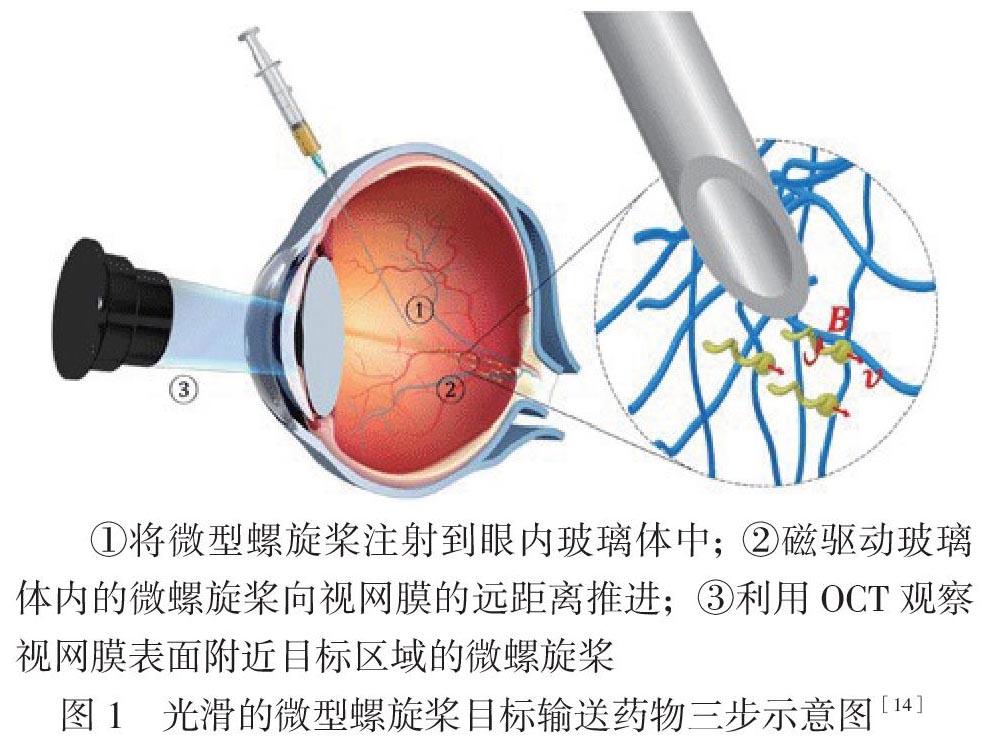
Wu等[14]应用磁场介导纳米硅和镍结合形成的微型螺旋桨样微车在玻璃体内的定向移动,使其定向到达视盘附近的视网膜,并用光学相干断层扫描监视其眼内运动及位置,见图1。Yee等[15]应用磁场驱动无针注射器进行体外玻璃体给药研究,并应用超声成像对药物进行定位。Ullrich等[16]开发了一种磁场控制眼内手术的微型机器人,研究其在玻璃体介质中进行手术操作,显示该微型机器人可应用于眼底相关疾病的靶向药物递送及眼内手术治疗。Dogangil等[17]研究出使用微型机器人进行体外视网膜药物递送,证实该可使用磁场控制微型机器人进行眼内靶向给药。
磁控机器人在眼部靶向给药及眼内手术治疗具有良好应用前景。但仍有一些问题需要解决,比如在相对弱的磁场中其作用的实施,特定环境使用时施加的力的大小以及速度,也就是磁场的大小及方向。在不干扰磁驱动情况下人眼内实时定位,以及磁控载体的安全性及其降解。
①将微型螺旋桨注射到眼内玻璃体中; ②磁驱动玻璃体内的微螺旋桨向视网膜的远距离推进;③利用OCT观察视网膜表面附近目标区域的微螺旋桨
图1 光滑的微型螺旋桨目标输送药物三步示意图[14]
3. 超声响应型
超声波具有非侵入性、无电离辐射、可穿透到达深部组织等优良特性,并能够通过超声频率、速率、时间进行便捷、灵活地调节。超声波通过热效应、机械效应产生的空化现象、能量辐射等效果触发药物从载体中释放出来。超声响应型载体在眼部药物控释的应用主要是经巩膜促进蛋白质等大分子的眼内可控扩散。
Huang等[18]将透明质酸包被的人血清白蛋白纳米粒,以1 MHz的频率、0.5 W/cm的强度和30 s的持续时间的超声,通过巩膜施用于离体牛眼,在预定的时间点分析玻璃体和视网膜中的人血清白蛋白的荧光强度。结果显示低频超声可安全、显著改善纳米粒通过玻璃体的扩散迁移率,以及促进它们穿过神经视网膜进入视网膜色素上皮和脉络膜的渗透型。Thakur等[19]评估了经巩膜或角膜超声应用对玻璃体内注射的罗丹明标记的纳米气泡分布影响,离体牛眼和猪眼实验证明超声处理显著增强了纳米气泡的定向迁移,多个角膜超声循环促进了染料向玻璃体后部的迁移。Cheung等[20]将兔眼浸入与异硫氰酸荧光素偶联的牛血清白蛋白溶液中,测量超声对血清白蛋白的眼内渗透影响,结果显示超声增强了巩膜内蛋白的渗透,使扩散率提高了1.6倍。
4. 光响应型
应用对特定波长光照(紫外区、可见光区或近红外区)反应的光敏系统可设计光响应型载体,用于控制药物释放,实现按需给药。光刺激响应系统具有非侵入性、患者易接受、灵活性高、精确性高等优势,能够进行非侵入性、远程、实时调控药物释放。可通过精确调节激活光的多个参数进行高精度的光敏系统的控制。这些参数包括光的波长、强度和极性以及辐照的时间、频率和位置。
Chen等[21]应用沸石咪唑的8-聚丙烯酸酸酯骨架(ZIF-8-PAA)载光敏剂甲基苯铵蓝(MB),而后用AgNO3/多巴胺将AgNO3原位还原为银纳米颗粒(AgNPs),最后用万古霉素/NH2-聚乙二醇(Van/NH2-PEG)进行二次修饰,形成复合纳米材料ZIF-8-PAA-MB @ AgNPs @ Van-PEG进行眼部抗菌治疗。结果表明在光的作用下,上述复合纳米颗粒可安全有效地释放出活性氧治疗眼内炎,可有效控制兔眼眼内炎。Mu等[22]应用光交联的作用使噻吗洛尔变构与接触镜单体结合载于接触镜,自然光照作用下,药量为滴眼液的5.7%时,可持续释放治疗剂量的噻吗洛尔达10 h,有效降低眼内压。Basuki等[23]应用带正电荷的聚(甲基丙烯酰氧基乙基三甲基氯化铵)[P(METAC)]修饰纳米金粒子,将其和anti-VEGF药物载于琼脂糖凝胶,在可见光的作用下,纳米金粒子吸收光产生热使得凝胶升温变软,释放出药物,达到药物的可控释放。
光刺激响应载药系统虽然有其独有的可控及易得优势,但其仍面临许多困难:①缺乏生物相容性和可生物降解的光反应材料;②紫外光激活相应缓释体系对人体具有基因毒性且穿透力弱,近红外光毒性低而组织穿透性强。因此,开发使用长波长或双光子响应的新型光敏剂,能够实现更深的组织穿透和更小的组织伤害,近红外光触发药物的可控载药体系更具临床应用前景。
二、内源刺激响应型
1. pH响应型
眼部应用的pH响应型载药缓释体系大都是原位凝胶系统。凝胶中的聚合物大分子含有可因pH改变而解离的基团,在眼表的pH改变作用下发生解离,使得聚合物发生相变形成凝胶。pH响应型眼部原位凝胶缓释系统载体主要是卡波姆、壳聚糖及HPMC构成,见表3。pH响应型原位凝胶载药系统与温度响应型原位凝胶载药系统的优缺点相似。
2. 离子强度响应型
离子强度响应型药物释放体系,是应用泪液中含有的阳离子(如Na+、Ca2+等)与聚合物发生络合反应使得聚合物构象发生改变而导致其相变形成凝胶。眼部离子强度响应型载药原位凝胶系统的响应聚合物主要包括结冷胶、黄原胶、藻酸盐等,见表4。离子强度响应型原位凝胶载药系统与温度响应型原位凝胶载药系统的优缺点相似。
3. 其他刺激响应型
Kim等[33]应用PEI包被的纳米金刚石(ND)在噻吗洛尔存在下与壳聚糖交联形成纳米凝胶而后载入接触镜,结果显示没有溶菌酶作用下无药物释放,溶菌酶作用下药物持续释放达24 h,见图2。Pornpattananangkul等[34]报道了通过细菌毒素来诱发磷脂脂质体释放万古霉素治疗耐甲氧西林金黄色葡萄球菌(MRSA)感染,在MRSA存在情况下,包载的万古霉素可在24 h内持续作用抑制细菌的增殖,抑菌效用与未包载万古霉素相当。眼部溶菌酶及细菌毒素响应型药物控释系统,可在眼部温和条件下发挥作用,并有高度选择性,由于刺激因素的持续存在,可以使得药物持续释放,但不同个体及部位的刺激的量及浓度不同,药物释放需要刺激响应载体具有相对高的灵敏度。
三、复合刺激响应型
复合刺激响应型载体,是指其对药物的释放受两种或以上刺激的控制。目前研究的眼部复合刺激响应型药物载体最为多的是温度及pH双敏感刺激响应型凝胶。该类凝胶同时含有温度及pH刺激響应成分,即壳聚糖及泊洛沙姆407而产生双刺激敏感响应,两种刺激结合最合适时达药物最佳释放。Gupta[35]等应用壳聚糖及结冷胶形成pH及离子强度双敏感响应型凝胶载噻吗洛尔,可安全有效延长其眼部作用时间。Rahanyan-K?gi等[36]使用单油精及3种脂质合成新型刺激-反应型纳米材料主客体脂质立方相(LCPs),结果表明其通过改变脂质体的种类形成了pH及紫外光控的亲水性缓释体系,该新型LCPs体系具有生物相容性、稳定、透明及非水溶性,可应用于眼部控释载药。复合刺激响应型系统可通过协同作用增强或持续递送药物,可以更好地控释药物。
四、小结与展望
如何有效地递送药物至靶组织,并高效释放药物治疗疾病的同时对其他邻近组织无毒性作用,这仍然是眼部给药体系面临的重要瓶颈,药物智能控释系统的载体是根据环境的变化而改变自身的物理行为或化学结构,从而实现定点、定量和定时的药物释放,达到药物高效靶向的治疗效果。现有的眼部智能控释系统包括凝胶、聚合物纳米粒、树枝状聚合物和无机纳米粒子等。尽管其在根据目的及需求控释药物方面有许多优势,但仍有一些问题需要解决,控释体系的毒性、稳定性、生物相容性及生物降解性,以及将药物有效递送至靶目标治疗区。合成的聚合物通常因为毒性限制了其应用,而无机纳米材料通常由于生物不相容及不可降解(如Au、Ag纳米粒, Au纳米棒)而限制其应用。构建的智能药物控释系统的药物载体需要具有生物相容和易降解、高载药量,在递送过程维持药物稳定不丢失等特性,并在各种环境刺激下释放的药物能够优先富集于病变部位[37]。此外,集成多种刺激响应为一体的智能控释系统药物载体,可以更加灵敏地根据不同的环境做出相适应的应答,精确地按需控制药物呈递和释放,是智能给药系统研究中的未来方向。
参 考 文 献
[1] Wehrung D, Chamsaz EA, Andrews JH, Joy A, Oyewumi MO. Engineering alkoxyphenacyl-polycarbonate nanoparticles for potential application in near-infrared light-modulated drug delivery via photon up-conversion process. J Nanosci Nanotechno, 2016,17(7):4867-4881.
[2] Wang W, Lee C, Pastuszka M, Laurie GW, MacKay JA. Thermally-responsive loading and release of elastin-like polypeptides from contact lenses. Pharmaceutics, 2019,11(5):221.
[3] Zhu M, Wang J, Li N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif Cells Nanomed Biotechnol,2018,46(6):1282-1287.
[4] Shi H, Wang Y, Bao Z, Lin D, Liu H, Yu A, Lei L, Li X, Xu X. Thermosensitive glycol chitosan-based hydrogel as a topical ocular drug delivery system for enhanced ocular bioavailability. Int J Pharm, 2019,570:118688.
[5] Wei Y, Li C, Zhu Q, Zhang X, Guan J, Mao S. Comparison of thermosensitive in situ gels and drug-resin complex for ocular drug delivery: in vitro drug release and in vivo tissue distribution. Int J Pharm, 2020,578:119184.
[6] Li J, Liu H, Liu LL, Cai CN, Xin HX, Liu W. Design and evaluation of a brinzolamide drug-resin in situ thermosensitive gelling system for sustained ophthalmic drug delivery. Chem Pharm Bull (Tokyo),2014, 62(10):1000-1008.
[7] Luo Z, Jin L, Xu L, Zhang ZL, Yu J, Shi S, Li X, Chen H. Thermosensitive PEG-PCL-PEG (PECE) hydrogel as an in situ gelling system for ocular drug delivery of diclofenac sodium. Drug Deliv, 2016,23(1):63-68.
[8] Xi L, Wang T, Zhao F, Zheng Q, Li X, Luo J, Liu J, Quan D, Ge J. Evaluation of an injectable thermosensitive hydrogel as drug delivery implant for ocular glaucoma surgery. PLoS One, 2014,9(6):e100632.
[9] Phua JL, Hou A, Lui YS, Bose T, Chandy GK, Tong L, Venkatraman S, Huang Y. Topical delivery of senicapoc nanoliposomal formulation for ocular surface treatments. Int J Mol Sci, 2018,19(10):2977.
[10] He W, Guo X, Feng M, Mao N. In vitro and in vivo studies on ocular vitamin A palmitate cationic liposomal in situ gels. Int J Pharm,2013,458(2):305-314.
[11] Liu R, Sun L, Fang S, Wang S, Chen J, Xiao X, Liu C. Thermosensitive in situ nanogel as ophthalmic delivery system of curcumin: development, characterization, in vitro permeation and in vivo pharmacokinetic studies. Pharm Dev Technol, 2016,21(5):576-582.
[12] Tan G, Yu S, Li J, Pan W. Development and characterization of nanostructured lipid carriers based chitosan thermosensitive hydrogel for delivery of dexamethasone. Int J Biol Macromol, 2017,103:941-947.
[13] Almeida H, Lob?o P, Frigerio C, Fonseca J, Silva R, Sousa Lobo JM, Amaral MH. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm Dev Technol, 2017,22(3):336-349.
[14] Wu Z, Troll J, Jeong HH, Wei Q, Stang M, Ziemssen F, Wang Z, Dong M, Schnichels S, Qiu T, Fischer P. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci Adv, 2018,4(11):eaat4388.
[15] Yee MQY, Yeow BS, Ren H. Dispersion characterization of magnetic actuated needleless injections with particle image velocimetry. Med Biol Eng Comput, 2019,57(11):2435-2447.
[16] Ullrich F, Bergeles C, Pokki J, Ergeneman O, Erni S, Chatzipirpiridis G, Pané S, Framme C, Nelson BJ. Mobility experiments with microrobots for minimally invasive intraocular surgery. Invest Ophthalmol Vis Sci, 2013,54(4):2853-2863.
[17] Dogangil G, Ergeneman O, Abbott JJ, Pan S, Hall H, Mun-twyler S, Nelson BJ. Toward targeted retinal drug delivery with wireless magnetic microrobots // 2008 {IEEE/RSJ} International Conference on Intelligent Robots and Systems. Nice: IEEE, 2008: 1921-1926.
[18] Huang D, Chen YS, Thakur SS, Rupenthal ID. Ultrasound-mediated nanoparticle delivery across ex vivo bovine retina after intravitreal injection. Eur J Pharm Biopharm, 2017, 119:125-136.
[19] Thakur SS, Chen YS, Houston ZH, Fletcher N, Barnett NL, Thurecht KJ, Rupenthal ID, Parekh HS. Ultrasound-responsive nanobubbles for enhanced intravitreal drug migration: an ex vivo evaluation. Eur J Pharm Biopharm,2019,136:102-107.
[20] Cheung AC, Yu Y, Tay D, Wong HS, Ellis-Behnke R, Chau Y. Ultrasound-enhanced intrascleral delivery of protein. Int J Pharm,2010,401(1-2):16-24.
[21] Chen H, Yang J, Sun L, Zhang H, Guo Y, Qu J, Jiang W, Chen W, Ji J, Yang YW, Wang B. Synergistic chemotherapy and photodynamic therapy of endophthalmitis mediated by zeolitic imidazolate framework-based drug delivery systems. Small, 2019, 15(47): e1903880.
[22] Mu C, Shi M, Liu P, Chen L, Marriott G. Daylight-mediated, passive, and sustained release of the glaucoma drug timolol from a contact lens. ACS Cent Sci, 2018,4(12):1677-1687.
[23] Basuki JS, Qie F, Mulet X, Suryadinata R, Vashi AV, Peng YY, Li L, Hao X, Tan T, Hughes TC. Photo-modulated therapeutic protein release from a hydrogel depot using visible light. Angew Chem Int Ed Engl, 2017,56(4):966-971.
[24] Gupta S, Vyas SP. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci Pharm,2010,78(4):959-976.
[25] Allam A, El-Mokhtar MA, Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J Pharm Pharmacol,2019,71(8):1209-1221.
[26] Singh J, Chhabra G, Pathak K. Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm, 2014,40(9):1223-1232.
[27] Maulvi FA, Choksi HH, Desai AR, Patel AS, Ranch KM, Vyas BA, Shah DO. pH triggered controlled drug delivery from contact lenses: addressing the challenges of drug leaching during sterilization and storage. Colloids Surf B Biointerfaces,2017,157:72-82.
[28] Geethalakshmi A, Karki R, Jha SK, Venkatesh DP, Nikunj B. Sustained ocular delivery of brimonidine tartrate using ion activated in situ gelling system. Curr Drug Deliv,2012,9(2):197-204.
[29] Morsi N, Ibrahim M, Refai H, EI Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci, 2017,104:302-314.
[30] Li P, Wang S, Chen H, Zhang S, Yu S, Li Y, Cui M, Pan W, Yang X. A novel ion-activated in situ gelling ophthalmic delivery system based on κ-carrageenan for acyclovir. Drug Dev Ind Pharm, 2018,44(5):829-836.
[31] Kesavan K, Kant S, Pandit JK. Therapeutic effectiveness in the treatment of experimental bacterial keratitis with ion-activated mucoadhesive hydrogel. Ocul Immunol Inflamm, 2016,24(5):489-492.
[32] Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 2: Precorneal retention and in vivo pharmacodynamic study. Int J Pharm, 2011,411(1-2):78-85.
[33] Kim HJ, Zhang K, Moore L, Ho D. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano, 2014,8(3):2998-3005.
[34] Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang L. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc,2011,133(11):4132-4139.
[35] Gupta H, Velpandian T, Jain S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target,2010,18(7):499-505.
[36] Rahanyan-K?gi N, Aleandri S, Speziale C, Mezzenga R, Landau EM. Stimuli-responsive lipidic cubic phase: triggered release and sequestration of guest molecules. Chemistry, 2015,21(5):1873-1877.
[37] 黃守坚.药物的控释制剂.新医学,1996,27(10):556-557.
(收稿日期:2020-07-25)
(本文编辑:郑巧兰)
