骨髓纤维化与非霍奇金淋巴瘤骨髓病理学特征及预后
2020-09-29吴春萌崔渤莉费海荣刘晓丹孙玲洁赵春亭
吴春萌 崔渤莉 费海荣 刘晓丹 孙玲洁 赵春亭

[摘要] 目的 探討骨髓纤维化(MF)与非霍奇金淋巴瘤(NHL)病人的骨髓病理特征及其对预后影响。方法对215例NHL病人(其中96例合并MF)的临床资料回顾分析,观察病人骨髓病理学特点,比较合并与未合并MF病人治疗6个周期缓解率、总生存率(OS)及无疾病进展生存率(PFS)的差异。结果 Ⅲ期和Ⅳ期NHL病人较Ⅰ期和Ⅱ期更易合并MF(Z=-2.548,P<0.05)。合并MF的NHL病人脾大例数较未合并MF的NHL病人多,两组比较差异有显著性(χ2=13.019,P<0.05)。合并MF病人化疗后3~4度骨髓抑制发生率较未合并MF者高(Z=-3.413,P<0.05)。合并和未合并MF的NHL病人治疗6周期完全缓解率差异无显著性(χ2=0.261,P>0.05)。寿命表分析显示,合并MF的NHL病人1、2、3年PFS及OS分别为83%、68%、55%和86%、85%、60%,未合并MF的NHL病人的1、2、3年PFS及OS分别为94%、81%、81%和96%、95%、95%,两组OS及PFS比较差异有显著性(χ2=6.077、5.443,P<0.05)。COX回归生存分析显示,合并MF是影响淋巴瘤病人OS(RR=0.357,95%CI=0.136~0.933,P<0.05)和PFS (RR=0.459,95%CI=0.239~0.884,P<0.05)的独立不良预后因素。结论 MF是NHL病人预后的危险因素,合并MF可降低NHL病人的PFS及OS,可能与化疗后严重骨髓抑制引起早期死亡有关。
[关键词] 淋巴瘤,非霍奇金;原发性骨髓纤维化;预后
[中图分类号] R733.4 [文献标志码] A [文章编号] 2096-5532(2020)05-0544-05
doi:10.11712/jms.2096-5532.2020.56.145 [开放科学(资源服务)标识码(OSID)]
[ABSTRACT] Objective To investigate the pathological features of bone marrow in patients with myelofibrosis (MF) and non-Hodgkin lymphoma (NHL) and their influence on prognosis. Methods A retrospective analysis was performed for the clinical data of 215 patients with NHL, among whom 96 patients had MF, and the pathological features of bone marrow were observed. Complete response (CR) rate, overall survival (OS) rate, and progression-free survival (PFS) rate were compared between the patients with MF and those without MF after 6 cycles of chemotherapy. Results The patients with stage Ⅲ/Ⅳ NHL were more likely to experience MF than those with stage Ⅰ/Ⅱ NHL (Z=-2.548,P<0.05). Compared with patients without secondary myelofibrosis, there are more symptoms of splenomegaly in patients with non-Hodgkins lymphoma with secondary myelofibrosis, the difference between the two is significant (χ2=13.019,P<0.05). The patients with MF had a significantly higher incidence rate of grade 3-4 myelosuppression than those without MF (Z=-3.413,P<0.05). There was no significant difference in CR rate after 6 cycles of treatment between NHL patients with MF and those without MF (χ2=0.261,P>0.05). The life table analysis showed that in the NHL patients with MF, the 1, 2, and 3 year PFS rates were 83%, 68%, and 55%, respectively, and the 1, 2, and 3 year OS rates were 86%, 85%, and 60%, respectively; in the NHL patients without MF, the 1, 2, and 3 year PFS rates were 94%, 81%, and 81%, respectively, and the 1, 2, and 3 year OS rates were 96%, 95%, and 95%, respectively; there were significant differences in OS and PFS rates between the two groups (χ2=6.077,5.443;P<0.05). The Cox regression survival analysis showed that in patients with NHL, MF was an independent negative prognostic factor for OS (RR=0.357,95%CI=0.136-0.933,P<0.05) and PFS (RR=0.459,95%CI=0.239-0.884,P<0.05). Conclusion MF is a risk factor for the prognosis of patients with NHL and can shorten PFS and OS of patients with NHL, which may be associated with early death caused by severe myelosuppression after chemotherapy.
[KEY WORDS] lymphoma, non-Hodgkin; primary myelofibrosis; prognosis
非霍奇金淋巴瘤(NHL)是一组异质性很大的淋巴增殖性疾病,绝大多数的NHL表现为淋巴结和(或)髓外淋巴组织受累。随着NHL的进展,逐渐出现骨髓侵犯。而合并骨髓纤维化(MF)是否会对NHL的预后有影响,目前国内外相关研究较少。本研究通过对NHL伴有MF病人的临床病理特征及其与预后的关系进行分析,探讨NHL合并MF对疾病的预后影响,为其治疗提供循证医学依据。
1 资料和方法
1.1 研究对象
收集我院2016年6月—2018年6月收治的215例NHL病人的临床资料。所有病人化疗后骨髓抑制分度按照WHO规定的抗肿瘤药物急性与亚急性毒副作用中血液学的分度标准;MF分级采用欧洲MF分级共识标准[1],MF-1级及以上即可判断为MF;淋巴瘤分类按照2019年WHO淋巴瘤分类[2]。NHL合并MF(A组)的96例病人中,前驱淋巴性肿瘤(T淋巴母细胞淋巴瘤)1例,成熟B细胞淋巴瘤74例(其中弥漫大B细胞淋巴瘤49例,滤泡性淋巴瘤9例,结外黏膜相关淋巴组织边缘带B细胞淋巴瘤5例,淋巴浆细胞淋巴瘤3例,结内边缘带B细胞淋巴瘤及套细胞淋巴瘤各2例,脾边缘带淋巴瘤1例,未明确分型B细胞淋巴瘤3例),成熟T/NK细胞淋巴瘤21例(血管免疫母细胞T细胞淋巴瘤6例,外周T细胞淋巴瘤6例,结外NK/T细胞淋巴瘤5例,ALK阳性间变性大细胞淋巴瘤和ALK阴性间变性大细胞淋巴瘤各2例)。NHL非合并MF(B组)病人119例。所有病人诊断和治疗均符合2018年版淋巴瘤诊疗规范[3]。
1.2 骨髓涂片和病理标本制备
骨髓涂片和骨髓活检标本取材于病人髂前上棘或髂后上棘,在采集骨髓液涂片同时取骨髓活组织送检,骨髓涂片采用瑞氏-吉姆萨染色,骨髓活检组织切片采用苏木精-吉姆萨-酸性品红(HGF)染色,观察病人骨髓病理学特点。
1.3 治疗及随访
病人的治疗采用以CHOP(环磷酰胺+长春新碱+表柔比星+泼尼松)为基础的方案化疗,平均治疗6周期。所有病人均通过门诊或电话随访至2018年12月,总生存时间为疾病确诊时间至死亡或随访结束。收集病人性别、年龄、分期、病理分类、MF分级、血常规、基因检测、治疗方案、化疗后骨髓抑制程度及转归等指标。
1.4 统计学分析
采用SPSS 17.0软件进行统计分析,分期及骨髓抑制程度比较采用秩和检验,率的比较采用卡方检验,生存率分析采用寿命表法,單因素生存分析采用Log-rank检验,多因素分析采用COX回归生存分析。P<0.05表示差异有统计学意义。
2 结 果
2.1 合并MF的NHL病人的临床及病理特征
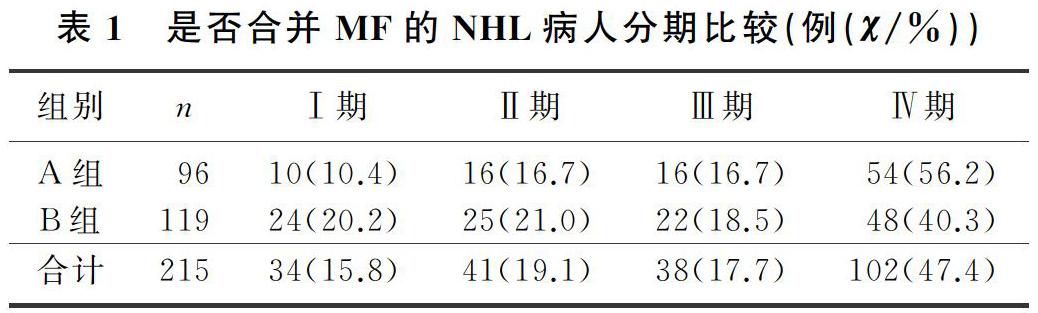
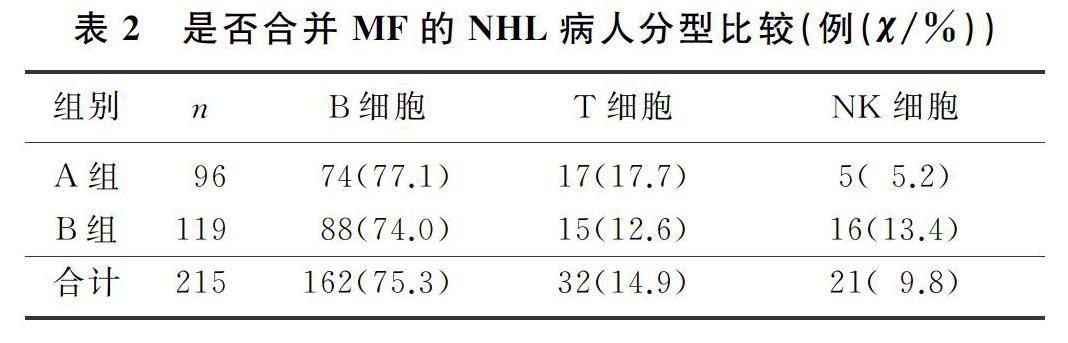
本文215例NHL病人中,男120例,女95例;年龄17~85岁,平均(57±13)岁。NHL分型:B细胞来源162例(75.3%),T细胞来源32例(14.9%),NK细胞来源21例(9.8%)。临床分期(Ann Arbor分期):Ⅰ期34例(15.8%),Ⅱ期41例(19.1%),Ⅲ期38例(17.7%),Ⅳ期102例(47.4%)。合并MF的NHL病人有96例,其中27例(28.1%)脾大;未合并MF的NHL病人有119例,其中11例(9.2%)脾大,两组脾大例数比较差异有统计学意义(χ2=13.019,P<0.05)。合并MF的NHL病人中,81例病人骨髓增生活跃或明显活跃,15例病人增生欠活跃或增生低下,表现为粒系增生正常,以中、晚期粒细胞为主;红系增生正常,以中、晚幼红细胞为主;分裂象可见,成熟红细胞大小不一。合并MF的NHL病人骨髓活检可见纤维组织不同程度增生,MF-1级78例(81.2%),MF-2级为16例(16.7%),MF-3级2例(2.1%)。
2.2 是否合并MF的NHL病人分期与分型比较
各分期NHL病人均可合并MF,Ⅰ期和Ⅱ期NHL病人75例,其中26例(34.7%)合并MF;Ⅲ期和Ⅳ期NHL病人140例,其中70例(50.0%)合并MF,Ⅲ期和Ⅳ期较Ⅰ期和Ⅱ期更易合并MF,二者比较差异有显著性(Z=-2.548,P<0.05)。215例NHL病人中,弥漫大B细胞淋巴瘤112例,其中合并MF者49例(43.7%);滤泡性淋巴瘤16例,其中合并MF者9例(56.3%);结外NK/T细胞淋巴瘤21例,其中合并MF者5例(23.8%),3种病理类型病人合并MF差异无统计学意义(χ2=4.314,P>0.05)。各种病理类型NHL均可合并MF,B细胞、T细胞、NK细胞来源NHL合并MF差异无显著性(P>0.05)。见表1、2。
2.3 是否合并MF的NHL病人治疗缓解率及骨髓抑制程度比较
本文215例NHL病人治疗6周期后,合并MF的NHL病人达到完全缓解33例(34.4%),未合并MF的NHL病人达到完全缓解37例(31.1%),两组比较差异无统计学意义(χ2=0.261,P>0.05)。合并MF的弥漫大B细胞淋巴瘤病人达到完全缓解23例(46.9%),未合并MF的弥漫大B细胞淋巴瘤病人达到完全缓解22例(34.9%),两组比较差异无统计学意义(χ2=1.656,P>0.05);合并MF的B细胞来源淋巴瘤病人达到完全缓解33例(44.6%),未合并MF的B细胞来源淋巴瘤病人达到完全缓解34例(38.6%),两组比较差异也无统计学意义(χ2=0.588,P>0.05)。
本文96例合并MF的NHL病人中,出现0~2度骨髓抑制23例(24.0%),3~4度骨髓抑制73例(76.0%);119例未合并MF的NHL病人中,出现0~2度骨髓抑制48例(40.3%),3~4度骨髓抑制71例(59.7%),合并MF的NHL病人更易发生较严重骨髓抑制,二者比较差异均具有显著意义(Z=-3.413,P<0.05)。见表3。
[参考文献]
[1] THIELE J, KVASNICKA H M, FACCHETTI F, et al. European consensus on grading bone marrow fibrosis and assessment of cellularity[J]. Haematologica, 2005,90(8):1128-1132.
[2] OTT G, KLAPPER W, FELLER A C, et al. Revised version of the 4th edition of the WHO classification of malignant lymphomas: what is new[J]? Pathologe, 2019,40(2):157-168.
[3] 中华人民共和国卫生和计划生育委员会医政医管局,中华医学会肿瘤学分会. 中国结直肠癌诊疗规范(2017年版)(摘编)[J]. 肿瘤综合治疗电子杂志, 2018,4(2):29-37.
[4] 肖志坚. 骨髓增生异常综合征和骨髓增殖性肿瘤的诊断分型应重视的一些问题[J]. 中华血液学杂志, 2010,31(4):217-218.
[5] 董家蔷,张剑,聂泽强,等. 80例继发性骨髓纤维化患者临床特点和骨髓活检分析[J]. 哈尔滨医科大学学报, 2009,43(6):603-608.
[6] 黄艳,孙嘉峰,杨佳,等. 继发性与原发性骨髓纤维化骨髓组织形态学观察及临床意义[J]. 山西医科大学学报, 2012,43(6):453-481.
[7] BHATT V R, BOCIEK R G, YUAN J, et al. Leukemic diffuse large B-cell lymphoma in a patient with myeloproliferative disorder[J]. J Natl Compr Cancer Netw: JNCCN, 2015,13(3):281-287.
[8] SHOUSE G, NIKOLAENKO L. Targeting the JAK/STAT pathway in T cell lymphoproliferative disorders[J]. Current Hematologic Malignancy Reports, 2019,14(6):570-576.
[9] WALDMANN T A, CHEN J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: implications for immunotherapy[J]. Annu Rev Immunol, 2017,35:533-550.
[10] RUMI E, BARAT C, BENEVOLO G, et al. Myeloproli-ferative and lymphoproliferative disorders: state of the art[J]. Hematological Oncology, 2020,38(2):121-128.
[11] TEFFERI A. Myelofibrosis with myeloid metaplasia[J]. The New England Journal of Medicine, 2000,342(17):1255-1265.
[12] CHOI J, CHO H, KANG S, et al. Intravascular large B-cell lymphoma associated with myelofibrosis:a case report[J]. Molecular and Clinical Oncology, 2017.doi:10.3892/mco. 2017.1398.
[13] FU R, YU H, WU Y H, et al. Hodgkins lymphoma associa-ted with myelofibrosis: a case report[J]. Oncol Lett, 2015,10(3):1551-1554.
[14] OKABE S, MIYAZAWA K, IGUCHI T, et al. Peripheral T-cell lymphoma together with myelofibrosis with elevated plasma transforming growth factor-beta1[J]. Leuk Lymphoma, 2005,46(4):599-602.
[15] MATSUNAGA T, TAKEMOTO N, MIYAJIMA N, et al. Splenic marginal zone lymphoma presenting as myelofibrosis associated with bone marrow involvement of lymphoma cells which secrete a large amount of TGF-β[J]. Annals of Hematology, 2004,83(5):322-325.
[16] 張怡安,刘澎. 骨髓增殖性肿瘤与淋巴系统肿瘤[J]. 中国实用内科杂志, 2019,39(2):135-138.
[17] CERVANTES F, MARTINEZ-TRILLOS A. Myelofibrosis:an update on current pharmacotherapy and future directions[J]. Expert Opinion on Pharmacotherapy, 2013,14(7):873-884.
[18] MATSUI K, ADACHI M, TOMINAGA T, et al. Angioimmunoblastic T cell lymphoma associated with reversible mye-lofibrosis[J]. Intern Med Tokyo Jpn, 2008,47(21):1921-1924.
[19] 许雯,李晓霞. 以骨髓受累为首发表现的非霍奇金淋巴瘤的临床特点[J]. 哈尔滨医科大学学报, 2018,52(1):45-48.
[20] 张文娟,丛琳,肖静,等. 以脾大为首发表现的恶性淋巴瘤24例临床分析[J]. 中外医学研究, 2014,12(12):15-16.
[21] 王文佳,董丽华,尹青松,等. 淋巴瘤合并骨髓纤维化五例临床分析并文献复习[J]. 中华血液学杂志, 2016,37(2):157-159.
[22] MASAROVA L, NEWBERRY K J, PIERCE S A, et al. Association of lymphoid malignancies and Philadelphia-chromosome negative myeloproliferative neoplasms: clinical characteristics, therapy and outcome[J]. Leuk Res, 2015,39(8):822-827.
[23] 劉亚琳,王雯娟,王晓宁. 非霍奇金淋巴瘤继发骨髓纤维化的骨髓病理学特征及其与疾病预后的关系[J]. 中国实验血液学杂志, 2015,23(3):674-678.
[24] 刘静,薛梅,阎洪敏,等. B淋巴母细胞淋巴瘤合并骨髓纤维化一例并文献复习[J]. 白血病·淋巴瘤, 2011,20(11):687-689.
[25] 祁明芳,郁知非. 继发于非霍奇金淋巴瘤的可逆性骨髓纤维化一例[J]. 临床血液学杂志, 1999,12(1):48.
[26] LARSON R A, HOCHHAUS A, HUGHES T P, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3 year follow-up [J]. Leukemia, 2012,26(10):2107-2203.
(本文编辑 黄建乡)
